Weed Control Efficacy and Crop-Weed Selectivity of a New Bioherbicide WeedLock
Abstract
1. Introduction
2. Materials and Methods
2.1. Glasshouse Experiment
2.1.1. Experimental Site
2.1.2. Test Plants
2.1.3. Experimental Treatments and Design
2.1.4. Cultural Practices
2.1.5. Data Collection
Plant Injury
Fresh and Dry Weight
Plant Height
2.2. Field Trial Experiment
2.2.1. Experimental Site and Field Setup
2.2.2. Initial Vegetation Analysis
2.2.3. Herbicide Treatments
2.2.4. Data Collection
Dominance of Weed Species
Evaluation of Treatment Efficacy
Fresh and Dry Weight
2.3. Statistical Analysis
3. Results
3.1. Glasshouse Experiment
3.1.1. Effects of WeedLock, Glyphosate Isopropyl-Amine, and Glufosinate-Ammonium on Visual Injury of Selected Weed Species
3.1.2. Effects of WeedLock, Glyphosate Isopropyl-Amine, and Glufosinate-Ammonium on Fresh Weight, Dry Weight, and Plant Height of Selected Weed Species
3.1.3. Effects of WeedLock, Glyphosate Isopropyl-Amine, and Glufosinate-Ammonium on Weed Control Efficiency of Selected Weed Species
3.1.4. Effects of WeedLock, Glyphosate Isopropyl-Amine, and Glufosinate-Ammonium on Injury Scale of Selected Crop Species
3.1.5. Effects of WeedLock, Glyphosate Isopropyl-Amine, and Glufosinate-Ammonium on Fresh Weight, Dry Weight, and Growth Reduction of Selected Crop Species
3.2. Field Trial Experiment
3.2.1. Floristic Weed Composition
3.2.2. Coefficient of Similarity
3.2.3. Effects of WeedLock, Glyphosate Isopropyl-Amine, and Glufosinate-Ammonium on Injury Scale of Weed Composition
3.2.4. Effects of WeedLock, Glyphosate Isopropyl-Amine, and Glufosinate-Ammonium on the Fresh and Dry Weight of Weed Composition
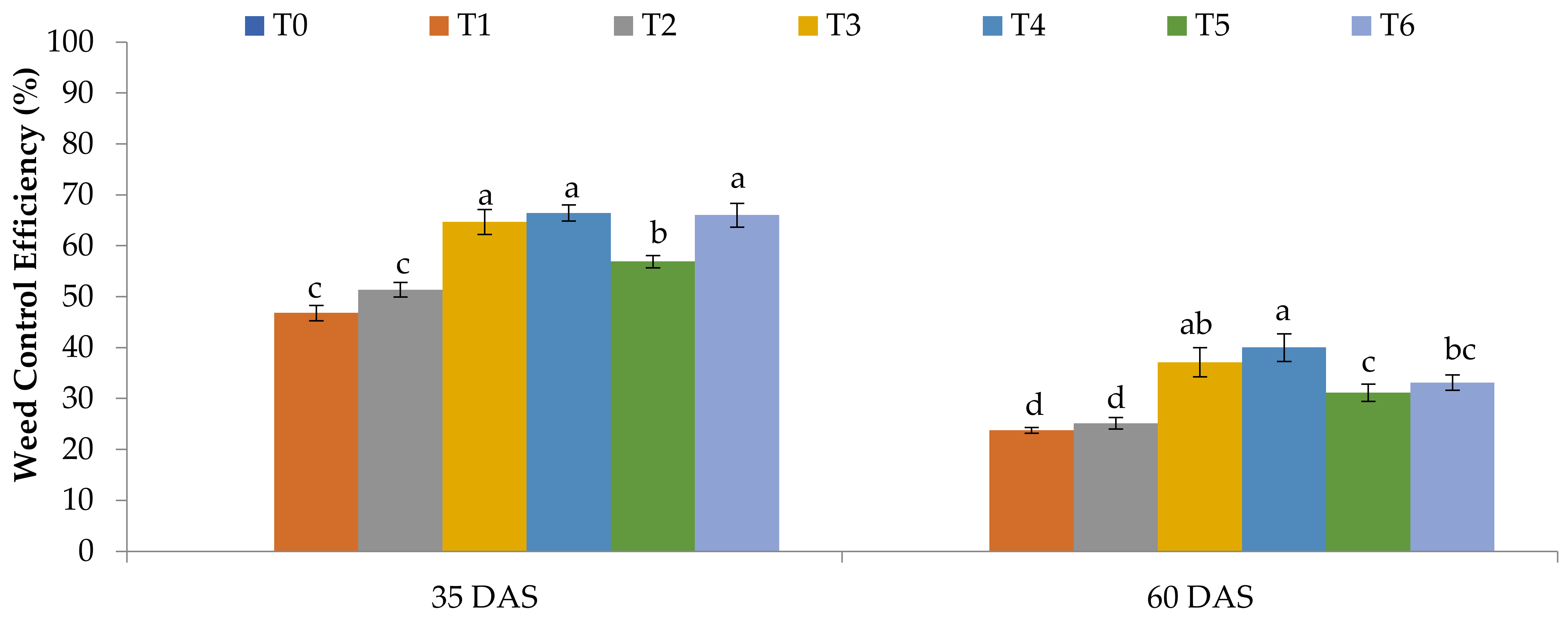
3.2.5. Effects of WeedLock, Glyphosate Isopropyl-Amine, and Glufosinate-Ammonium on Weed Control Efficiency of Weed Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juraimi, A.S.; Uddin, M.K.; Anwar, M.P.; Mohamed, M.T.M.; Ismail, M.R.; Man, A. Sustainable weed management in direct seeded rice culture: A review. Aust. J. Crop Sci. 2013, 7, 989–1002. [Google Scholar]
- Abouziena, H.F.; Haggag, W.M. Weed control in clean agriculture: A review. Planta Daninha 2016, 34, 377–392. [Google Scholar] [CrossRef]
- Heap, I. Herbicide resistant weeds. In Integrated Pest Management; Springer: Dordrecht, The Netherlands, 2014; pp. 281–301. [Google Scholar]
- Ruzmi, R.; Ahmad-Hamdani, M.S.; Bakar, B.B. Prevalence of herbicide-resistant weed species in Malaysian rice fields: A review. Weed Biol. Manag. 2017, 17, 3–16. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. Higher peroxidase activity, leaf nutrient contents and carbon isotope composition changes in Arabidopsis thaliana are related to rutin stress. J. Plant Physiol. 2014, 171, 1325–1333. [Google Scholar] [CrossRef]
- Islam, A.K.M.M.; Yeasmin, S.; Qasem, J.R.S.; Juraimi, A.S.; Anwar, M.P. Allelopathy of Medicinal Plants: Current Status and Future Prospects in Weed Management. Agric. Sci. 2018, 9, 1569–1588. [Google Scholar] [CrossRef]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Hasan, M. Assessment of allelopathic compounds to develop new natural herbicides: A review. Allelopath. J. 2021, 52, 19–37. [Google Scholar] [CrossRef]
- El-Darier, S.M.; Abdelaziz, H.A.; ZeinEl-Dien, M.H. Effect of soil type on the allelotoxic activity of Medicago sativa L. residues in Vicia faba L. agroecosystems. J. Taibah Univ. Sci. 2014, 8, 84–89. [Google Scholar] [CrossRef][Green Version]
- Hosni, K.; Hassen, I.; Sebei, H.; Casabianca, H. Secondary metabolites from Chrysanthemum coronarium (Garland) flowerheads: Chemical composition and biological activities. Ind. Crop Prod. 2013, 44, 263–271. [Google Scholar] [CrossRef]
- Kremer, R.J. The role of bioherbicides in weed management. Biopestic. Int. 2005, 1, 127–141. [Google Scholar]
- Bailey, K.L. The bioherbicide approach to weed control using plant pathogens. In Integrated Pest Management; Academic Press: Cambridge, CA, USA, 2014; pp. 245–266. [Google Scholar]
- Travlos, I.; Rapti, E.; Gazoulis, I.; Kanatas, P.; Tataridas, A.; Kakabouki, I.; Papastylianou, P. The herbicidal potential of different pelargonic acid products and essential oils against several important weed species. Agronomy 2020, 10, 1687. [Google Scholar] [CrossRef]
- Muñoz, M.; Torres-Pagán, N.; Peiró, R.; Guijarro, R.; Sánchez-Moreiras, A.M.; Verdeguer, M. Phytotoxic effects of three natural compounds: Pelargonic acid, carvacrol, and cinnamic aldehyde, against problematic weeds in Mediterranean crops. Agronomy 2020, 10, 791. [Google Scholar] [CrossRef]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An Eco-Friendly Tool for Sustainable Weed Management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Travaini, M.L.; Sosa, G.M.; Ceccarelli, E.A.; Walter, H.; Cantrell, C.L.; Carrillo, N.J.; Dayan, F.E.; Meepagala, K.M.; Duke, S.O. Khellin and Visnagin, Furanochromones from Ammi visnaga (L.) Lam., as Potential Bioherbicides. J. Agric. Food Chem. 2016, 64, 9475–9487. [Google Scholar] [CrossRef]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Hasan, M. Allelopathic potential of Malaysian invasive weed species on Weedy rice (Oryza sativa f. spontanea Roshev). Allelopath. J. 2021, 53, 53–68. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Ahmad-Hamdani, M.S.; Berahim, Z.; Hasan, M. Physiological and Biochemical Responses of Ageratum conyzoides, Oryza sativa f. spontanea (Weedy Rice) and Cyperus iria to Parthenium hysterophorus Methanol Extract. Plants 2021, 10, 1205. [Google Scholar] [CrossRef]
- Jursík, M.; Soukup, J.; Holec, J.; Andr, J. Important aspects of chemical weed control: Environmental factors affecting herbicide efficacy. Listy Cukrov. Rep. 2011, 127, 348. [Google Scholar]
- Van Alfen, N.K. Encyclopedia of Agriculture and Food Systems; Academic Press: Cambridge, CA, USA, 2014. [Google Scholar]
- Ozkan, T.G.I.; Urusak, E.A.; Appiah, K.S.; Fujii, Y.; Ozkan, I. First Broad Screening of Allelopathic Potential of Wild and Cultivated Plants in Turkey. Plants 2019, 8, 532. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.K.M.M.; Hasan, M.M.; Yeasmin, S.; Abedin, M.A.; Kader, M.A.; Rashid, M.H.; Anwar, M.P. Bioassay screening of tropical tree sawdust for allelopathic properties and their field performance against paddy weeds. Fundam. Appl. Agric. 2019, 4, 906–915. [Google Scholar]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Hasan, M. Bioherbicidal Properties of Parthenium hysterophorus, Cleome rutidosperma and Borreria alata Extracts on Selected Crop and Weed Species. Agronomy 2021, 11, 643. [Google Scholar] [CrossRef]
- Burrill, L.C.; Cárdenas, J.; Locatelli, E. Field Manual for Weed Control Research; International Plant Protection Center, Oregon State University: Corvallis, OR, USA, 1976; p. 63. [Google Scholar]
- Abdullah, M.R.; Zakaria, N.; Ahmad-Hamdani, M.S.; Juraimi, A.S. Evaluation of Herbicide Efficacy on Weed Control and Grain Yield in Rice Field under Flooded Condition. Plant Arch. 2020, 20, 8163–8169. [Google Scholar]
- Barnes, D.E.; Chan, L.G. Common Weeds of Malaysia and Their Control; Ancom Berhad: Petaling Jaya, Malaysia, 1990. [Google Scholar]
- Fee, C.G.; Tui, L.C.; Bin, C.S.; Hoy, C.K. Pictorial Guide to Common Weeds of Plantations and Their Control (No. L-0987); Agricultural Crop Trust, ACT: Bonn, Germany, 2013. [Google Scholar]
- Juraimi, A.S.; Muhammad Saiful, A.H.; Uddin, M.K.; Anuar, A.R.; Azmi, M. Diversity of weed communities under different water regimes in bertam irrigated direct seeded rice field. Aust. J. Crop Sci. 2011, 5, 595–604. [Google Scholar]
- Janiya, J.D.; Moody, K. Weed populations in transplanted and wet-seeded rice as affected by weed control method. Int. J. Pest Manag. 1989, 35, 8–11. [Google Scholar] [CrossRef]
- Goldsmith, F.B.; Harisson, C.M.; Morton, A.J. Description and analysis of vegetation. In Methods in Plant Ecology; Moore, P.D., Chapman, S.B., Eds.; Blackwell Scientific Publication: London, UK, 1986; pp. 437–521. [Google Scholar]
- Garba, J.; Othman, R.; Hamdani, M.S.A. Adsorption-desorption and leaching potential of glyphosate and aminomethylphosphonic acid in acidic Malaysian soil amended with cow dung and rice husk ash. Environ. Monit. Assess. 2018, 190, 1–15. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Arif, I.A.; Khan, H.A. Modes of action of different classes of herbicides. In Herbicides: Physiology of Action, and Safety; IntechOpen: London, UK, 2015; pp. 165–186. [Google Scholar] [CrossRef]
- Takano, H.K.; Dayan, F.E. Glufosinate-ammonium: A review of the current state of knowledge. Pest Manag. Sci. 2020, 76, 3911–3925. [Google Scholar] [CrossRef]
- Reddy, K.N.; Zablotowicz, R.M.; Bellaloui, N.; Ding, W. Glufosinate effects on nitrogen nutrition, growth, yield, and seed composition in glufosinate-resistant and glufosinate-sensitive soybean. Int. J. Agron. 2011, 1–9. [Google Scholar] [CrossRef]
- Chang, S.Y.; Liao, C.H. Analysis of glyphosate, glufosinate and aminomethylphosphonic acid by capillary electrophoresis with indirect fluorescence detection. J. Chromatogr. A 2002, 959, 309–315. [Google Scholar] [CrossRef]
- Heide, H.; Kalisz, H.M.; Follmann, H. The oxygen evolving enhancer protein 1 (OEE) of photosystem II in green algae exhibits thioredoxin activity. J. Plant Physiol. 2004, 161, 139–149. [Google Scholar] [CrossRef]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Ahmad-Hamdani, M.S.; Hasan, M. Phytochemical Constituents and Allelopathic Potential of Parthenium hysterophorus L. in Comparison to Commercial Herbicides to Control Weeds. Plants 2021, 10, 1445. [Google Scholar] [CrossRef]
- Lee, S.M.; Radhakrishnan, R.; Kang, S.M.; Kim, J.H.; Lee, I.Y.; Moon, B.Y.; Yoon, B.W.; Lee, I.J. Phytotoxic mechanisms of bur cucumber seed extracts on lettuce with special reference to analysis of chloroplast proteins, phytohormones, and nutritional elements. Ecotoxicol. Environ. Saf. 2015, 122, 230–237. [Google Scholar] [CrossRef]
- Cai, X.; Gu, M. Bioherbicides in organic horticulture. Horticulturae 2016, 2, 3. [Google Scholar] [CrossRef]
- Wibawa, W.; Mohamad, R.B.; Omar, D.; Zain, N.M.; Puteh, A.B.; Awang, Y. Comparative impact of a single application of selected broad spectrum herbicides on ecological components of oil palm plantation. Afr. J. Agric. Res. 2010, 5, 2097–2102. [Google Scholar] [CrossRef]
- Hoerlein, G. Glufosinate (phosphinothricin), a natural amino acid with unexpected herbicidal properties. Rev. Environ. Contam. Toxicol. 1994, 138, 73–145. [Google Scholar] [CrossRef]
- Riley, P.; Warhurst, M.; Diamand, E.; Barron, H. Health and Environmental Impacts of Glufosinate-Ammonium; PAN UK: Brighton, UK, 2001. [Google Scholar]
- Garba, J.; Abd Samsuri, W.; Othman, R.; Hamdani, M.S.A. Evaluation of Adsorptive Characteristics of Cow Dung and Rice Husk Ash for Removal of Aqueous Glyphosate and Aminomethylphoshonic Acid. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.C. Chemical control of grassy weeds. In Tropical Grassy Weeds; Baker, F.W.G., Terry, P.J., Eds.; CAB: Wallingford, UK, 1991. [Google Scholar]
- Hoss, N.E.; Al-Khatib, K.; Peterson, D.E.; Loughin, T.M. Efficacy of glyphosate, glufosinate, and imazethapyr on selected weed species. Weed Sci. 2003, 51, 110–117. [Google Scholar] [CrossRef]
- Mohamad, R.; Mohayidin, M.G.; Wibaya, W.; Juraimi, A.S.; Lassim, M.M. Management of mixed weeds in young oil-palm plantation with selected broad-spectrum herbicides. Pertanika J. Trop. Agric. Sci. 2010, 33, 193–203. [Google Scholar]
- Wibawa, W.; Mohayidin, M.G.; Mohamad, R.B.; Juraimi, A.S.; Omar, D. Efficacy and cost-effectiveness of three broad-spectrum herbicides to control weeds in immature oil palm plantation. Pertanika J. Trop. Agric. Sci. 2010, 33, 233–241. [Google Scholar]
- Ghorbani, R.; Seel, W.; Rashed, M.H.; Leifert, C. Effect of plant age, temperature and humidity on virulence of Ascochyta caulina on common lambsquarters (Chenopodium album). Weed Sci. 2006, 54, 526–531. [Google Scholar] [CrossRef]
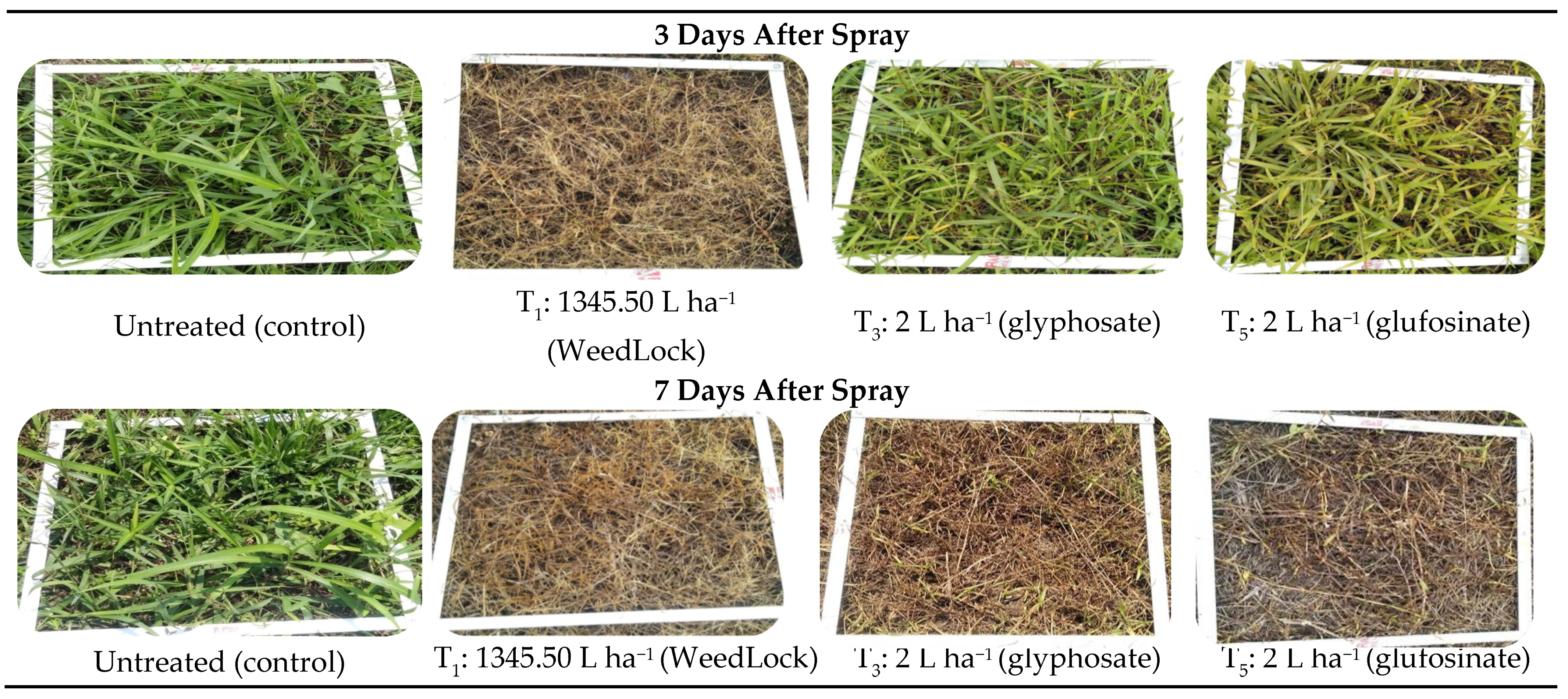
| Scale | Injury (%) | Effects on Weeds |
|---|---|---|
| 1 | 0 | No effect (all foliage green and alive) |
| 2 | 1–10 | Very light symptoms (very minor chlorosis and/or leaf curling) |
| 3 | 11–30 | Light symptoms |
| 4 | 31–49 | Symptoms not reflected in the yield |
| 5 | 50 | Medium (moderate chlorosis and/or leaf curling) |
| 6 | 51–70 | Fairly heavy damage |
| 7 | 71–90 | Heavy damage |
| 8 | 91–99 | Very heavy damage (severe chlorosis and/or dead leaves) |
| 9 | 100 | Complete kill (dead) |
| Test Weeds | Doses | Injury Scale | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 DAS | 7 DAS | 14 DAS | 21 DAS | ||||||||||
| WL | GLY | GLU | WL | GLY | GLU | WL | GLY | GLU | WL | GLY | GLU | ||
| Ageratum conyzoides | T0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 |
| T1 | 7.25a ± 0.25 | 2.00b ± 0 | 2.00b ± 0 | 6.75b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 6.75b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 6.75b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | |
| T2 | 8.00a ± 0 | 2.00b ± 0 | 2.50b ± 0.29 | 8.25a ± 0.48 | 9.00a ± 0 | 9.00a ± 0 | 8.25a ± 0.48 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| T3 | 8.00a ± 0 | 2.00c ± 0 | 2.75b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| Euphorbia hirta | T0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 |
| T1 | 7.25a ± 0.25 | 2.00b ± 0 | 2.00b ± 0 | 6.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 6.75b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 6.75b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | |
| T2 | 8.00a ± 0 | 2.00b ± 0 | 2.50b ± 0.29 | 7.75b ± 0.48 | 9.00a ± 0 | 9.00a ± 0 | 7.75b ± 0.48 | 9.00a ± 0 | 9.00a ± 0 | 7.75b ± 0.48 | 9.00a ± 0 | 9.00a ± 0 | |
| T3 | 8.00a ± 0 | 2.00b ± 0 | 2.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| Eleusine indica | T0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 |
| T1 | 7.50a ± 0.29 | 2.00b ± 0 | 2.00b ± 0 | 7.00b ± 0.41 | 9.00a ± 0 | 9.00a ± 0 | 6.75b ± 0.48 | 9.00a ± 0 | 9.00a ± 0 | 6.75b ± 0.48 | 9.00a ± 0 | 9.00a ± 0 | |
| T2 | 8.00a ± 0 | 2.00b ± 0 | 2.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| T3 | 8.00a ± 0 | 2.00c ± 0 | 2.75b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| Axonopus compressus | T0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 |
| T1 | 4.75a ± 0.48 | 2.00b ± 0 | 2.00b ± 0 | 4.50b ± 0.64 | 9.00a ± 0 | 9.00a ± 0 | 4.25b ± 0.48 | 9.00a ± 0 | 9.00a ± 0 | 4.25b ± 0.48 | 9.00a ± 0 | 9.00a ± 0 | |
| T2 | 7.75a ± 0.25 | 2.00b ± 0 | 2.50b ± 0.29 | 7.75b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 7.75b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 7.75b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | |
| T3 | 8.00a ± 0 | 2.00b ± 0 | 2.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| Cyperus iria | T0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0.0 | 1.00b ± 0 | 1.00b ± 0 |
| T1 | 7.00a ± 0.41 | 2.00b ± 0 | 2.00b ± 0 | 6.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 6.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 6.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | |
| T2 | 8.00a ± 0 | 2.00b ± 0 | 2.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| T3 | 8.00a ± 0 | 2.00b ± 0 | 2.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| Fimbristylis miliacea | T0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 |
| T1 | 5.25a ± 0.48 | 2.00b ± 0 | 2.00b ± 0 | 4.50b ± 0.64 | 9.00a ± 0 | 9.00a ± 0 | 4.50b ± 0.64 | 9.00a ± 0 | 9.00a ± 0 | 4.50b ± 0.64 | 9.00a ± 0 | 9.00a ± 0 | |
| T2 | 7.50a ± 0.29 | 2.00b ± 0 | 2.50b ± 0.29 | 8.50a ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 8.50a ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 8.50a ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | |
| T3 | 8.00a ± 0 | 2.00b ± 0 | 2.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| Test Weeds | Doses | Fresh Weight (g) | Dry Weight (g) | Plant Height (cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WL | GLY | GLU | WL | GLY | GLU | WL | GLY | GLU | ||
| Ageratum conyzoides | T0 | 35.68a ± 0.93 | 36.21a ± 0.66 | 35.88a ± 0.72 | 8.62a ± 0.15 | 8.44a ± 0.11 | 8.26a ± 0.06 | 43.3a ± 0.52 | 41.0b ± 0.65 | 41.2b ± 0.74 |
| T1 | 5.30a ± 0.49 | 1.91b ± 0.11 | 2.07b ± 0.47 | 0.80a ± 0.07 | 0.77a ± 0.07 | 0.90a ± 0.07 | 21.3a ± 0.84 | 0.00a ± 0 | 0.00b ± 0 | |
| T2 | 1.93a ± 0.32 | 0.99b ± 0.09 | 1.29ab ± 0.13 | 0.21a ± 0.03 | 0.20a ± 0.03 | 0.23a ± 0.03 | 4.27a ± 2.49 | 0.00a ± 0 | 0.00a ± 0 | |
| T3 | 0.85a ± 0.08 | 0.84a ± 0.07 | 0.88a ± 0.08 | 0.13a ± 0.01 | 0.12a ± 0.01 | 0.15a ± 0.01 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 | |
| Euphorbia hirta | T0 | 42.00a ± 0.30 | 40.20b ± 0.62 | 40.88ab ± 0.51 | 12.13a ± 0.32 | 11.69a ± 0.25 | 11.84a ± 0.11 | 30.66a ± 0.56 | 29.79a ± 0.33 | 30.77a ± 0.37 |
| T1 | 14.02a ± 0.64 | 11.81b ± 0.42 | 12.10b ± 0.29 | 1.84a ± 0.06 | 1.18b ± 0.05 | 1.26b ± 0.06 | 17.19a ± 0.30 | 0.00b ± 0 | 0.00b ± 0 | |
| T2 | 9.82a ± 0.89 | 4.68c ± 0.37 | 7.87b ± 0.37 | 1.32a ± 0.05 | 1.02b ± 0.05 | 1.11b ± 0.05 | 5.73a ± 0.21 | 0.00b ± 0 | 0.00b ± 0 | |
| T3 | 3.89a ± 0.16 | 1.74b ± 0.16 | 2.06b ± 0.22 | 0.95a ± 0.04 | 0.35b ± 0.05 | 0.45b ± 0.06 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 | |
| Eleusine indica | T0 | 79.51a ± 0.29 | 80.51a ± 0.50 | 80.30a ± 0.32 | 19.75a ± 0.46 | 19.05a ± 0.51 | 19.35a ± 0.14 | 71.47a ± 0.31 | 71.88a ± 0.39 | 70.56a ± 0.64 |
| T1 | 9.14a ± 0.62 | 6.87b ± 0.28 | 7.69b ± 0.22 | 1.88a ± 0.12 | 1.49a ± 0.18 | 1.61a ± 0.10 | 30.68a ± 0.88 | 0.00b ± 0 | 0.00b ± 0 | |
| T2 | 2.91a ± 0.11 | 1.58b ± 0.11 | 2.25c ± 0.12 | 0.52b ± 0.05 | 0.63ab ± 0.04 | 0.70a ± 0.05 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 | |
| T3 | 1.73a ± 0.11 | 1.24b ± 0.06 | 1.45ab ± 0.10 | 0.33b ± 0.02 | 0.30b ± 0.03 | 0.49a ± 0.06 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 | |
| Axonopus compressus | T0 | 66.14a ± 0.61 | 64.37b ± 0.40 | 64.96ab ± 0.39 | 14.21a ± 0.13 | 13.77a ± 0.23 | 13.94a ± 0.21 | 34.36a ± 0.46 | 34.05a ± 0.45 | 34.40a ± 0.28 |
| T1 | 15.74a ± 0.38 | 4.78b ± 0.16 | 5.02b ± 0.27 | 2.36a ± 0.11 | 1.31b ± 0.05 | 1.11b ± 0.06 | 13.87a ± 0.46 | 0.00b ± 0 | 0.00b ± 0 | |
| T2 | 6.72a ± 0.33 | 2.08b ± 0.06 | 2.23b ± 0.17 | 1.29a ± 0.06 | 0.78b ± 0.07 | 1.09a ± 0.07 | 5.82a ± 0.52 | 0.00a ± 0 | 0.00a ± 0 | |
| T3 | 2.22a ± 0.12 | 1.73b ± 0.08 | 1.85b ± 0.11 | 0.42a ± 0.06 | 0.45a ± 0.04 | 0.60a ± 0.07 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 | |
| Cyperus iria | T0 | 45.22b ± 0.53 | 47.16a ± 0.25 | 47.03a ± 0.71 | 8.72a ± 0.31 | 9.17a ± 0.26 | 9.13a ± 0.09 | 54.88a ± 0.76 | 56.59a ± 0.72 | 55.80a ± 0.56 |
| T1 | 6.48a ± 0.27 | 2.32c ± 0.17 | 3.11b ± 0.12 | 1.29a ± 0.15 | 0.58b ± 0.04 | 1.15a ± 0.03 | 21.65a ± 1.05 | 0.00b ± 0 | 0.00b ± 0 | |
| T2 | 2.37a ± 0.25 | 1.39b ± 0.05 | 2.26a ± 0.12 | 0.43ab ± 0.05 | 0.33b ± 0.04 | 0.68a ± 0.14 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 | |
| T3 | 1.45a ± 0.09 | 1.01b ± 0.06 | 1.37a ± 0.08 | 0.16b ± 0.02 | 0.15b ± 0.02 | 0.33a ± 0.04 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 | |
| Fimbristylis miliacea | T0 | 24.74b ± 0.44 | 25.76ab ± 0.31 | 25.95a ± 0.27 | 3.48a ± 0.07 | 3.52a ± 0.05 | 3.55a ± 0.04 | 46.48a ± 0.79 | 47.56a ± 0.49 | 47.65a ± 0.49 |
| T1 | 10.63a ± 0.61 | 4.36b ± 0.23 | 4.43b ± 0.30 | 0.74a ± 0.05 | 0.51b ± 0.06 | 0.52ab ± 0.09 | 29.05a ± 0.48 | 0.00b ± 0 | 0.00b ± 0 | |
| T2 | 4.04a ± 0.21 | 2.43b ± 0.18 | 2.53b ± 0.27 | 0.57a ± 0.03 | 0.37b ± 0.05 | 0.38b ± 0.05 | 4.30a ± 2.48 | 0.00a ± 0 | 0.00a ± 0 | |
| T3 | 1.92a ± 0.08 | 1.27b ± 0.11 | 1.30b ± 0.14 | 0.28a ± 0.05 | 0.20a ± 0.04 | 0.27a ± 0.04 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 | |
| Test Weeds | Doses | Weed Control Efficiency (%) | ||
|---|---|---|---|---|
| WeedLock | Glyphosate Isopropyl-Amine | Glufosinate-Ammonium | ||
| Ageratum conyzoides | T0 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 |
| T1 | 90.66a ± 0.86 | 90.86a ± 0.84 | 89.09a ± 0.93 | |
| T2 | 97.56a ± 0.38 | 97.61a ± 0.32 | 97.19a ± 0.36 | |
| T3 | 98.49ab ± 0.08 | 98.55a ± 0.09 | 98.16b ± 0.17 | |
| Euphorbia hirta | T0 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 |
| T1 | 84.82b ± 0.45 | 89.88a ± 0.36 | 89.32a ± 0.56 | |
| T2 | 89.04b ± 0.55 | 91.30a ± 0.31 | 90.65a ± 0.34 | |
| T3 | 92.12b ± 0.40 | 96.99a ± 0.45 | 96.18a ± 0.55 | |
| Eleusine indica | T0 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 |
| T1 | 90.43a ± 0.79 | 92.12a ± 1.09 | 91.68a ± 0.50 | |
| T2 | 97.36a ± 0.32 | 96.69ab ± 0.18 | 96.38b ± 0.27 | |
| T3 | 98.29a ± 0.14 | 98.42a ± 0.12 | 97.47b ± 0.34 | |
| Axonopus compressus | T0 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 |
| T1 | 83.39b ± 0.64 | 91.93a ± 0.22 | 90.57a ± 0.38 | |
| T2 | 90.92b ± 0.36 | 94.36a ± 0.44 | 92.16b ± 0.61 | |
| T3 | 97.07a ± 0.39 | 96.73ab ± 0.29 | 95.69b ± 0.48 | |
| Cyperus iria | T0 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 |
| T1 | 85.21b ± 1.53 | 93.71a ± 0.35 | 87.37b ± 0.33 | |
| T2 | 95.08ab ± 0.40 | 96.42a ± 0.36 | 92.55b ± 1.51 | |
| T3 | 98.20a ± 0.19 | 98.35a ± 0.23 | 96.32b ± 0.44 | |
| Fimbristylis miliacea | T0 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 |
| T1 | 78.70b ± 1.62 | 85.66a ± 1.41 | 85.41a ± 2.44 | |
| T2 | 83.62b ± 1.32 | 89.45a ± 1.46 | 89.39a ± 1.56 | |
| T3 | 91.87a ± 1.45 | 94.34a ± 1.13 | 92.20a ± 1.31 | |
| Test Crops | Doses | Visual Injury (Scale) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 DAS | 7 DAS | 14 DAS | 21 DAS | ||||||||||
| WL | GLY | GLU | WL | GLY | GLU | WL | GLY | GLU | WL | GLY | GLU | ||
| Zea mays | T0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 |
| T1 | 3.75a ± 0.48 | 2.00b ± 0 | 2.00b ± 0 | 3.25b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 3.25b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 3.25b ± 0.25 | 9.00a ± 0 | 9.00 ± 0 | |
| T2 | 7.75a ± 0.25 | 2.00c ± 0 | 2.75b ± 0.25 | 8.75a ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 8.75a ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 8.75a ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | |
| T3 | 8.00a ± 0 | 2.00c ± 0 | 2.75b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| Oryza sativa | T0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 |
| T1 | 5.75a ± 0.48 | 2.00b ± 0 | 2.00b ± 0 | 5.75b ± 0.48 | 9.00a ± 0 | 9.00a ± 0 | 4.75b ± 0.48 | 9.00a ± 0 | 9.00a ± 0 | 4.75b ± 0.48 | 9.00a ± 0 | 9.00a ± 0 | |
| T2 | 8.00a ± 0 | 2.00b ± 0 | 2.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| T3 | 8.00a ± 0 | 2.00b ± 0 | 2.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| Abelmoschus esculentus | T0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 |
| T1 | 7.50a ± 0.29 | 2.00b ± 0 | 2.00b ± 0 | 7.25b ± 0.48 | 9.00a ± 0 | 9.00a ± 0 | 6.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 6.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | |
| T2 | 8.00a ± 0 | 2.00b ± 0 | 2.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| T3 | 8.00a ± 0 | 2.00c ± 0 | 2.75b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| Amaranthus gangeticus | T0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 | 1.00a ± 0 |
| T1 | 5.00a ± 0.41 | 2.00b ± 0 | 2.00b ± 0 | 4.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 4.25b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 4.25b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | |
| T2 | 8.00a ± 0 | 2.00b ± 0 | 2.50b ± 0.29 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| T3 | 8.00a ± 0 | 2.00c ± 0 | 2.75b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | |
| Test Crops | Doses | Fresh Weight (g) | Dry Weight (g) | Growth Reduction (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WL | GLY | GLU | WL | GLY | GLU | WL | GLY | GLU | ||
| Zea mays | T0 | 542.89b ± 3.47 | 549.34ab ± 3.18 | 556.72a ± 3.82 | 93.58b ± 3.52 | 100.86ab ± 2.02 | 103.08a ± 1.44 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 |
| T1 | 221.52a ± 1.89 | 23.12b ± 0.64 | 20.28b ± 0.61 | 62.89a ± 2.13 | 2.45b ± 0.36 | 2.27b ± 0.22 | 32.26b ± 4.81 | 97.55a ± 0.40 | 97.80a ± 0.19 | |
| T2 | 31.47a ± 1.23 | 14.13b ± 0.17 | 15.26b ± 0.55 | 3.17a ± 1.16 | 1.95a ± 0.11 | 2.09a ± 0.22 | 96.64a ± 1.16 | 98.06a ± 0.12 | 97.98a ± 0.19 | |
| T3 | 7.93b ± 0.39 | 8.62b ± 0.20 | 10.96a ± 0.66 | 0.94a ± 0.24 | 1.00a ± 0.24 | 1.36a ± 0.29 | 98.99a ± 0.27 | 99.00a ± 0.25 | 98.69a ± 0.26 | |
| Oryza sativa | T0 | 45.13a ± 0.49 | 43.56a ± 0.62 | 44.74a ± 0.35 | 11.22a ± 0.13 | 10.93a ± 0 | 11.16a ± 0.20 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 |
| T1 | 16.96a ± 0.47 | 4.20b ± 0.25 | 4.32b ± 0.22 | 3.71a ± 0.10 | 1.22b ± 0.21 | 1.29b ± 0.08 | 66.88b ± 1.03 | 88.85a ± 0.58 | 88.38a ± 0.79 | |
| T2 | 6.71a ± 0.33 | 2.12b ± 0.19 | 2.04b ± 0.12 | 1.65a ± 0.09 | 0.79b ± 0.08 | 0.84b ± 0.04 | 85.31b ± 0.86 | 92.71a ± 0.71 | 92.50a ± 0.26 | |
| T3 | 2.09a ± 0.11 | 1.12b ± 0.10 | 1.36b ± 0.06 | 0.83a ± 0.07 | 0.41b ± 0.04 | 0.50b ± 0.05 | 92.59b ± 0.53 | 96.23a ± 0.44 | 95.48a ± 0.37 | |
| Abelmoschus esculentus | T0 | 355.68a ± 3.47 | 342.53b ± 3.90 | 341.83b ± 3.39 | 70.49a ± 1.66 | 68.27a ± 3.36 | 66.95a ± 2.85 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 |
| T1 | 26.24a ± 0.50 | 10.34c ± 0.35 | 12.59b ± 0.25 | 2.95a ± 0.28 | 1.94b ± 0.11 | 2.02b ± 0.07 | 95.84b ± 0.31 | 97.16a ± 0.05 | 96.98a ± 0.06 | |
| T2 | 12.22a ± 0.56 | 6.90b ± 0.30 | 7.22b ± 0.21 | 1.76a ± 0.15 | 1.15b ± 0.08 | 1.18b ± 0.09 | 97.51b ± 0.17 | 98.32a ± 0.05 | 98.24a ± 0.08 | |
| T3 | 6.33a ± 0.28 | 4.35b ± 0.17 | 3.85b ± 0.13 | 0.77a ± 0.09 | 0.79a ± 0.02 | 0.73a ± 0.07 | 98.90a ± 0.10 | 98.83a ± 0.05 | 98.92a ± 0.07 | |
| Amaranthus gangeticus | T0 | 86.02a ± 1.00 | 84.42a ± 0.97 | 85.09a ± 1.05 | 21.10a ± 0.68 | 20.42a ± 0.48 | 20.49a ± 0.27 | 0.00a ± 0 | 0.00a ± 0 | 0.00a ± 0 |
| T1 | 39.14a ± 0.64 | 5.29b ± 0.20 | 6.67b ± 0.40 | 10.23a ± 0.52 | 1.79b ± 0.06 | 1.82b ± 0.07 | 51.42b ± 2.84 | 91.20a ± 0.41 | 91.09a ± 0.43 | |
| T2 | 6.39a ± 0.23 | 3.38c ± 0.21 | 4.52b ± 0.23 | 1.39a ± 0.05 | 1.36a ± 0.04 | 1.39a ± 0.03 | 93.38a ± 0.27 | 93.31a ± 0.28 | 93.20a ± 0.20 | |
| T3 | 4.07a ± 0.10 | 1.09c ± 0.10 | 1.66b ± 0.12 | 0.95a ± 0.04 | 0.92a ± 0.04 | 0.96a ± 0.04 | 95.49a ± 0.24 | 95.46a ± 0.21 | 95.33a ± 0.19 | |
| Scientific Name | Family Name | Summed Dominance Ratio (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Broadleaves | T0 | T1 | T2 | T3 | T4 | T5 | T6 | |
| Ageratum conyzoides | Asteraceae | 14.91 | 14.22 | 9.02 | 12.91 | 10.06 | 6.97 | 12.63 |
| Ageratum houstonianum Mill. | Asteraceae | 4.53 | 14.35 | 12.25 | 1.69 | 5.05 | 5.58 | - |
| Cleome rutidosperma DC. | Cleomaceae | 10.41 | 16.00 | 13.13 | 14.15 | 18.72 | 1.77 | 12.05 |
| Desmodium triflorum | Fabaceae | - | - | 12.08 | - | 14.34 | 14.25 | 17.36 |
| Eleutheranthera ruderalis | Asteraceae | 16.86 | 12.24 | 12.24 | 12.20 | 17.04 | 10.17 | 9.19 |
| Euphorbia hirta | Euphorbiaceae | 6.81 | 8.27 | 7.68 | 9.58 | 8.99 | 5.01 | 6.23 |
| Hedyotis corymbosa (L.) Lam. | Rubiaceae | 3.35 | 7.36 | 4.72 | - | - | 4.11 | 20.31 |
| Ipomoea aquatica Forssk. | Convolvulaceae | 2.04 | 5.77 | 2.28 | 4.45 | 9.22 | 3.16 | - |
| Ipomoea triloba L. | Convolvulaceae | 4.10 | 7.07 | 7.55 | 6.13 | 5.00 | 10.32 | 10.24 |
| Melochia corchorifolia L. | Malvaceae | 7.60 | 7.92 | 6.75 | 3.68 | 9.44 | 4.31 | - |
| Mimosa invisa | Fabaceae | 8.04 | 2.64 | 4.41 | - | 7.32 | - | 7.07 |
| Mimosa pudica | Fabaceae | 1.80 | 5.73 | 5.53 | - | 4.50 | 6.58 | 4.87 |
| Phyllanthus amarus | Phyllanthaceae | 7.18 | 16.75 | 13.34 | 10.33 | 11.00 | 11.50 | 8.32 |
| Grasses | ||||||||
| Brachiaria mutica | Poaceae | 11.57 | 6.20 | 14.78 | - | 11.03 | 12.78 | - |
| Cynodon dactylon (L.) Pers. | Poaceae | 6.23 | 14.62 | 7.51 | 8.27 | 6.41 | 9.60 | 10.85 |
| Digitaria ciliaris (Retz.) Koeler | Poaceae | 6.97 | 9.24 | 8.19 | 8.03 | 6.87 | 8.50 | 9.69 |
| Digitaria fuscescens (J.Presl) Henrard | Poaceae | 9.23 | - | 11.21 | - | - | 9.68 | - |
| Digitaria longiflora (Retz.) Pers. | Poaceae | 12.49 | 8.29 | 5.54 | 8.08 | - | 4.88 | 5.84 |
| Echinochloa colona (L.) Link | Poaceae | 9.21 | - | 6.02 | 8.49 | 6.81 | 7.89 | 7.76 |
| Eleusine indica | Poaceae | - | - | 6.02 | 6.05 | 7.39 | 1.52 | - |
| Ottochloa nodosa (Kunth) Dandy | Poaceae | 4.51 | 14.34 | - | - | 8.04 | 7.81 | 10.54 |
| Panicum maximum | Poaceae | 9.92 | 9.81 | 6.84 | 3.83 | 12.84 | 8.26 | - |
| Paspalum conjugatum | Poaceae | 8.90 | - | 7.73 | - | 14.57 | 4.74 | 11.43 |
| Paspalum distichum L. | Poaceae | 11.69 | - | - | 6.68 | - | 12.76 | 8.70 |
| Paspalum scrobiculatum L. | Poaceae | - | - | 8.47 | 9.92 | 9.67 | 6.77 | 7.39 |
| Sporobolus diander (Retz.) P.Beauv. | Poaceae | 4.65 | 6.63 | 4.90 | 6.05 | 9.86 | 9.67 | 9.86 |
| Sedges | ||||||||
| Cyperus digitatus | Cyperaceae | 5.26 | - | 10.10 | 6.68 | 6.44 | 18.07 | 9.02 |
| Cyperus esculentus L. | Cyperaceae | - | 4.07 | 13.95 | - | 12.01 | - | 8.13 |
| Cyperus iria | Cyperaceae | 9.16 | 11.22 | 6.91 | 7.69 | 6.25 | 9.75 | 10.94 |
| Cyperus rotundus L. | Cyperaceae | 9.69 | - | 5.94 | 9.91 | 10.56 | 12.88 | 6.96 |
| Fimbristylis miliacea | Cyperaceae | 14.90 | 12.90 | - | - | 9.57 | 8.13 | 7.62 |
| Rhynchospora corymbosa (L.) Britton | Cyperaceae | 7.38 | 4.06 | - | - | 7.42 | 15.99 | 9.47 |
| Treatments | T0 | T1 | T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|---|
| T0 | - | 59.95 | 59.27 | 53.72 | 60.81 | 67.79 | 45.96 |
| T1 | 59.95 | - | 56.46 | 46.35 | 57.93 | 56.52 | 38.94 |
| T2 | 59.27 | 56.46 | - | 61.19 | 59.88 | 66.29 | 49.11 |
| T3 | 53.72 | 46.35 | 61.19 | - | 50.85 | 53.67 | 46.87 |
| T4 | 60.81 | 57.93 | 59.88 | 50.85 | - | 59.80 | 61.36 |
| T5 | 67.79 | 56.52 | 66.29 | 53.67 | 59.80 | - | 57.44 |
| T6 | 45.96 | 38.94 | 49.11 | 46.87 | 61.36 | 57.44 | - |
| Treatments | Visual Injury (Scale) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 DAS | 3 DAS | 7 DAS | 14 DAS | 21 DAS | 28 DAS | 35 DAS | 60 DAS | |
| T0 | 1.00c ± 0 | 1.00d ± 0 | 1.00b ± 0 | 1.00d ± 0 | 1.00e ± 0 | 1.00e ± 0 | 1.00e ± 0 | 1.00a ± 0 |
| T1 | 7.75a ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 7.75c ± 0.25 | 6.75d ± 0.25 | 6.25d ± 0.25 | 5.00d ± 0.41 | 1.00a ± 0 |
| T2 | 8.00a ± 0 | 9.00a ± 0 | 9.00a ± 0 | 8.25b ± 0.25 | 7.25cd ± 0.25 | 6.50cd ± 0.29 | 5.75cd ± 0.25 | 1.00a ± 0 |
| T3 | 2.00b ± 0 | 2.00c ± 0 | 9.00a ± 0 | 9.00a ± 0 | 8.25ab ± 0.25 | 7.50ab ± 0.29 | 7.00ab ± 0.41 | 1.00a ± 0 |
| T4 | 2.00b ± 0 | 2.00c ± 0 | 9.00a ± 0 | 9.00a ± 0 | 8.75a ± 0.25 | 7.75a ± 0.25 | 7.25a ± 0.25 | 1.00a ± 0 |
| T5 | 2.25b ± 0.25 | 3.00b ± 0 | 9.00a ± 0 | 9.00a ± 0 | 7.75bc ± 0.25 | 6.75bcd ± 0.25 | 6.25bc ± 0.25 | 1.00a ± 0 |
| T6 | 2.50b ± 0.29 | 3.25b ± 0.25 | 9.00a ± 0 | 9.00a ± 0 | 8.25ab ± 0.25 | 7.25abc ± 0.48 | 6.75ab ± 0.25 | 1.00a ± 0 |
| Treatments | 35 DAS | 60 DAS | ||
|---|---|---|---|---|
| Fresh Weight (g) | Dry Weight (g) | Fresh Weight (g) | Dry Weight (g) | |
| T0 | 657.98a ± 3.38 | 82.98a ± 1.67 | 861.48a ± 3.31 | 121.22a ± 2.28 |
| T1 | 373.09b ± 2.15 | 44.16b ± 1.84 | 688.23b ± 2.29 | 92.41b ± 1.53 |
| T2 | 296.19c ± 2.48 | 40.38bc ± 1.77 | 664.38c ± 2.07 | 90.69b ± 1.81 |
| T3 | 261.31d ± 3.24 | 29.16d ± 1.45 | 566.32f ± 2.21 | 76.02de ± 2.41 |
| T4 | 222.49e ± 2.88 | 27.79d ± 1.24 | 527.76g ± 3.25 | 72.51e ± 2.00 |
| T5 | 264.43d ± 2.98 | 35.79c ± 1.57 | 601.01d ± 3.61 | 83.35c ± 1.70 |
| T6 | 228.12e ± 2.63 | 28.07d ± 1.45 | 584.56e ± 2.94 | 80.96cd ± 1.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.; Mokhtar, A.S.; Rosli, A.M.; Hamdan, H.; Motmainna, M.; Ahmad-Hamdani, M.S. Weed Control Efficacy and Crop-Weed Selectivity of a New Bioherbicide WeedLock. Agronomy 2021, 11, 1488. https://doi.org/10.3390/agronomy11081488
Hasan M, Mokhtar AS, Rosli AM, Hamdan H, Motmainna M, Ahmad-Hamdani MS. Weed Control Efficacy and Crop-Weed Selectivity of a New Bioherbicide WeedLock. Agronomy. 2021; 11(8):1488. https://doi.org/10.3390/agronomy11081488
Chicago/Turabian StyleHasan, Mahmudul, Anis Syahirah Mokhtar, Adam Mustafa Rosli, Hafizuddin Hamdan, Mst. Motmainna, and Muhammad Saiful Ahmad-Hamdani. 2021. "Weed Control Efficacy and Crop-Weed Selectivity of a New Bioherbicide WeedLock" Agronomy 11, no. 8: 1488. https://doi.org/10.3390/agronomy11081488
APA StyleHasan, M., Mokhtar, A. S., Rosli, A. M., Hamdan, H., Motmainna, M., & Ahmad-Hamdani, M. S. (2021). Weed Control Efficacy and Crop-Weed Selectivity of a New Bioherbicide WeedLock. Agronomy, 11(8), 1488. https://doi.org/10.3390/agronomy11081488






