Nanostructured Gas Sensors: From Air Quality and Environmental Monitoring to Healthcare and Medical Applications
Abstract
:1. Introduction
2. Air Quality and Environmental Monitoring
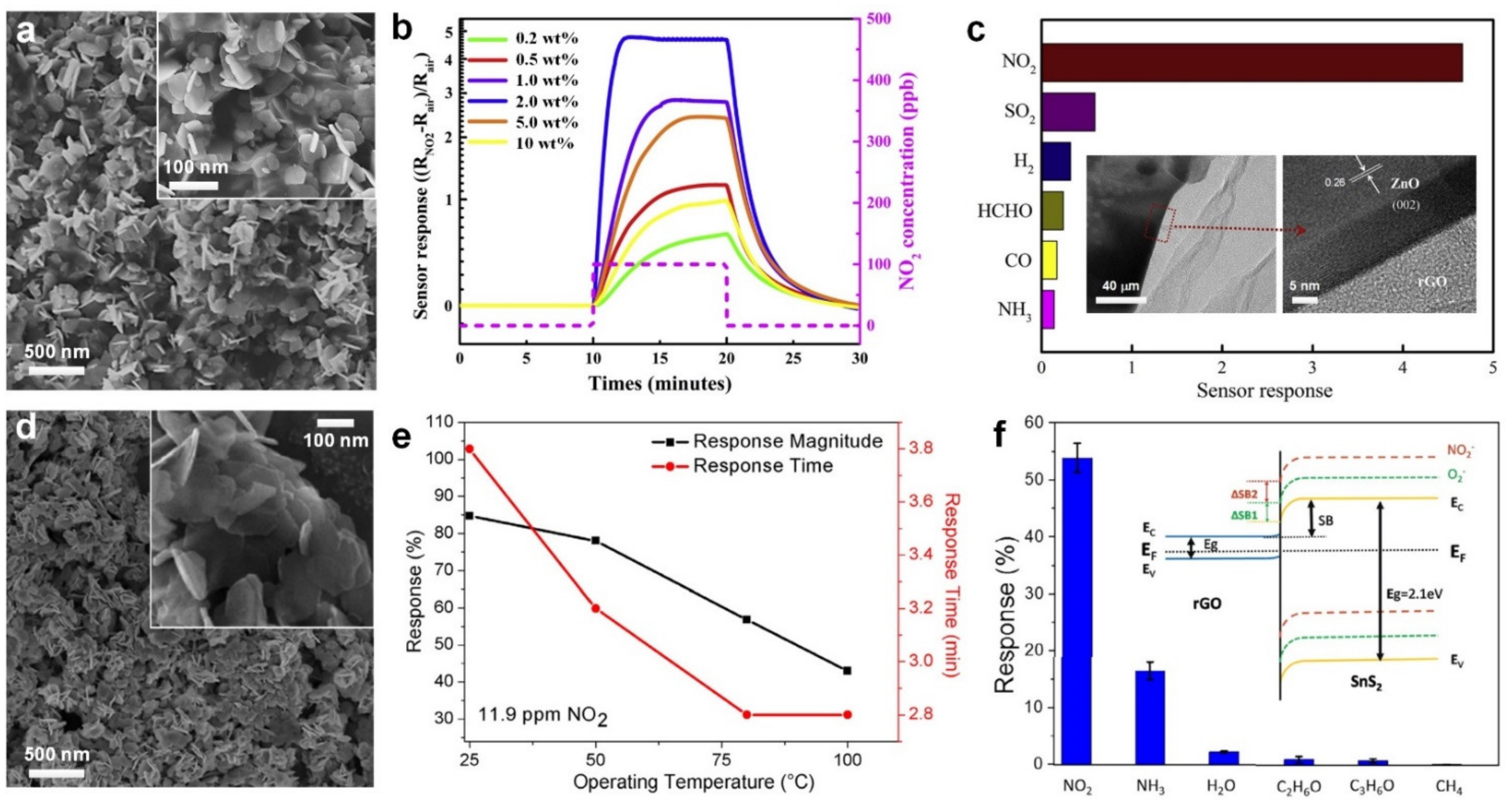
2.1. Nitrogen Dioxide (NO2)
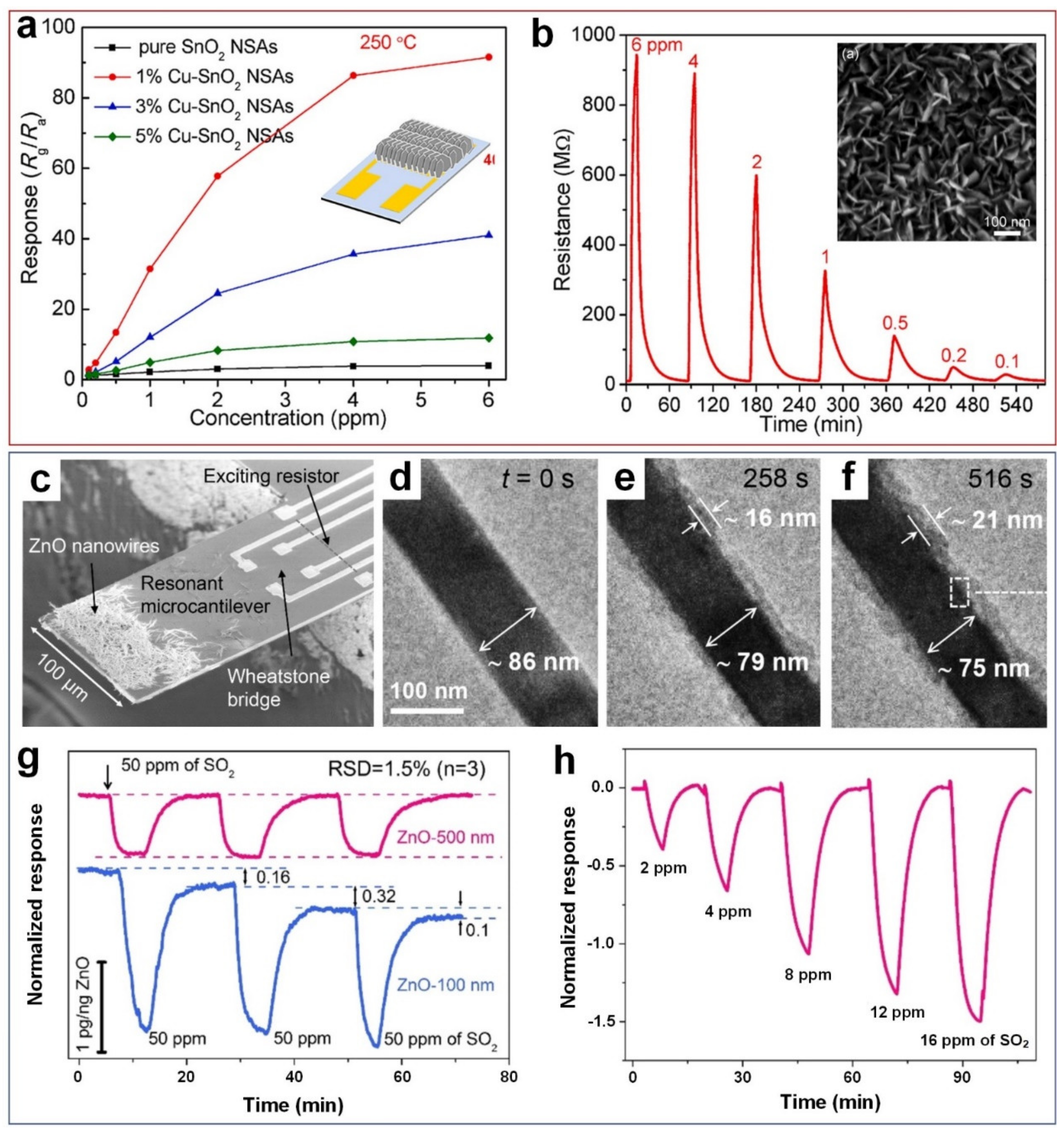
2.2. Sulphur Dioxide (SO2)
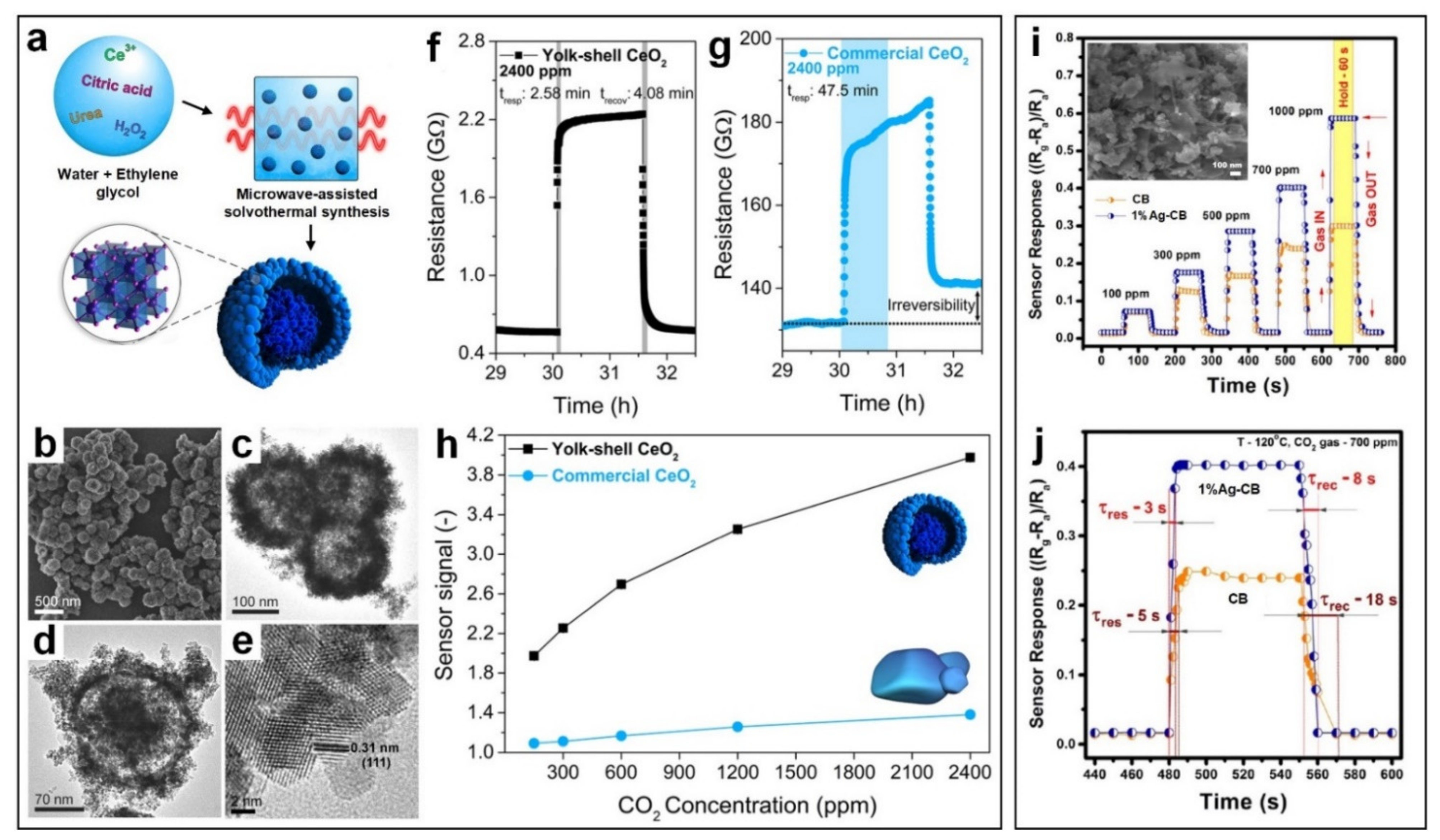
2.3. Carbon Dioxide (CO2)
3. Health and Medical Monitoring
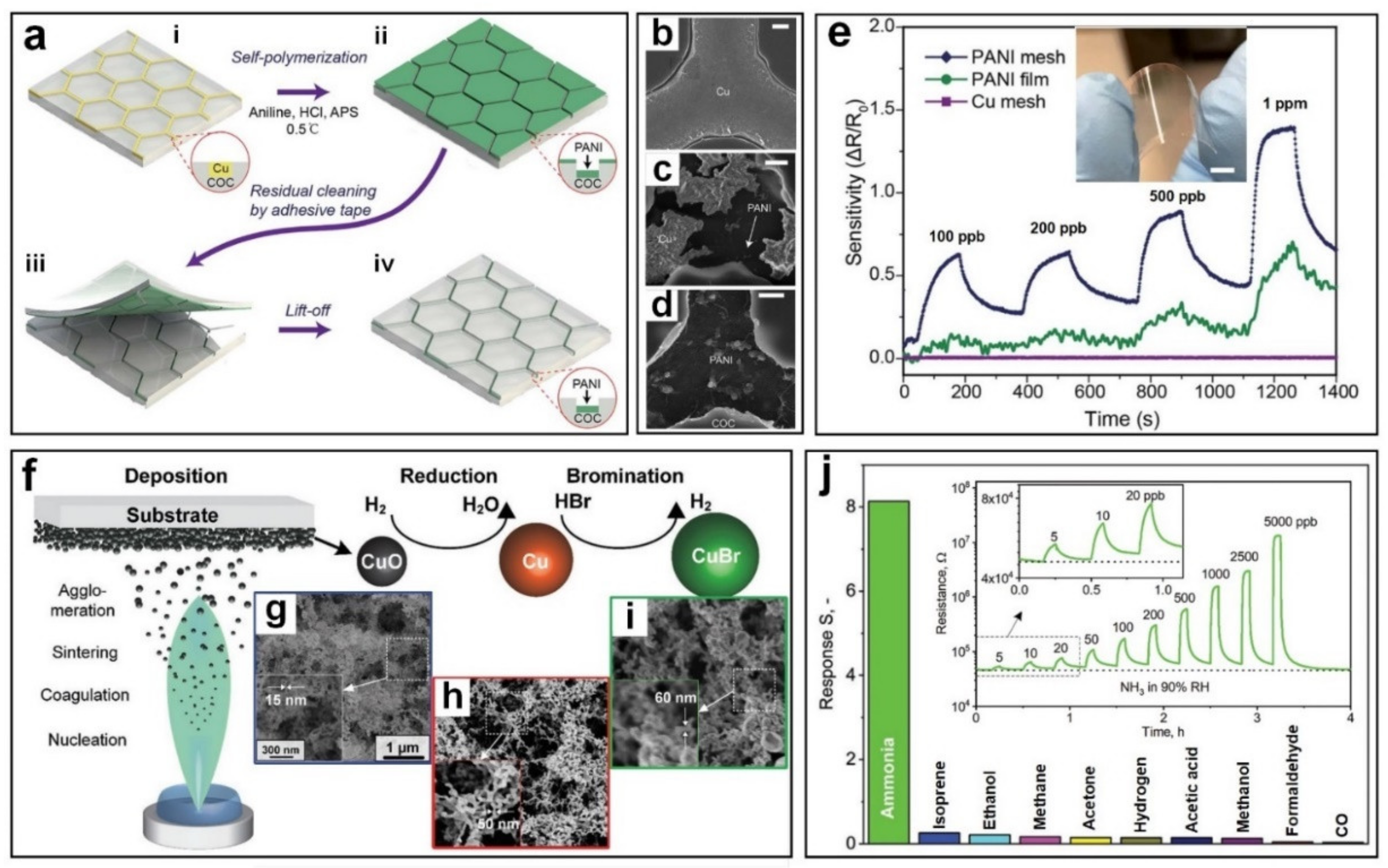
3.1. Ammonia (NH3)
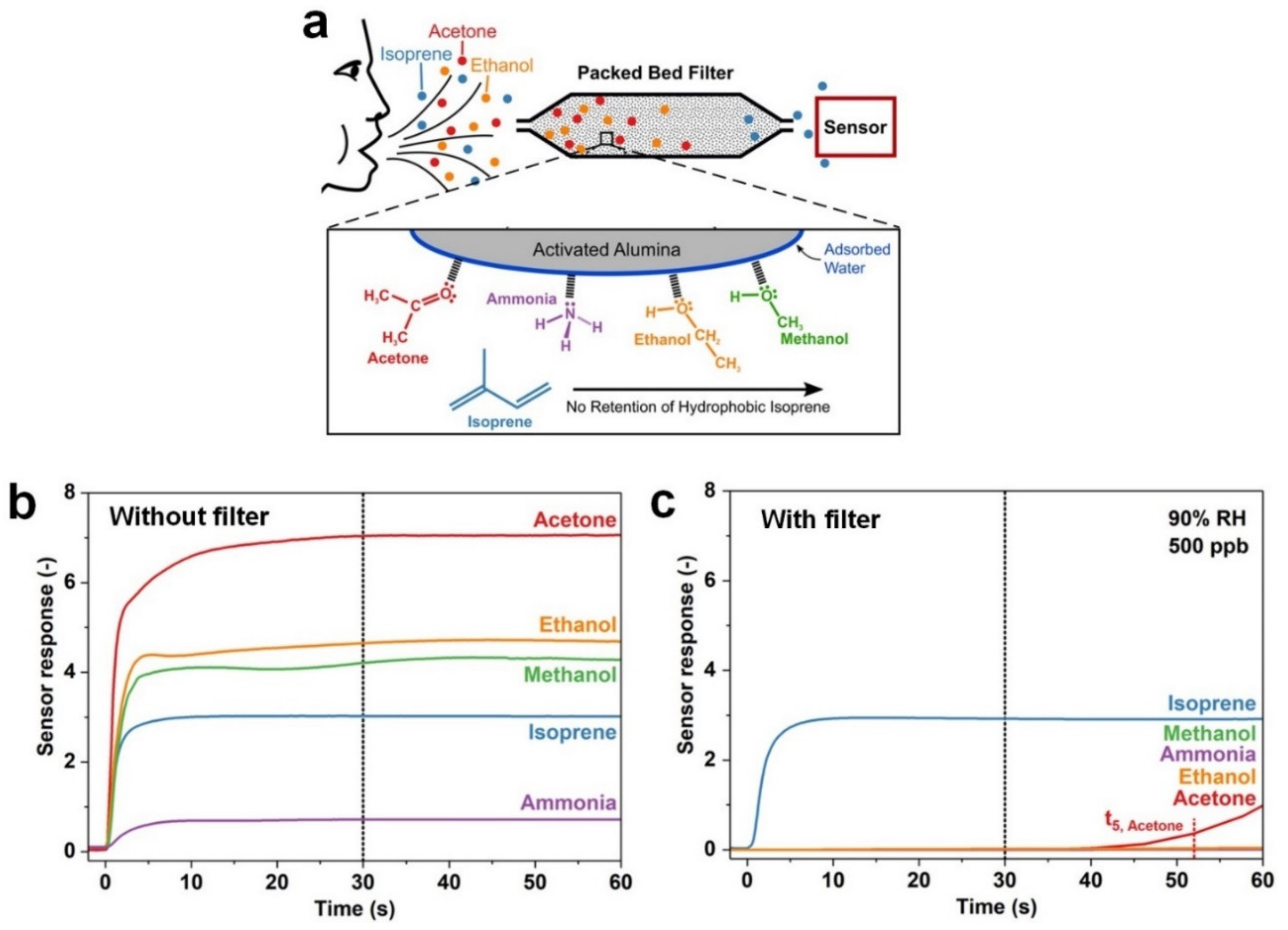
3.2. Isoprene (C5H8)
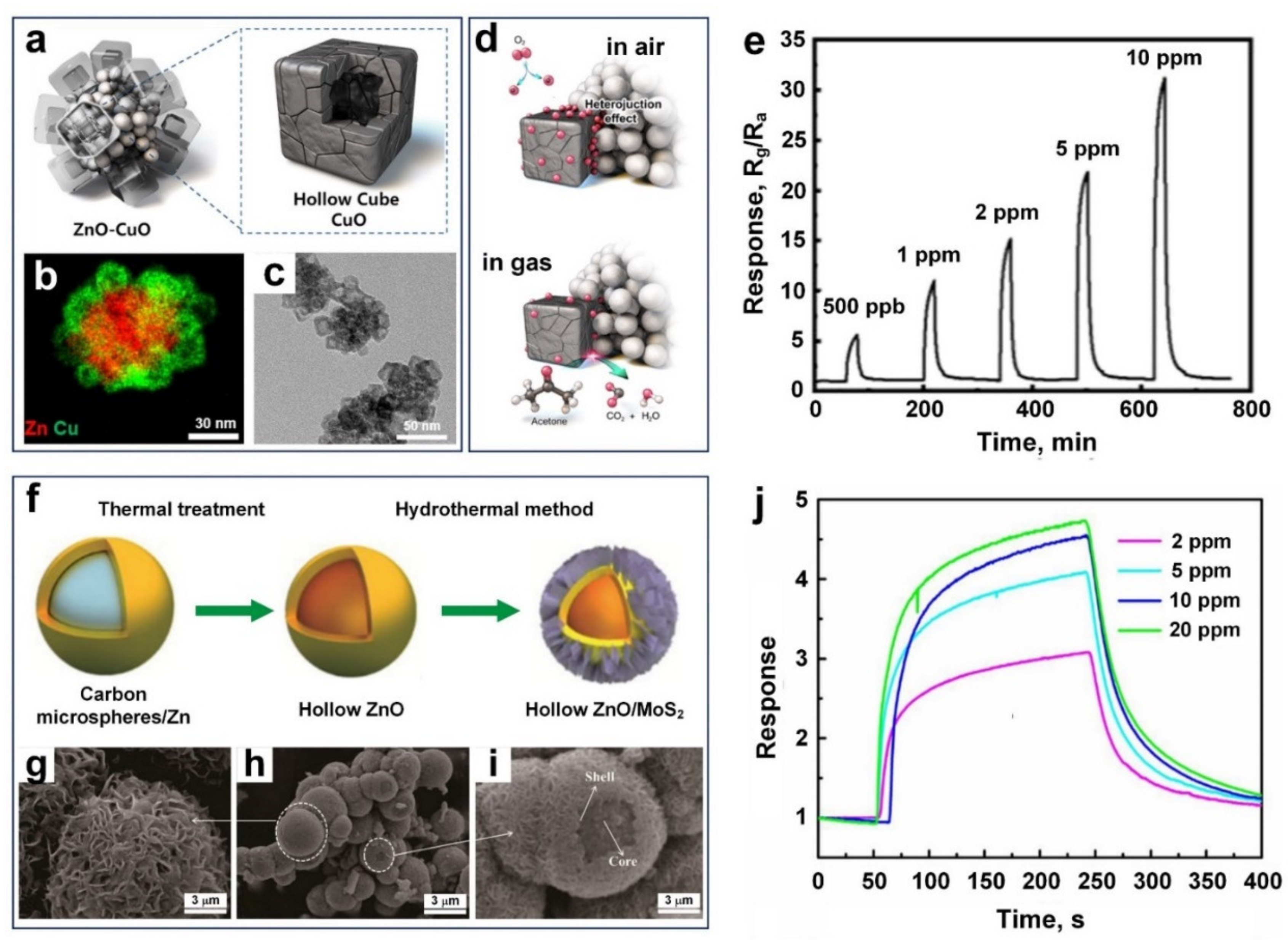
3.3. Acetone (C3H6O)
4. Summary and Outlook
| Target Gas | Materials | Methods | Working Temp. (°C) | Con. (ppm) | Response # | Response/Recovery Time | Ref. |
|---|---|---|---|---|---|---|---|
| NO2 | SnS2/rGO | Hydrothermal | RT | 1 | 6.5 a | 75 s/~180 s * | [18] |
| rGO/ZnO1−x | Hydrothermal | 25 | 0.1 | 4.66 b | 1.5 min/2.5 min | [26] | |
| ZnO/rGO | Solvothermal | 110 | 2.5 | 32.11 | 182 s/234 s | [29] | |
| SnS2/rGO | Wet chemistry | 80 | 10 | 0.618 c | 6 min/~53 min | [30] | |
| SnS2 | Wet chemistry | 120 | 10 | ~35 | ~170 s/~140 s | [31] | |
| ZnO/rGO | Thermal reduction, soft solution | RT | 5 | 2.5 | 25 s/15 s | [87] | |
| g-C3N4/rGO | Layer-by-layer self-assembly | RT | 2 | 0.52 | 138 s/318 s | [88] | |
| Zn2SnO4/rGO | Hydrothermal | 30 | 1 | 0.83 | - | [89] | |
| S-rGO/SnS2 | Hydrothermal | RT | 1 | 0.72 | - | [90] | |
| CdS/ZnO | Liquid plasma spray | RT | 1 | 30.9 d | 18.2 min/>70 min | [91] | |
| In/Ga/ZnO | RF sputtering | RT | 5 | 0.5 e | -/5 min e | [92] | |
| SnO/SnO2 | Hydrothermal | RT | 0.2 | 1.5 | 57 s/5 min | [93] | |
| Si/WO3 | Chemical etching & annealing | RT | 5 | 0.92 | 1 s/31 s | [94] | |
| NiO/CuO | Reflux, hydrothermal | RT | 100 | 0.772 | 2 s/- | [95] | |
| α-Fe2O3/PANI | Polymerization | RT | 20 | 228 | 2.3 min/2.4 min | [96] | |
| CuPcTS/SnO2 | Spin coating | 50 | 1 | 2399 | 5 min/10 min | [97] | |
| SO2 | Ni3BTC2/OH-SWNTs | Solvothermal | RT | 15 | 0.85 * | 5.59 s/11.04 s | [33] |
| Cu:SnO2 | Precipitation | 250 | 6 | 90.51 | 4.5 min/15 min | [34] | |
| g-C3N4/rGO | Layer-by-layer self-assembly | RT | 20 | 0.09 f | 140 s f/130 s f | [88] | |
| rGO/WO3 | Metal organic decomposition | 25 | 0.3 | 0.027 | 66 s/298 s | [98] | |
| Au/SnO2−X | Hydrothermal | 200 | 20 | 0.904 | 34 s/14 s | [99] | |
| PANI | Template-free | RT | 5 | 0.045 | 185 s/<200 s | [100] | |
| Ru/Al2O3/ZnO | Hydrothermal & inkjet printing | 350 | 25 | 0.2 | ~1 min/~6 min | [101] | |
| NH4+ZSM-5 (23) | Ion exchange | RT | 4200 | 0.85 | 63 min/3 min | [102] | |
| CO2 | CeO2 | Solvothermal | 100 | 2400 | 2.9 | 2.58 min/4.08 min | [48] |
| Ag/CuO/BaTiO3 | Hydrothermal | 120 | 700 | 0.4 | 3 s/8 s | [49] | |
| Graphene PEI/PEG | CVD & e-beam evaporation | RT | 5000 | 0.3 g | “several tens of seconds” | [52] | |
| SWCNTs/Q4VP−VBAm | Spray coating | 21 | 20000 | 0.25 * | <1000 s */~3000 s | [53] | |
| Pd/La2O3 | Spray pyrolysis | 250 | 500 | 2.57 | 105 s/145 s | [103] | |
| NH3 | PANI | Polymerization | 24 | 0.0025 | 0.03 | - | [65] |
| CuBr | FSP | RT | 5 | 276 | 2.2 min/50 s | [66] | |
| PET/MWCNTs/PANI | EDA modification | ~15–18 | 50 | 1.17 | 47 s/- | [67] | |
| SnO2/PANI | Hydrothermal, polymerization | 28 | 100 | 28.8 | 125 s/167 s | [68] | |
| S-rGO/SnS2 | Hydrothermal | RT | 20 | 0.45 | - | [90] | |
| SWCNTs | Hydrothermal | RT | 1.5 | 0.032 | 10 min/- h | [104] | |
| C5H8 | Ti:ZnO | FSP | 325 | 0.02 | 0.26 | ~1 min/~5 min | [70] |
| Au/ZnO | Solvothermal, annealing | 350 | 1 | ~1 | ~2 min */- | [74] | |
| In2O3 | Hydrothermal | 190 | 5 | 0.75 | 53 s/299 s | [75] | |
| Pt:SnO2 | FSP | 400 | 0.5 | ~0.75 | 10 s/20 s * | [76] | |
| Pt/In2O3 | Hydrothermal | 200 | 5 | 0.99 | 124 s/204 s | [105] | |
| Cr2O3:In2O3 | Hydrothermal | 240 | 0.5 | 0.95 | 135 s/830 s | [106] | |
| C3H6O | ZnO/CuO | Thermal oxidation | 200 | 1 | 10.14 | - | [46] |
| In/Ga/ZnO | Photochemical activation | RT | 750 | 0.27 i | 37 s/53 s | [81] | |
| Au/ZnO | Precipitation | 172 | 100 | 0.98 | 1 s/20 s | [82] | |
| WO3 | Hydrothermal | 320 | 0.25 | 0.97 | 5 s/5 s | [83] | |
| HZnO/MoS2 | Hydrothermal | 30 | 50 | ~0.25 j | 19 s/97 s | [84] | |
| Co3O4/SnO2 | Hydrothermal | 220 | 50 | 0.92 | 12 s/18 s | [85] | |
| Co3O4/CoWO4 | Hydrothermal | 255 | 20 | 8.9 | 22 s/12 s | [86] | |
| Au/ZnO | Pyrolysis, sonication | 365 | 100 | ~1 | 5 s/- | [107] |
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rim-Rukeh, A. An Assessment of the Contribution of Municipal Solid Waste Dump Sites Fire to Atmospheric Pollution. Open J. Air Pollut. 2014, 3, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Morillas, H.; Maguregui, M.; Gallego-Cartagena, E.; Marcaida, I.; Carral, N.; Madariaga, J.M. The influence of marine environment on the conservation state of Built Heritage: An overview study. Sci. Total Environ. 2020, 745, 140899. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Acid rain-the major cause of pollution: Its causes, effects. Int. J. Appl. Chem 2017, 13, 53–58. [Google Scholar]
- Wu, R.; Xie, S. Spatial Distribution of Secondary Organic Aerosol Formation Potential in China Derived from Speciated Anthropogenic Volatile Organic Compound Emissions. Environ. Sci. Technol. 2018, 52, 8146–8156. [Google Scholar] [CrossRef]
- Raza, W.; Saeed, S.; Saulat, H.; Gul, H.; Sarfraz, M.; Sonne, C.; Sohn, Z.H.; Brown, R.J.C.; Kim, K.-H. A review on the deteriorating situation of smog and its preventive measures in Pakistan. J. Clean. Prod. 2021, 279, 123676. [Google Scholar] [CrossRef]
- Nie, E.; Zheng, G.; Shao, Z.; Yang, J.; Chen, T. Emission characteristics and health risk assessment of volatile organic compounds produced during municipal solid waste composting. Waste Manage. 2018, 79, 188–195. [Google Scholar] [CrossRef]
- WHO. Burden of Disease from the Joint Effects of Household and Ambient Air Pollution for 2016; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Nasiri, N.; Clarke, C. Nanostructured Chemiresistive Gas Sensors for Medical Applications. Sensors 2019, 19, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tricoli, A.; Nasiri, N.; De, S. Wearable and Miniaturized Sensor Technologies for Personalized and Preventive Medicine. Adv. Funct. Mater. 2017, 27, 1605271. [Google Scholar] [CrossRef]
- Kharitonov, S.A.; Barnes, P.J. Biomarkers of some pulmonary diseases in exhaled breath. Biomarkers 2002, 7, 1–32. [Google Scholar] [CrossRef]
- Nasiri, N. Introductory Chapter: Wearable Technologies for Healthcare Monitoring. In Wearable Devices: The Big Wave of Innovation; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Adir, O.; Poley, M.; Chen, G.; Froim, S.; Krinsky, N.; Shklover, J.; Shainsky-Roitman, J.; Lammers, T.; Schroeder, A. Integrating Artificial Intelligence and Nanotechnology for Precision Cancer Medicine. Adv. Mater. 2020, 32, 1901989. [Google Scholar] [CrossRef] [PubMed]
- Sayago, I.; Aleixandre, M.; Santos, J.P. Development of Tin Oxide-Based Nanosensors for Electronic Nose Environmental Applications. Biosensors 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasiri, N.; Clarke, C. Nanostructured Gas Sensors for Medical and Health Applications: Low to High Dimensional Materials. Biosensors 2019, 9, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Semanjski, I.; Gautama, S.; Tsiligianni, E.; Deligiannis, N.; Rajan, R.; Pasveer, F.; Philips, W. A Review of Urban Air Pollution Monitoring and Exposure Assessment Methods. ISPRS Int. J. Geoinf. 2017, 6, 389. [Google Scholar] [CrossRef] [Green Version]
- Yi, W.; Lo, K.; Mak, T.; Leung, K.; Leung, Y.; Meng, M. A Survey of Wireless Sensor Network Based Air Pollution Monitoring Systems. Sensors 2015, 15, 31392–31427. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Jiao, W.; Chu, Z.; Ding, G.; Yan, M.; Zhong, X.; Wang, R. Ultrasensitive room temperature ppb-level NO2 gas sensors based on SnS2/rGO nanohybrids with P-N transition and optoelectronic visible light enhancement performance. J. Mater. Chem. C 2019, 7, 8616–8625. [Google Scholar] [CrossRef]
- Chen, T.-M.; Kuschner, W.G.; Gokhale, J.; Shofer, S. Outdoor Air Pollution: Nitrogen Dioxide, Sulfur Dioxide, and Carbon Monoxide Health Effects. Am. J. Med. Sci. 2007, 333, 249–256. [Google Scholar] [CrossRef]
- WHO. Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Richter, A.; Burrows, J.P.; Nüß, H.; Granier, C.; Niemeier, U. Increase in tropospheric nitrogen dioxide over China observed from space. Nature 2005, 437, 129–132. [Google Scholar] [CrossRef]
- Lerdau, M.T.; Munger, J.W. The NO2 flux conundrum. Science 2000, 289, 2000. [Google Scholar] [CrossRef] [Green Version]
- Shukla, J.B.; Sundar, S.; Shivangi; Naresh, R. Modeling and analysis of the acid rain formation due to precipitation and its effect on plant species. Nat. Resour. Model. 2013, 26, 53–65. [Google Scholar] [CrossRef]
- Lyimo, T.J. Microbial and nutrient pollution in the coastal bathing waters of Dar es Salaam. Aquat. Conserv. 2009, 19, S27–S37. [Google Scholar] [CrossRef]
- Korotchenkov, G.S. Handbook of Gas Sensor Materials; Spinger: New York, NY, USA, 2013; Volume 1. [Google Scholar]
- Geng, X.; Lu, P.; Zhang, C.; Lahem, D.; Olivier, M.-G.; Debliquy, M. Room-temperature NO2 gas sensors based on rGO@ZnO1-x composites: Experiments and molecular dynamics simulation. Sens. Actuators B Chem. 2019, 282, 690–702. [Google Scholar] [CrossRef]
- Nasiri, N.; Bo, R.H.; Wang, F.; Fu, L.; Tricoli, A. Ultraporous Electron-Depleted ZnO Nanoparticle Networks for Highly Sensitive Portable Visible-Blind UV Photodetectors. Adv. Mater. 2015, 27, 4336–4343. [Google Scholar] [CrossRef]
- Nasiri, N.; Bo, R.; Hung, T.F.; Roy, V.A.L.; Fu, L.; Tricoli, A. Tunable Band-Selective UV-Photodetectors by 3D Self-Assembly of Heterogeneous Nanoparticle Networks. Adv. Funct. Mater. 2016, 26, 7359–7366. [Google Scholar] [CrossRef]
- Cao, P.; Cai, Y.; Pawar, D.; Navale, S.T.; Rao, C.N.; Han, S.; Xu, W.; Fang, M.; Liu, X.; Zeng, Y.; et al. Down to ppb level NO2 detection by ZnO/rGO heterojunction based chemiresistive sensors. Chem. Eng. J. 2020, 401, 125491. [Google Scholar] [CrossRef]
- Shafiei, M.; Bradford, J.; Khan, H.; Piloto, C.; Wlodarski, W.; Li, Y.X.; Motta, N. Low-operating temperature NO2 gas sensors based on hybrid two-dimensional SnS2-reduced graphene oxide. Appl. Surf. Sci. 2018, 462, 330–336. [Google Scholar] [CrossRef]
- Ou, J.Z.; Ge, W.; Carey, B.; Daeneke, T.; Rotbart, A.; Shan, W.; Wang, Y.; Fu, Z.; Chrimes, A.F.; Wlodarski, W.; et al. Physisorption-Based Charge Transfer in Two-Dimensional SnS2 for Selective and Reversible NO2 Gas Sensing. Acs Nano 2015, 9, 10313–10323. [Google Scholar] [CrossRef]
- Das, S.; Chakraborty, S.; Parkash, O.; Kumar, D.; Bandyopadhyay, S.; Samudrala, S.K.; Sen, A.; Maiti, H.S. Vanadium doped tin dioxide as a novel sulfur dioxide sensor. Talanta 2008, 75, 385–389. [Google Scholar] [CrossRef]
- Ingle, N.; Mane, S.; Sayyad, P.; Bodkhe, G.; Al-Gahouari, T.; Mahadik, M.; Shirsat, S.; Shirsat, M.D. Sulfur Dioxide (SO2) Detection Using Composite of Nickel Benzene Carboxylic (Ni3BTC2) and OH-Functionalized Single Walled Carbon Nanotubes (OH-SWNTs). Front. Mater. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Zhao, C.H.; Gong, H.M.; Niu, G.Q.; Wang, F. Ultrasensitive SO2 sensor for sub-ppm detection using Cu-doped SnO2 nanosheet arrays directly grown on chip. Sens. Actuators B Chem. 2020, 324. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Wang, X.; Yao, F.; Xu, P.; Li, M.; Yu, H.; Li, X. Quantitative Structure–Activity Relationship of Nanowire Adsorption to SO2 Revealed by In Situ TEM Technique. Nano Lett. 2021, 21, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Chuang, S.S.C. The Nature of Adsorbed Carbon Dioxide on Immobilized Amines during Carbon Dioxide Capture from Air and Simulated Flue Gas. Energy Technol. 2017, 5, 510–519. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. CO2 and Greenhouse Gas Emission. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 13 May 2021).
- Lacis, A.A.; Schmidt, G.A.; Rind, D.; Ruedy, R.A. Atmospheric CO2: Principal Control Knob Governing Earth’s Temperature. Science 2010, 330, 356–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [Green Version]
- Ramalho, O.; Wyart, G.; Mandin, C.; Blondeau, P.; Cabanes, P.-A.; Leclerc, N.; Mullot, J.-U.; Boulanger, G.; Redaelli, M. Association of carbon dioxide with indoor air pollutants and exceedance of health guideline values. Build Environ. 2015, 93, 115–124. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Kler, J.S.; Hernke, M.T.; Braun, R.K.; Meyer, K.C.; Funk, W.E. Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustain. 2019, 2, 691–701. [Google Scholar] [CrossRef]
- Griffiths, S.; Sovacool, B.K. Rethinking the future low-carbon city: Carbon neutrality, green design, and sustainability tensions in the making of Masdar City. Energy Res. Soc. Sci. 2020, 62, 101368. [Google Scholar] [CrossRef]
- Molina, A.; Escobar-Barrios, V.; Oliva, J. A review on hybrid and flexible CO2 gas sensors. Synth. Met. 2020, 270, 116602. [Google Scholar] [CrossRef]
- Jeong, H.-M.; Kim, J.-H.; Jeong, S.-Y.; Kwak, C.-H.; Lee, J.-H. Co3O4 –SnO2 Hollow Heteronanostructures: Facile Control of Gas Selectivity by Compositional Tuning of Sensing Materials via Galvanic Replacement. ACS Appl. Mater. Interfaces 2016, 8, 7877–7883. [Google Scholar] [CrossRef]
- Lee, J.E.; Lim, C.K.; Park, H.J.; Song, H.; Choi, S.-Y.; Lee, D.-S. ZnO–CuO Core-Hollow Cube Nanostructures for Highly Sensitive Acetone Gas Sensors at the ppb Level. ACS Appl. Mater. Interfaces 2020, 12, 35688–35697. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, D.; Shao, C.; Lu, G.; Li, X.; Liu, Y. Hollow CuFe2O4/α-Fe2O3 composite with ultrathin porous shell for acetone detection at ppb levels. Sens. Actuators B Chem. 2018, 258, 436–446. [Google Scholar] [CrossRef]
- Zito, C.A.; Perfecto, T.M.; Dippel, A.-C.; Volanti, D.P.; Koziej, D. Low-Temperature Carbon Dioxide Gas Sensor Based on Yolk–Shell Ceria Nanospheres. ACS Appl. Mater. Interfaces 2020, 12, 17745–17751. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Ippolito, S.J.; Periasamy, S.; Sabri, Y.M.; Sunkara, M.V. Efficient Heterostructures of Ag@CuO/BaTiO3 for Low-Temperature CO2 Gas Detection: Assessing the Role of Nanointerfaces during Sensing by Operando DRIFTS Technique. ACS Appl. Mater. Interfaces 2017, 9, 27014–27026. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.H.S.; de Lazaro, S.R. A theoretical investigation of the Zn-doping influence on structural and electronic properties of BaTiO3. Solid State Ion. 2016, 297, 36–42. [Google Scholar] [CrossRef]
- Lee, S.; Bock, J.A.; Trolier-Mckinstry, S.; Randall, C.A. Ferroelectric-thermoelectricity and Mott transition of ferroelectric oxides with high electronic conductivity. J. Eur. Ceram. 2012, 32, 3971–3988. [Google Scholar] [CrossRef]
- Son, M.; Pak, Y.; Chee, S.-S.; Auxilia, F.M.; Kim, K.; Lee, B.-K.; Lee, S.; Kang, S.K.; Lee, C.; Lee, J.S.; et al. Charge transfer in graphene/polymer interfaces for CO2 detection. Nano Res. 2018, 11, 3529–3536. [Google Scholar] [CrossRef]
- Yoon, B.; Choi, S.-J.; Swager, T.M.; Walsh, G.F. Switchable Single-Walled Carbon Nanotube–Polymer Composites for CO2 Sensing. ACS Appl. Mater. Interfaces 2018, 10, 33373–33379. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Pereira, J.; Porto-Figueira, P.; Cavaco, C.; Taunk, K.; Rapole, S.; Dhakne, R.; Nagarajaram, H.; Câmara, J. Breath Analysis as a Potential and Non-Invasive Frontier in Disease Diagnosis: An Overview. Metabolites 2015, 5, 3–55. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Tian, H.; Pan, S.; Prior, S.A.; Feng, Y.; Batchelor, W.D.; Chen, J.; Yang, J. Global ammonia emissions from synthetic nitrogen fertilizer applications in agricultural systems: Empirical and process-based estimates and uncertainty. Glob. Chang. Biol. 2019, 25, 314–326. [Google Scholar] [CrossRef] [Green Version]
- Ghavam, S.; Vahdati, M.; Wilson, I.A.G.; Styring, P. Sustainable Ammonia Production Processes. Front. Energy Res. 2021, 9. [Google Scholar] [CrossRef]
- Liu, G.K.; Zhu, L.J.; Yu, Y.M.; Qiu, M.; Gao, H.J.; Chen, D.Y. WO3 nanoplates for sensitive and selective detections of both acetone and NH3 gases at different operating temperatures. J. Alloys Compd. 2021, 858. [Google Scholar] [CrossRef]
- Saidi, T.; Zaim, O.; Moufid, M.; El Bari, N.; Ionescu, R.; Bouchikhi, B. Exhaled breath analysis using electronic nose and gas chromatography–mass spectrometry for non-invasive diagnosis of chronic kidney disease, diabetes mellitus and healthy subjects. Sens. Actuators B Chem. 2018, 257, 178–188. [Google Scholar] [CrossRef]
- Mitrayana, M.; Ma’arif, M.A.; Wasono, M.A.J.; Satriawan, M.; Ikhsan, M.R. Application of the CO2 laser photoacoustic spectroscopy in detecting ammonia gas (NH3) in liver disease patients breath. Key Eng. Mater. 2020, 840, 399–405. [Google Scholar] [CrossRef]
- Yang, T.; Jiang, X.; Zhong, Y.; Zhao, X.; Lin, S.; Li, J.; Li, X.; Xu, J.; Li, Z.; Zhu, H. A Wearable and Highly Sensitive Graphene Strain Sensor for Precise Home-Based Pulse Wave Monitoring. ACS Sens. 2017, 2, 967–974. [Google Scholar] [CrossRef]
- Subbiah, D.K.; Mani, G.K.; Babu, K.J.; Das, A.; Rayappan, J.B.B. Nanostructured ZnO on cotton fabrics A novel flexible gas sensor & UV filter. J. Clean. Prod. 2018, 194, 372–382. [Google Scholar] [CrossRef]
- Kassem, O.; Saadaoui, M.; Rieu, M.; Viricelle, J.-P. A novel approach to a fully inkjet printed SnO2-based gas sensor on a flexible foil. J. Mater. Chem. C 2019, 7, 12343–12353. [Google Scholar] [CrossRef]
- Yu, Z.; Tang, Y.; Cai, G.; Ren, R.; Tang, D. Paper Electrode-Based Flexible Pressure Sensor for Point-of-Care Immunoassay with Digital Multimeter. Anal. Chem. 2019, 91, 1222–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.; Zhang, C.; Khan, A.; Liang, C.; Li, W.-D. Highly transparent and flexible polyaniline mesh sensor for chemiresistive sensing of ammonia gas. RSC Adv. 2018, 8, 5312–5320. [Google Scholar] [CrossRef] [Green Version]
- Güntner, A.T.; Wied, M.; Pineau, N.J.; Pratsinis, S.E. Rapid and Selective NH3 Sensing by Porous CuBr. Adv. Sci. 2020, 7, 1903390. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Fan, H.; Li, Z.; Jia, Y.; Yadav, A.K.; Dong, G.; Wang, W.; Dong, W.; Wang, S. Multi-walled carbon nanotubes/polyaniline on the ethylenediamine modified polyethylene terephthalate fibers for a flexible room temperature ammonia gas sensor with high responses. Sens. Actuators B Chem. 2021, 334, 129677. [Google Scholar] [CrossRef]
- Li, S.; Liu, A.; Yang, Z.; He, J.; Wang, J.; Liu, F.; Lu, H.; Yan, X.; Sun, P.; Liang, X.; et al. Room temperature gas sensor based on tin dioxide@polyaniline nanocomposite assembled on flexible substrate: Ppb-level detection of NH3. Sens. Actuators B Chem. 2019, 299, 126970. [Google Scholar] [CrossRef]
- Karl, T.; Prazeller, P.; Mayr, D.; Jordan, A.; Rieder, J.; Fall, R.; Lindinger, W. Human breath isoprene and its relation to blood cholesterol levels: New measurements and modeling. J. Appl. Physiol. 2001, 91, 762–770. [Google Scholar] [CrossRef] [Green Version]
- Güntner, A.T.; Pineau, N.J.; Chie, D.; Krumeich, F.; Pratsinis, S.E. Selective sensing of isoprene by Ti-doped ZnO for breath diagnostics. J. Mater. Chem. B 2016, 4, 5358–5366. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, D.; Jamnig, H.; Heininger, P.; Klieber, M.; Schroecksnadel, S.; Fiegl, M.; Hackl, M.; Denz, H.; Amann, A. Decline of exhaled isoprene in lung cancer patients correlates with immune activation. J. Breath Res. 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Schwarz, K.; Ligor, M.; Ligor, T.; Filipiak, W.; Denz, H.; Fiegl, M.; et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 2009, 9, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhouri, N.; Singh, T.; Alsabbagh, E.; Guirguis, J.; Chami, T.; Hanouneh, I.; Grove, D.; Lopez, R.; Dweik, R. Isoprene in the Exhaled Breath is a Novel Biomarker for Advanced Fibrosis in Patients with Chronic Liver Disease: A Pilot Study. Clin. Transl. Gastroenterol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Haneda, H.; Watanabe, K.; Shimanoe, K.; Sakaguchi, I. Highly sensitive isoprene gas sensor using Au-loaded pyramid-shaped ZnO particles. Sens. Actuators B Chem. 2021, 326, 128999. [Google Scholar] [CrossRef]
- Han, B.; Wang, J.; Yang, W.; Chen, X.; Wang, H.; Chen, J.; Zhang, C.; Sun, J.; Wei, X. Hydrothermal synthesis of flower-like In2O3 as a chemiresistive isoprene sensor for breath analysis. Sens. Actuators B Chem. 2020, 309, 127788. [Google Scholar] [CrossRef]
- Van Den Broek, J.; Güntner, A.T.; Pratsinis, S.E. Highly Selective and Rapid Breath Isoprene Sensing Enabled by Activated Alumina Filter. ACS Sens. 2018, 3, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.N.; Wang, C.J. Is breath acetone a biomarker of diabetes? A historical review on breath acetone measurements. J. Breath Res. 2013, 7. [Google Scholar] [CrossRef] [Green Version]
- Deng, C.; Zhang, J.; Yu, X.; Zhang, W.; Zhang, X. Determination of acetone in human breath by gas chromatography–mass spectrometry and solid-phase microextraction with on-fiber derivatization. J. Chromatogr. B Biomed. Appl. 2004, 810, 269–275. [Google Scholar] [CrossRef]
- Diskin, A.M.; Spanel, P.; Smith, D. Time variation of ammonia, acetone, isoprene and ethanol in breath: A quantitative SIFT-MS study over 30 days. Physiol. Meas. 2003, 24, 107–119. [Google Scholar] [CrossRef]
- WHO. World Health Statistics 2016: Monitoring Health for the Sustainable Development Goals (SDGs); WHO: Geneva, Switzerland, 2016; ISBN 9789240695696. [Google Scholar]
- Jaisutti, R.; Kim, J.; Park, S.K.; Kim, Y.-H. Low-Temperature Photochemically Activated Amorphous Indium-Gallium-Zinc Oxide for Highly Stable Room-Temperature Gas Sensors. ACS Appl. Mater. Interfaces 2016, 8, 20192–20199. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, S.; Qu, F.; Gong, S.; Wang, C.; Qiu, L.; Yang, M.; Cheng, W. High performance acetone sensor based on ZnO nanorods modified by Au nanoparticles. J. Alloys Compd. 2019, 797, 246–252. [Google Scholar] [CrossRef]
- Lu, J.; Xu, C.; Cheng, L.; Jia, N.; Huang, J.; Li, C. Acetone sensor based on WO3 nanocrystallines with oxygen defects for low concentration detection. Mater. Sci. Semicond. Process. 2019, 101, 214–222. [Google Scholar] [CrossRef]
- Chang, X.; Qiao, X.; Li, K.; Wang, P.; Xiong, Y.; Li, X.; Xia, F.; Xue, Q. UV assisted ppb-level acetone detection based on hollow ZnO/MoS2 nanosheets core/shell heterostructures at low temperature. Sens. Actuators B Chem. 2020, 317, 128208. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, L.; Yang, C.; Liu, X.; Zhang, J. Highly sensitive and selective electronic sensor based on Co catalyzed SnO2 nanospheres for acetone detection. Sens. Actuators B Chem. 2020, 304, 127237. [Google Scholar] [CrossRef]
- Qu, F.; Zhang, N.; Zhang, S.; Zhao, R.; Yao, D.; Ruan, S.; Yang, M. Construction of Co3O4/CoWO4 core-shell urchin-like microspheres through ion-exchange method for high-performance acetone gas sensing performance. Sens. Actuators B Chem. 2020, 309, 127711. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, L.; Guo, F.; Liu, S.; Qi, L.; Shan, M.; Fan, X. Facial development of high performance room temperature NO2 gas sensors based on ZnO nanowalls decorated rGO nanosheets. Appl. Surf. Sci. 2017, 423, 721–727. [Google Scholar] [CrossRef]
- Chen, A.; Liu, R.; Peng, X.; Chen, Q.; Wu, J. 2D Hybrid Nanomaterials for Selective Detection of NO2 and SO2 Using “Light On and Off” Strategy. ACS Appl. Mater. Interfaces 2017, 9, 37191–37200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sackmann, A.; Gao, S.; Weimar, U.; Lu, G.; Liu, S.; Zhang, T.; Barsan, N. Study on highly selective sensing behavior of ppb-level oxidizing gas sensors based on Zn2SnO4 nanoparticles immobilized on reduced graphene oxide under humidity conditions. Sens. Actuators B Chem. 2019, 285, 590–600. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, W.; Chu, Z.; Wang, S.; Chen, L.; Nie, X.; Wang, R.; He, X. High Sensitivity, Humidity-Independent, Flexible NO2 and NH3 Gas Sensors Based on SnS2 Hybrid Functional Graphene Ink. ACS Appl. Mater. Interfaces 2020, 12, 997–1004. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, C.; Debliquy, M. Cadmium sulfide activated zinc oxide coatings deposited by liquid plasma spray for room temperature nitrogen dioxide detection under visible light illumination. Ceram. Int. 2016, 42, 4845–4852. [Google Scholar] [CrossRef]
- Vijjapu, M.T.; Surya, S.G.; Yuvaraja, S.; Zhang, X.; Alshareef, H.N.; Salama, K.N. Fully Integrated Indium Gallium Zinc Oxide NO2 Gas Detector. ACS Sens. 2020, 5, 984–993. [Google Scholar] [CrossRef]
- Yu, H.; Yang, T.; Wang, Z.; Li, Z.; Zhao, Q.; Zhang, M. p-N heterostructural sensor with SnO-SnO2 for fast NO2 sensing response properties at room temperature. Sens. Actuators B Chem. 2018, 258, 517–526. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Z.; Liu, D.; Wang, K. Dendritic composite array of silicon nanowires/WO3 nanowires for sensitive detection of NO2 at room temperature. Mater. Lett. 2017, 207, 29–32. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, J.; Rehman, A.U.; Gong, L.; Kan, K.; Li, L.; Shi, K. Synthesis of NiO@CuO nanocomposite as high-performance gas sensing material for NO2 at room temperature. Appl. Surf. Sci. 2017, 412, 230–237. [Google Scholar] [CrossRef]
- Sonker, R.K.; Yadav, B.C. Development of Fe2O3–PANI nanocomposite thin film based sensor for NO2 detection. J. Taiwan Inst. Chem. Eng. 2017, 77, 276–281. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, X.; Huo, L.; Tian, X.; Qi, T.; Yang, F.; Wang, X.; Yu, K.; Ma, F.; Sun, J. P-CuPcTS/n-SnO2 organic-inorganic hybrid film for ppb-level NO2 gas sensing at low operating temperature. Sens. Actuators B Chem. 2017, 248, 324–331. [Google Scholar] [CrossRef]
- Su, P.-G.; Zheng, Y.-L. Room-temperature ppb-level SO2 gas sensors based on RGO/WO3 and MWCNTs/WO3 nanocomposites. Anal. Methods. 2021, 13, 782–788. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S. Oxygen Vacancies as an Efficient Strategy for Promotion of Low Concentration SO2 Gas Sensing: The Case of Au-Modified SnO2. ACS Sustain. Chem. Eng. 2018, 6, 13427–13434. [Google Scholar] [CrossRef]
- Chaudhary, V.; Singh, H.; Kaur, A. Effect of charge carrier transport on sulfur dioxide monitoring performance of highly porous polyaniline nanofibres. Polym. Int. 2017, 66, 699–704. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Chen, Y.; Zhang, Y.; Gao, X.; Xu, P.; Li, X.; Fang, J.; Wen, W. An integrated micro-chip with Ru/Al2O3/ZnO as sensing material for SO2 detection. Sens. Actuators B Chem. 2018, 262, 26–34. [Google Scholar] [CrossRef]
- Choeichom, P.; Sirivat, A. Discriminative sensing performances of ZSM-5, Y, mordenite, ferrierite, beta, 3A, 4A, 5A, and 13X zeolites towards sulfur dioxide. Ionics 2018, 24, 2829–2841. [Google Scholar] [CrossRef]
- Yadav, A.A.; Lokhande, A.C.; Kim, J.H.; Lokhande, C.D. Improvement in CO2 sensing characteristics using Pd nanoparticles decorated La2O3 thin films. J. Ind. Eng. Chem. 2017, 49, 76–81. [Google Scholar] [CrossRef]
- Panes-Ruiz, L.A.; Shaygan, M.; Fu, Y.; Liu, Y.; Khavrus, V.; Oswald, S.; Gemming, T.; Baraban, L.; Bezugly, V.; Cuniberti, G. Toward Highly Sensitive and Energy Efficient Ammonia Gas Detection with Modified Single-Walled Carbon Nanotubes at Room Temperature. ACS Sens. 2018, 3, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, B.; Wang, H.; Yang, W.; Wang, J.; Wei, X. Hierarchical Pt-decorated In2O3 microspheres with highly enhanced isoprene sensing properties. Ceram. Int. 2021, 47, 9477–9485. [Google Scholar] [CrossRef]
- Wu, X.; Wang, H.; Wang, J.; Chen, J.; Shi, L.; Han, B.; Tian, X. Hydrothermal synthesis of flower-like Cr2O3-doped In2O3 nanorods clusters for ultra-low isoprene detection. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126606. [Google Scholar] [CrossRef]
- Wang, P.; Dong, T.; Jia, C.; Yang, P. Ultraselective acetone-gas sensor based ZnO flowers functionalized by Au nanoparticle loading on certain facet. Sens. Actuators B Chem. 2019, 288, 1–11. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Leishman, M.; Bagnall, D.; Nasiri, N. Nanostructured Gas Sensors: From Air Quality and Environmental Monitoring to Healthcare and Medical Applications. Nanomaterials 2021, 11, 1927. https://doi.org/10.3390/nano11081927
Chen X, Leishman M, Bagnall D, Nasiri N. Nanostructured Gas Sensors: From Air Quality and Environmental Monitoring to Healthcare and Medical Applications. Nanomaterials. 2021; 11(8):1927. https://doi.org/10.3390/nano11081927
Chicago/Turabian StyleChen, Xiaohu, Michelle Leishman, Darren Bagnall, and Noushin Nasiri. 2021. "Nanostructured Gas Sensors: From Air Quality and Environmental Monitoring to Healthcare and Medical Applications" Nanomaterials 11, no. 8: 1927. https://doi.org/10.3390/nano11081927
APA StyleChen, X., Leishman, M., Bagnall, D., & Nasiri, N. (2021). Nanostructured Gas Sensors: From Air Quality and Environmental Monitoring to Healthcare and Medical Applications. Nanomaterials, 11(8), 1927. https://doi.org/10.3390/nano11081927







