Hydrogen Storage in Untreated/Ammonia-Treated and Transition Metal-Decorated (Pt, Pd, Ni, Rh, Ir and Ru) Activated Carbons
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Crystallinity and Morphological Characterization
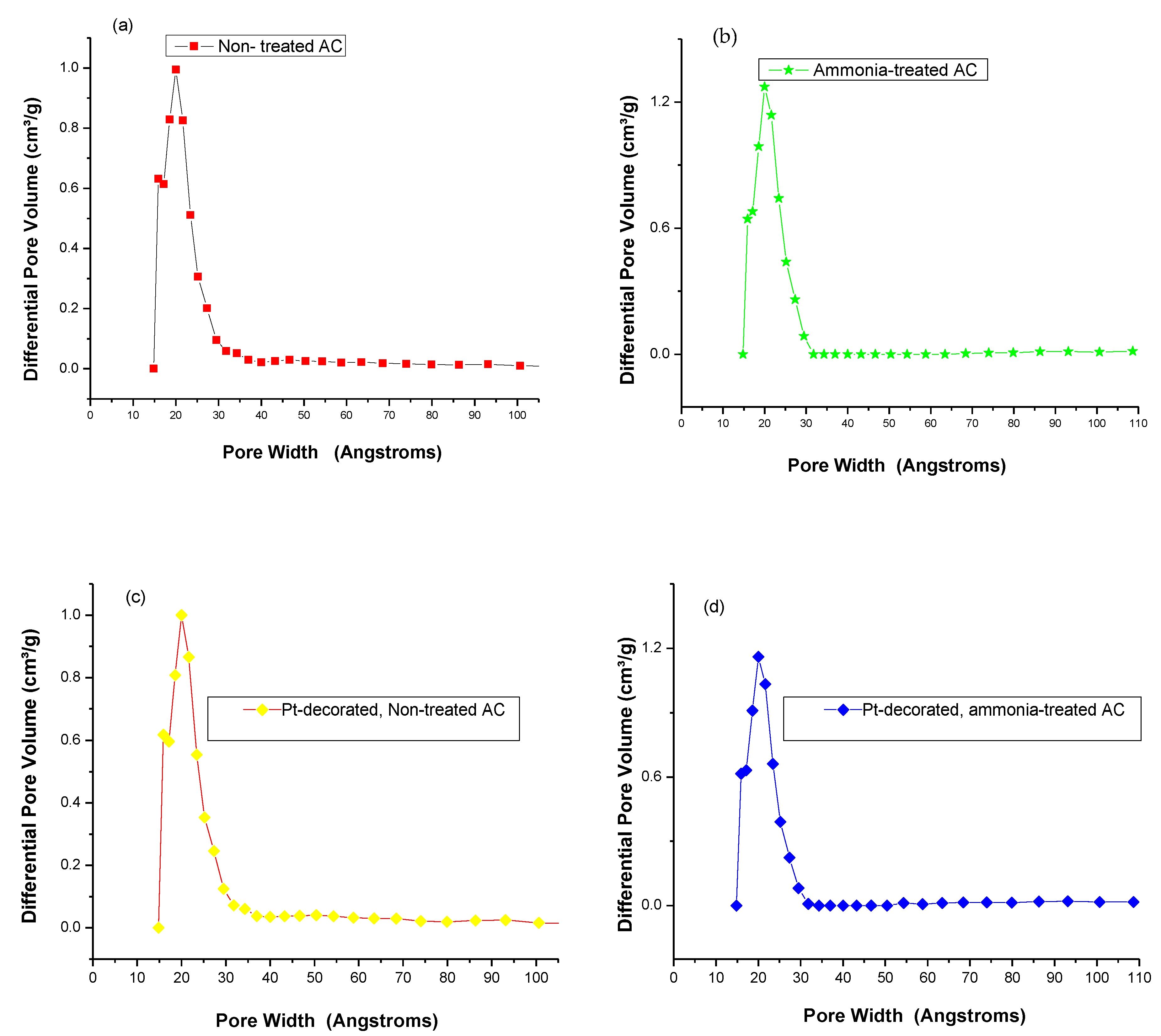
3.2. Textural Characterization
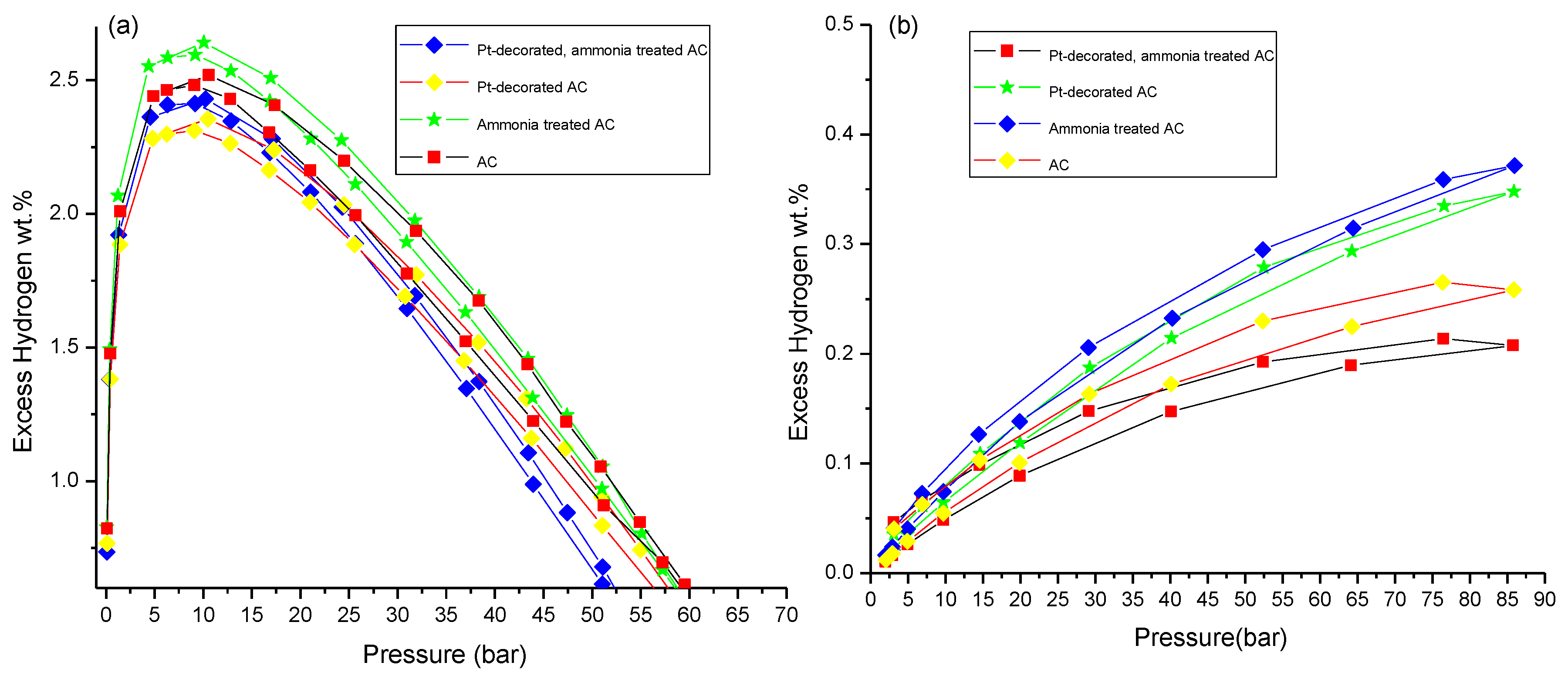
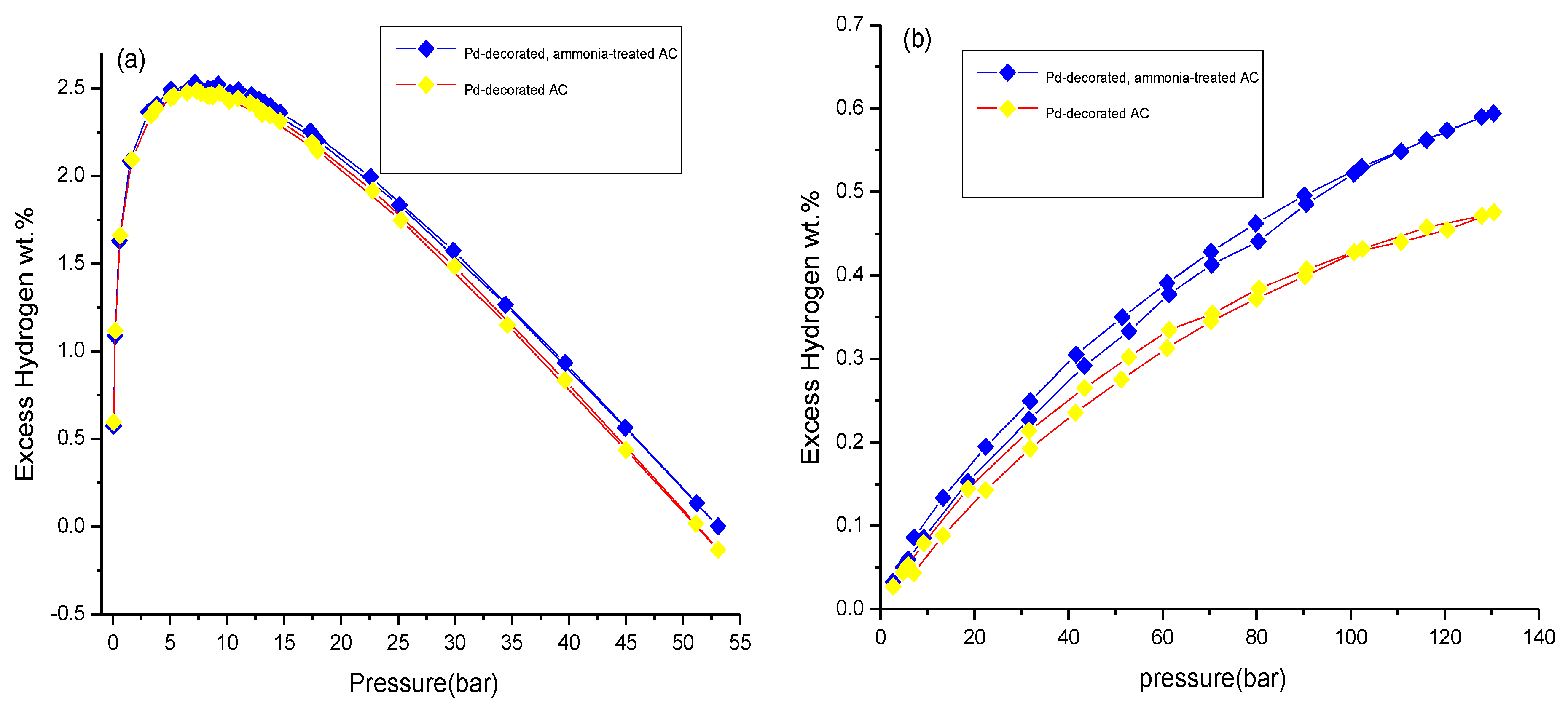
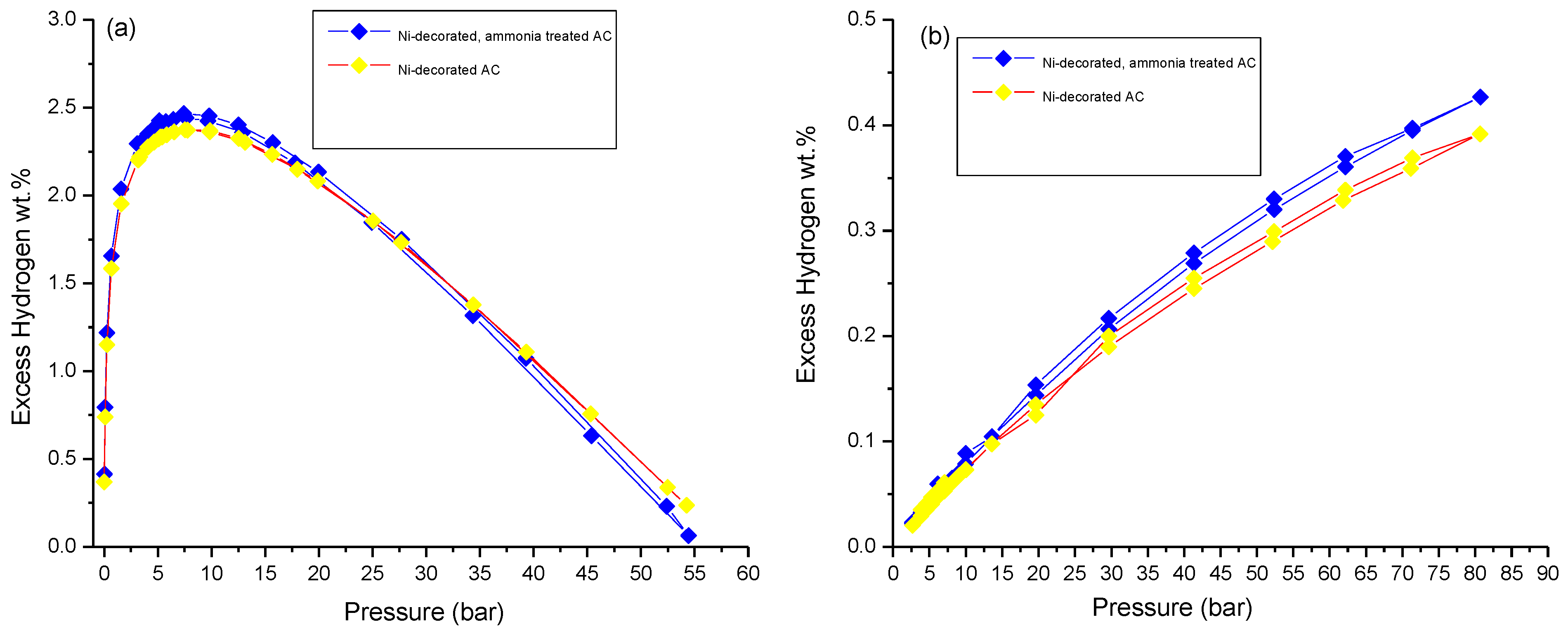
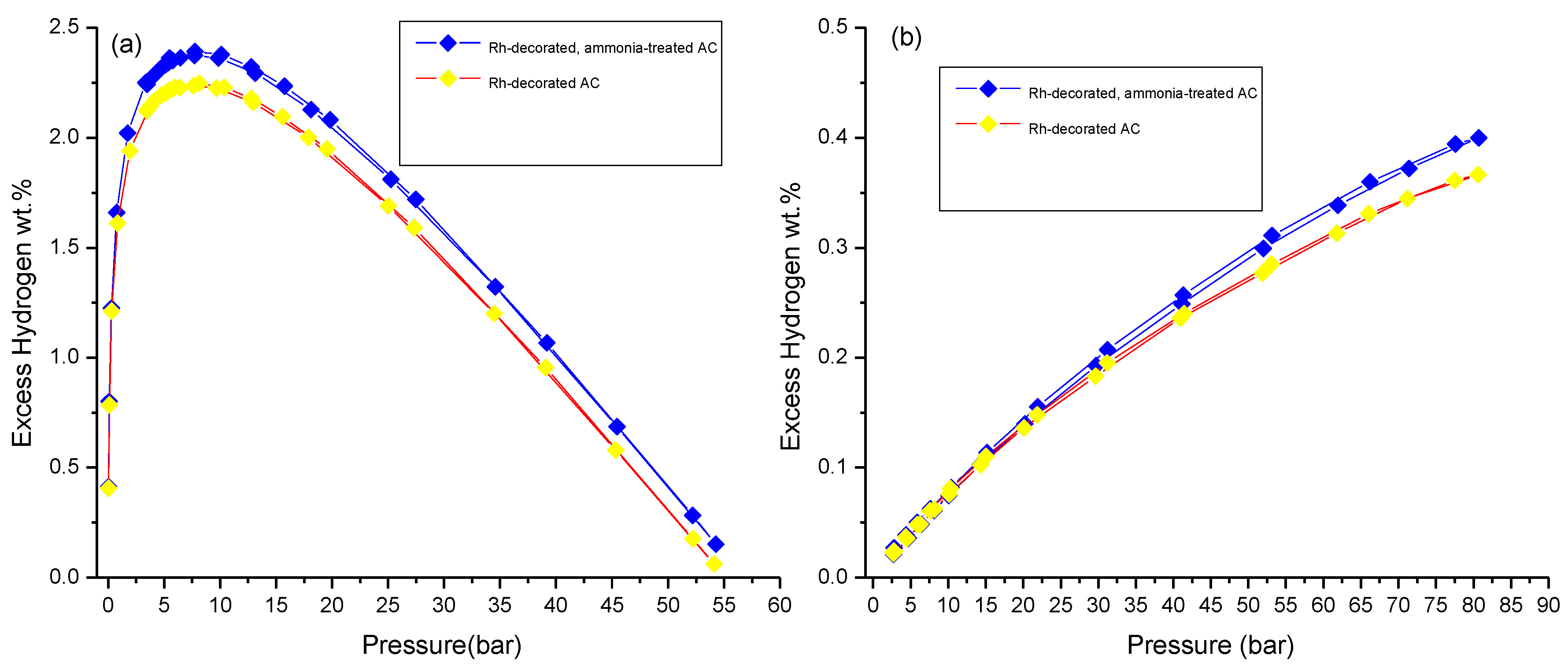
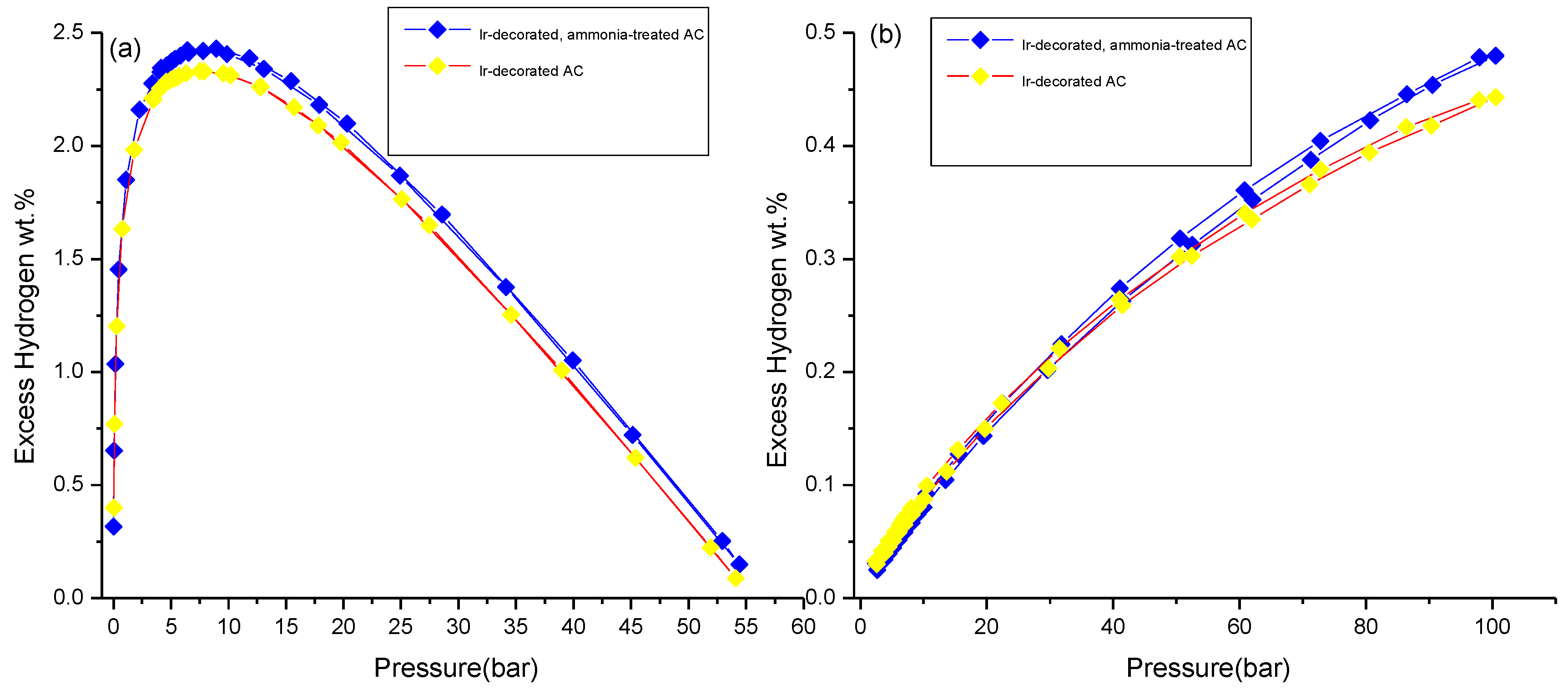
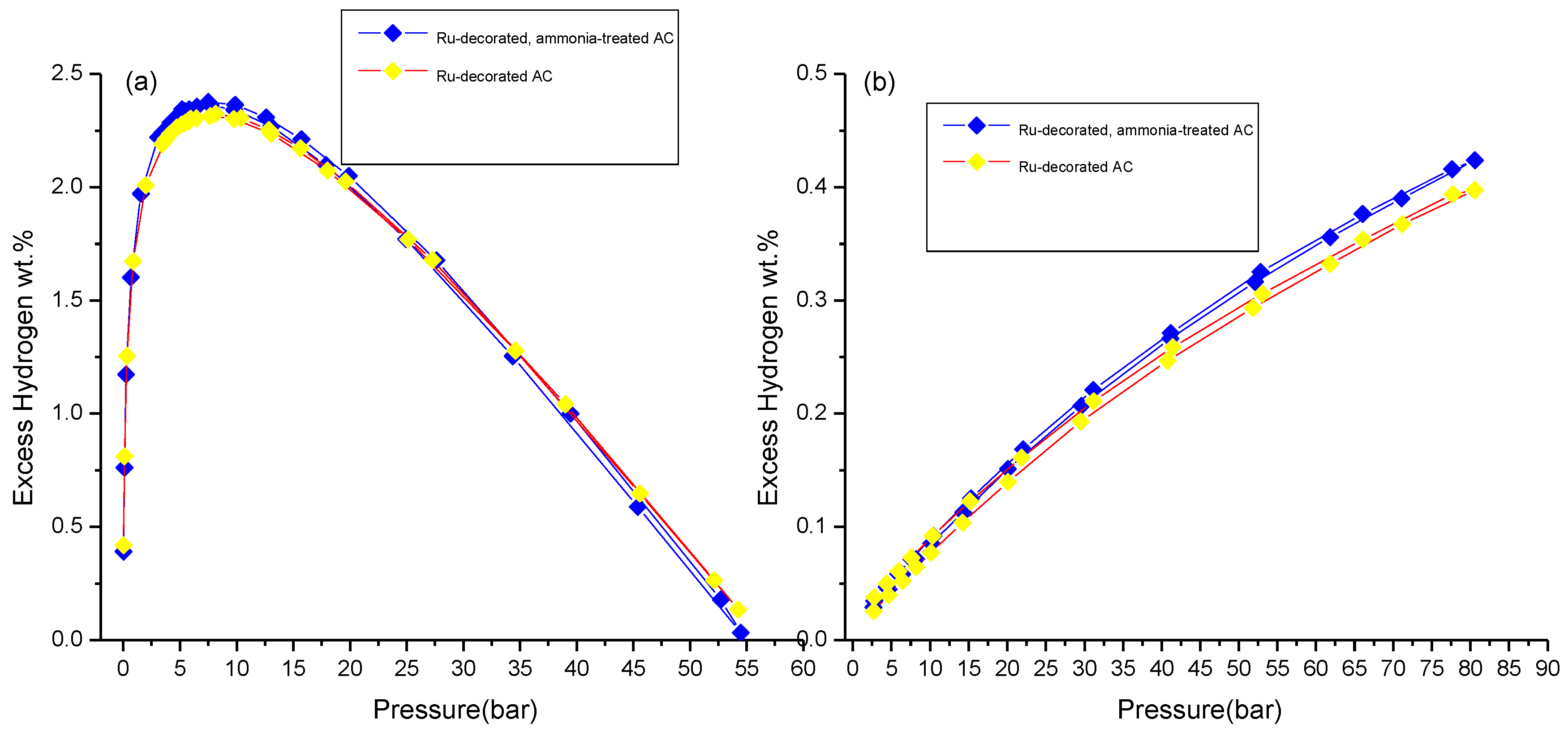
3.3. Hydrogen Storage Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlapbach, L.; Züttel, A. Hydrogen-Storage Materials for Mobile Applications. Nat. Cell Biol. 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Rostrup-Nielsen, J.R.; Rostrup-Nielsen, T. Large-Scale Hydrogen Production. Cattech 2002, 6, 150–159. [Google Scholar] [CrossRef]
- Rosen, M.A.; Koohi-Fayegh, S. The Prospects for Hydrogen as an Energy Carrier: An Overview of Hydrogen Energy and Hydrogen Energy Systems. Energy Ecol. Environ. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Rivera, X.C.S.; Topriska, E.; Kolokotroni, M.; Azapagic, A. Environmental Sustainability of Renewable Hydrogen in Comparison with Conventional Cooking Fuels. J. Clean. Prod. 2018, 196, 863–879. [Google Scholar] [CrossRef]
- Logan, K.G.; Nelson, J.D.; Hastings, A. Electric and Hydrogen Buses: Shifting from Conventionally Fuelled Cars in the UK. Transp. Res. Part D Transp. Environ. 2020, 85, 102350. [Google Scholar] [CrossRef]
- Post, L.E.M. Fuel Cell Electric Buses in U.S. Transit Fleets: Current Status 2020. NREL/TP-5400-75583. 2021. Available online: https://www.nrel.gov/docs/fy21osti/75583.pdf (accessed on 1 May 2021).
- Kapelewski, M.T.; Runcevski, T.; Tarver, J.D.; Jiang, H.Z.H.; Hurst, K.E.; Parilla, P.A.; Ayala, A.; Gennett, T.; Fitzgerald, S.A.; Brown, C.M.; et al. Record High Hydrogen Storage Capacity in the Metal–Organic Framework Ni2 (m-dobdc) at Near-Ambient Temperatures. Chem. Mater. 2018, 30, 8179–8189. [Google Scholar] [CrossRef]
- The Engineering ToolBox. Hydrogen-Density and Specific Weight. Available online: https://www.engineeringtoolbox.com/hydrogen-H2-density-specific-weight-temperature-pressure-d_2044.html (accessed on 1 May 2021).
- Barthélémy, H. Effects of Pressure and Purity on the Hydrogen Embrittlement of Steels. Int. J. Hydrog. Energy 2011, 36, 2750–2758. [Google Scholar] [CrossRef]
- Krasae-In, S.; Stang, J.H.; Neksa, P. Simulation on a Proposed Large-Scale Liquid Hydrogen Plant Using a Multi-Component Refrigerant Refrigeration System. Int. J. Hydrog. Energy 2010, 35, 12531–12544. [Google Scholar] [CrossRef]
- David, A.C.; Bebon, L.; Thiebaud, F. Hydrogen Storage: The Different Technologies, the Challenges and the Stakes. In Proceedings of the International Symposiumon Sustainable Hydrogen, Alger, Algeria, 15 June 2020; pp. 1–9. [Google Scholar]
- von Colbe, J.B.; Ares, J.-R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.; Guzik, M.N.; Jacob, I.; et al. Application of Hydrides in Hydrogen Storage and Compression: Achievements, Outlook and Perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Somo, T.R.; Maponya, T.C.; Davids, M.W.; Hato, M.J.; Lototskyy, M.V.; Modibane, K.D. A Comprehensive Review on Hydrogen Absorption Behaviour of Metal Alloys Prepared through Mechanical Alloying. Metals 2020, 10, 562. [Google Scholar] [CrossRef]
- Andersson, J.; Grönkvist, S. Large-Scale Storage of Hydrogen. Int. J. Hydrog. Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Hu, X.; Qi, Z.; Qin, F.; Chen, J. Mechanism Analysis on Stress Accumulation in Cylindrical Vertical-Placed Metal Hydride Reactor. Energy Power Eng. 2011, 03, 490–498. [Google Scholar] [CrossRef]
- Lohstroh, W.; Fichtner, M.; Breitung, W. Complex Hydrides as Solid Storage Materials: First Safety Tests. Int. J. Hydrog. Energy 2009, 34, 5981–5985. [Google Scholar] [CrossRef]
- Ovshinsky, S.R.; Fetcenko, M.A.; Ross, J. A Nickel Metal Hydride Battery for Electric Vehicles. Science 1993, 260, 176–181. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.; Williams, C.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef]
- Dong, J.; Wang, X.; Xu, H.; Zhao, Q.; Li, J. Hydrogen Storage in Several Microporous Zeolites. Int. J. Hydrog. Energy 2007, 32, 4998–5004. [Google Scholar] [CrossRef]
- Uzun, A.; Keskin, S. Site Characteristics in Metal Organic Frameworks for Gas Adsorption. Prog. Surf. Sci. 2014, 89, 56–79. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen Storage in Carbon Materials—A Review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical Conductivity of Individual Carbon Nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Wong, E.W.; Sheehan, P.; Lieber, C.M. Nanobeam Mechanics: Elasticity, Strength, and Toughness of Nanorods and Nanotubes. Science 1997, 277, 1971–1975. [Google Scholar] [CrossRef]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef]
- Liang, W.; Yokojima, S.; Ng, M.-F.; Chen, G.; He, G. Optical Properties of Single-Walled 4 Å Carbon Nanotubes. J. Am. Chem. Soc. 2001, 123, 9830–9836. [Google Scholar] [CrossRef] [PubMed]
- Minot, E.D.; Yaish, Y.; Sazonova, V.; McEuen, P.L. Determination of Electron Orbital Magnetic Moments in Carbon Nanotubes. Nat. Cell Biol. 2004, 428, 536–539. [Google Scholar] [CrossRef]
- Motta, M.; Kinloch, I.; Windle, A.; Li, Y.-L. Mechanical Properties of Continuously Spun Fibers of Carbon Nanotubes. Nano Lett. 2005, 5, 1529–1533. [Google Scholar] [CrossRef] [PubMed]
- Aboud, M.F.A.; Alothman, Z.A.; Habila, M.A.; Zlotea, C.; Latroche, M.; Cuevas, F. Hydrogen Storage in Pristine and d10-Block Metal-Anchored Activated Carbon Made from Local Wastes. Energies 2015, 8, 3578–3590. [Google Scholar] [CrossRef]
- Mukherjee, B.; Kalita, G.; Sharon, M.; Sharon, M. Hydrogen Storage by Carbon Fibers from Cotton. QSci. Connect. 2013, 2013. [Google Scholar] [CrossRef]
- Dodziuk, H.; Dolgonos, G. Molecular Modeling Study of Hydrogen Storage in Carbon Nanotubes. Chem. Phys. Lett. 2002, 356, 79–83. [Google Scholar] [CrossRef]
- Lee, S.M.; An, K.H.; Lee, Y.H.; Seifert, G.; Frauenheim, T. A Hydrogen Storage Mechanism in Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2001, 123, 5059–5063. [Google Scholar] [CrossRef]
- Dillon, A.C.; Jones, K.M.; Bekkedahl, T.A.; Kiang, C.H.; Bethune, D.S.; Heben, M.J. Storage of Hydrogen in Single-Walled Carbon Nanotubes. Nat. Cell Biol. 1997, 386, 377–379. [Google Scholar] [CrossRef]
- Liu, C.; Fan, Y.Y.; Liu, M.; Cong, H.T.; Cheng, H.-M.; Dresselhaus, M.S. Hydrogen Storage in Single-Walled Carbon Nanotubes at Room Temperature. Science 1999, 286, 1127–1129. [Google Scholar] [CrossRef]
- Zhou, L. Progress and Problems in Hydrogen Storage Methods. Renew. Sustain. Energy Rev. 2005, 9, 395–408. [Google Scholar] [CrossRef]
- Shaijumon, M.; Ramaprabhu, S. Studies of Yield and Nature of Carbon Nanostructures Synthesized by Pyrolysis of Ferrocene and Hydrogen Adsorption Studies of Carbon Nanotubes. Int. J. Hydrog. Energy 2005, 30, 311–317. [Google Scholar] [CrossRef]
- Lee, H.; Kang, Y.-S.; Kim, S.-H.; Lee, J.-Y. Hydrogen Desorption Properties of Multiwall Carbon Nanotubes with Closed and Open Structures. Appl. Phys. Lett. 2002, 80, 577–579. [Google Scholar] [CrossRef]
- Yürüm, Y.; Taralp, A.; Veziroglu, T.N. Storage of Hydrogen in Nanostructured Carbon Materials. Int. J. Hydrogen Energy 2009, 34, 3784–3798. [Google Scholar] [CrossRef]
- Hou, P.-X.; Yang, Q.-H.; Bai, S.; Xu, S.-T.; Liu, A.M.; Cheng, H.-M. Bulk Storage Capacity of Hydrogen in Purified Multiwalled Carbon Nanotubes. J. Phys. Chem. B 2002, 106, 963–966. [Google Scholar] [CrossRef]
- Shiraishi, M.; Takenobu, T.; Kataura, H.; Ata, M. Hydrogen Adsorption and Desorption in Carbon Nanotube Systems and Its Mechanisms. Appl. Phys. A 2004, 78, 947–953. [Google Scholar] [CrossRef]
- Ding, R.G.; Lu, G.Q.; Yan, Z.F.; Wilson, M.A. Recent Advances in the Preparation and Utilization of Carbon Nanotubes for Hydrogen Storage. J. Nanosci. Nanotechnol. 2001, 1, 7–29. [Google Scholar] [CrossRef]
- Tibbetts, G.G.; Meisner, G.P.; Olk, C.H. Hydrogen Storage Capacity of Some Carbon Nanotubes and Filaments; Canadian Hydrogen Association: Vancouver, BC, Canada, 2000. [Google Scholar]
- Frankland, S.; Brenner, D. Hydrogen Raman Shifts in Carbon Nanotubes from Molecular Dynamics Simulation. Chem. Phys. Lett. 2001, 334, 18–23. [Google Scholar] [CrossRef]
- Tibbetts, G.G.; Meisner, G.P.; Olk, C.H. Hydrogen Storage Capacity of Carbon Nanotubes, Filaments, and Vapor-Grown Fibers. Carbon 2001, 39, 2291–2301. [Google Scholar] [CrossRef]
- Shiraishi, M.; Takenobu, T.; Ata, M. Gas–Solid Interactions in the Hydrogen/Single-Walled Carbon Nanotube System. Chem. Phys. Lett. 2003, 367, 633–636. [Google Scholar] [CrossRef]
- Bénard, P.; Chahine, R. Storage of Hydrogen by Physisorption on Carbon and Nanostructured Materials. Scr. Mater. 2007, 56, 803–808. [Google Scholar] [CrossRef]
- Gadiou, R.; Saadallah, S.-E.; Piquero, T.; David, P.; Parmentier, J.; Vix-Guterl, C. The Influence of Textural Properties on the Adsorption of Hydrogen on Ordered Nanostructured Carbons. Microporous Mesoporous Mater. 2005, 79, 121–128. [Google Scholar] [CrossRef]
- Georgakis, M.; Stavropoulos, G.; Sakellaropoulos, G. Molecular Dynamics Study of Hydrogen Adsorption in Carbonaceous Microporous Materials and the Effect of Oxygen Functional Groups. Int. J. Hydrog. Energy 2007, 32, 1999–2004. [Google Scholar] [CrossRef]
- Yildirim, T.; Ciraci, S. Titanium-Decorated Carbon Nanotubes as a Potential High-Capacity Hydrogen Storage Medium. Phys. Rev. Lett. 2005, 94, 175501. [Google Scholar] [CrossRef]
- Zhan, L.; Li, K.; Zhu, X.; Lv, C.; Ling, L. Adsorption Limit of Supercritical Hydrogen on Super-Activated Carbon. Carbon 2002, 40, 455–457. [Google Scholar] [CrossRef]
- Wang, Q.; Johnson, J.K.; Broughton, J.Q. Path Integral Grand Canonical Monte Carlo. J. Chem. Phys. 1997, 107, 5108–5117. [Google Scholar] [CrossRef]
- Cheng, H.; Pez, G.; Kern, G.; Kresse, G.; Hafner, J. Hydrogen Adsorption in Potassium-Intercalated Graphite of Second Stage: An ab Initio Molecular Dynamics Study. J. Phys. Chem. B 2001, 105, 736–742. [Google Scholar] [CrossRef]
- Chambers, A.; Park, C.; Baker, A.R.T.K.; Rodriguez, N.M. Hydrogen Storage in Graphite Nanofibers. J. Phys. Chem. B 1998, 102, 4253–4256. [Google Scholar] [CrossRef]
- Gupta, B.K.; Tiwari, R.; Srivastava, O. Studies on Synthesis and Hydrogenation Behaviour of Graphitic Nanofibres Prepared Through Palladium Catalyst Assisted Thermal Cracking of Acetylene. J. Alloy. Compd. 2004, 381, 301–308. [Google Scholar] [CrossRef]
- Browning, D.J.; Gerrard, M.L.; Lakeman, J.B.; Mellor, I.; Mortimer, R.J.; Turpin, M.C. Studies into the Storage of Hydrogen in Carbon Nanofibers: Proposal of a Possible Reaction Mechanism. Nano Lett. 2002, 2, 201–205. [Google Scholar] [CrossRef]
- Bénard, P.; Chahine, R. Determination of the Adsorption Isotherms of Hydrogen on Activated Carbons above the Critical Temperature of the Adsorbate over Wide Temperature and Pressure Ranges. Langmuir 2001, 17, 1950–1955. [Google Scholar] [CrossRef]
- Nard, P.B.; Chahine, R. Modeling of Adsorption Storage of Hydrogen on Activated Carbons. Int. J. Hydrog. Energy 2001, 26, 849–855. [Google Scholar]
- Bhatia, S.K.; Myers, A.L. Optimum Conditions for Adsorptive Storage. Langmuir 2006, 22, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Gigras, A.; Bhatia, S.K.; Kumar, A.A.; Myers, A.L. Feasibility of Tailoring for High Isosteric Heat to Improve Effectiveness of Hydrogen Storage in Carbons. Carbon 2007, 45, 1043–1050. [Google Scholar] [CrossRef]
- Thomas, K.M. Hydrogen Adsorption and Storage on Porous Materials. Catal. Today 2007, 120, 389–398. [Google Scholar] [CrossRef]
- Psofogiannakis, G.M.; Froudakis, G.E. DFT Study of the Hydrogen Spillover Mechanism on Pt-Doped Graphite. J. Phys. Chem. C 2009, 113, 14908–14915. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, R.T. Enhanced Hydrogen Storage on Pt-Doped Carbon by Plasma Reduction. J. Phys. Chem. C 2010, 114, 5956–5963. [Google Scholar] [CrossRef]
- Prins, R. Hydrogen Spillover. Facts and Fiction. Chem. Rev. 2012, 112, 2714–2738. [Google Scholar] [CrossRef]
- Psofogiannakis, G.M.; Froudakis, G.E. Theoretical Explanation of Hydrogen Spillover in Metal−Organic Frameworks. J. Phys. Chem. C 2011, 115, 4047–4053. [Google Scholar] [CrossRef]
- Robell, A.J.; Ballou, E.V.; Boudart, M. Surface Diffusion of Hydrogen on Carbon. J. Phys. Chem. 1964, 68, 2748–2753. [Google Scholar] [CrossRef]
- Guo, J.-H.; Li, X.-D.; Liu, H.; Li, S.-J.; Chen, G. Combined Density Functional Theory and Kinetic Monte Carlo Study of Hydrogen Spillover on Fluorine-Decorating Covalent Organic Frameworks. J. Phys. Chem. C 2019, 123, 15935–15943. [Google Scholar] [CrossRef]
- Sabo, M.; Henschel, A.; Fröde, H.; Klemm, E.; Kaskel, S. Solution Infiltration of Palladium into MOF-5: Synthesis, Physisorption and Catalytic Properties. J. Mater. Chem. 2007, 17, 3827–3832. [Google Scholar] [CrossRef]
- Zhao, W.; Fierro, V.; Zlotea, C.; Izquierdo, M.T.; Chevalier-César, C.; Latroche, M.; Celzard, A. Activated Carbons Doped with Pd Nanoparticles for Hydrogen Storage. Int. J. Hydrog. Energy 2012, 37, 5072–5080. [Google Scholar] [CrossRef]
- Dibandjo, P.; Zlotea, C.; Gadiou, R.; Ghimbeu, C.M.; Cuevas, F.; Latroche, M.; Leroy, E.; Vix-Guterl, C. Hydrogen Storage in Hybrid Nanostructured Carbon/Palladium Materials: Influence of Particle Size and Surface Chemistry. Int. J. Hydrog. Energy 2013, 38, 952–965. [Google Scholar] [CrossRef]
- Nag, N.K. A Study on the Formation of Palladium Hydride in a Carbon-Supported Palladium Catalyst. J. Phys. Chem. B 2001, 105, 5945–5949. [Google Scholar] [CrossRef]
- Zlotea, C.; Cuevas, F.; Paul-Boncour, V.; Leroy, E.; Dibandjo, P.; Gadiou, R.; Vix-Guterl, C.; Latroche, M. Size-Dependent Hydrogen Sorption in Ultrasmall Pd Clusters Embedded in a Mesoporous Carbon Template. J. Am. Chem. Soc. 2010, 132, 7720–7729. [Google Scholar] [CrossRef]
- Stoyanov, S.R.; Titov, A.V.; Král, P. Transition Metal and Nitrogen Doped Carbon Nanostructures. Coord. Chem. Rev. 2009, 253, 2852–2871. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Gray, J.L.; Gong, Q.; Zhao, Y.; Li, J.; Klontzas, E.; Psofogiannakis, G.; Froudakis, G.; Lueking, A. Hydrogen Storage with Spectroscopic Identification of Chemisorption Sites in Cu-TDPAT via Spillover from a Pt/Activated Carbon Catalyst. J. Phys. Chem. C 2014, 118, 26750–26763. [Google Scholar] [CrossRef]
- Nishihara, H.; Ittisanronnachai, S.; Itoi, H.; Li, L.-X.; Suzuki, K.; Nagashima, U.; Ogawa, H.; Kyotani, T.; Ito, M. Experimental and Theoretical Studies of Hydrogen/Deuterium Spillover on Pt-Loaded Zeolite-Templated Carbon. J. Phys. Chem. C 2014, 118, 9551–9559. [Google Scholar] [CrossRef]
- Stevens, D.; Zhang, S.; Chen, Z.; Dahn, J. On the Determination of Platinum Particle Size in Carbon-Supported Platinum Electrocatalysts for Fuel Cell Applications. Carbon 2003, 41, 2769–2777. [Google Scholar] [CrossRef]
- Chen, L.; Cooper, A.C.; Pez, G.P.; Cheng, H. Mechanistic Study on Hydrogen Spillover onto Graphitic Carbon Materials. J. Phys. Chem. C 2007, 111, 18995–19000. [Google Scholar] [CrossRef]
- Rey, M.B.; Juaristi, J.I.; Alducin, M.; López, M.J.; Alonso, J.A. Is Spillover Relevant for Hydrogen Adsorption and Storage in Porous Carbons Doped with Palladium Nanoparticles? J. Phys. Chem. C 2016, 120, 17357–17364. [Google Scholar] [CrossRef]
- Srinivas, S.; Rao, P. Direct Observation of Hydrogen Spillover on Carbon-Supported Platinum and Its Influence on the Hydrogenation of Benzene. J. Catal. 1994, 148, 470–477. [Google Scholar] [CrossRef]
- Psofogiannakis, G.M.; Froudakis, G.E. DFT Study of Hydrogen Storage by Spillover on Graphite with Oxygen Surface Groups. J. Am. Chem. Soc. 2009, 131, 15133–15135. [Google Scholar] [CrossRef]
- Ayala, P.; Arenal, R.; Rümmeli, M.H.; Rubio, A.; Pichler, T. The Doping of Carbon Nanotubes with Nitrogen and Their Potential Applications. Carbon 2010, 48, 575–586. [Google Scholar] [CrossRef]
- Park, H.-L.; Chung, Y.-C. Hydrogen Storage in Al and Ti Dispersed on Graphene with Boron Substitution: First-Principles Calculations. Comput. Mater. Sci. 2010, 49, S297–S301. [Google Scholar] [CrossRef]
- Park, H.-L.; Yi, S.-C.; Chung, Y.-C. Hydrogen Adsorption on Li Metal in Boron-Substituted Graphene: An ab Initio Approach. Int. J. Hydrogen Energy 2010, 35, 3583–3587. [Google Scholar] [CrossRef]
- Lv, Y.-A.; Cui, Y.-H.; Xiang, Y.-Z.; Wang, J.-G.; Li, X.-N. Modulation of Bonding between Noble Metal Monomers and CNTs by B-, N-doping. Comput. Mater. Sci. 2010, 48, 621–625. [Google Scholar] [CrossRef]
- Wang, K.; Shi, C.; Zhao, N.; Du, X.; Li, J. First-Principles Study of the B- or N-Doping Effects on Chemical Bonding Characteristics between Magnesium and Single-Walled Carbon Nanotubes. Chem. Phys. Lett. 2009, 469, 145–148. [Google Scholar] [CrossRef]
- Warczinski, L.; Hättig, C. How Nitrogen Doping Affects Hydrogen Spillover on Carbon-Supported Pd Nanoparticles: New Insights from DFT. J. Phys. Chem. C 2021, 125, 9020–9031. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Fan, X.; Kuo, J.-L.; Deng, W.-Q. DFT Study of Hydrogen Storage by Spillover on Graphene with Boron Substitution. J. Phys. Chem. C 2011, 115, 9241–9249. [Google Scholar] [CrossRef]
- Nachimuthu, S.; He, L.; Cheng, H.-J.; Tiono, R.D.; Jiang, J.-C. A First-Principles Study on Double-Sided Decorated Boron–Nitrogen Codoped Graphene by Vanadium for Enhanced Low-Temperature Reversible Hydrogen Storage. Sustain. Energy Fuels 2021, 2159–2168. [Google Scholar] [CrossRef]
- Kim, G.; Jhi, S.-H.; Park, N.; Louie, S.G.; Cohen, M.L. Optimization of Metal Dispersion in Doped Graphitic Materials for Hydrogen Storage. Phys. Rev. B 2008, 78, 085408. [Google Scholar] [CrossRef]
- Kang, K.Y.; Lee, B.I.; Lee, J.S. Hydrogen Adsorption on Nitrogen-Doped Carbon Xerogels. Carbon 2009, 47, 1171–1180. [Google Scholar] [CrossRef]
- Chizari, K.; Janowska, I.; Houllé, M.; Florea, I.; Ersen, O.; Romero, T.; Bernhardt, P.; Ledoux, M.J.; Pham-Huu, C. Tuning of Nitrogen-Doped Carbon Nanotubes as Catalyst Support for Liquid-Phase Reaction. Appl. Catal. A Gen. 2010, 380, 72–80. [Google Scholar] [CrossRef]
- Aboud, M.F.A.; Alothman, Z.A.; Bagabas, A.A. Storage and Separation of Methane and Carbon Dioxide Using Platinum-Decorated Activated Carbons Treated with Ammonia. Mater. Res. Express 2021, 8, 025503. [Google Scholar] [CrossRef]
- Mangun, C.L.; Benak, K.R.; Economy, J.; Foster, K.L. Surface Chemistry, Pore Sizes and Adsorption Properties of Activated Carbon Fibers and Precursors Treated with Ammonia. Carbon 2001, 39, 1809–1820. [Google Scholar] [CrossRef]
- Chen, B.; Bao, S.; Zhang, Y.; Zheng, R. Ultrasound-Assisted Synthesis of N235-Impregnated Resins for Vanadium (V) Adsorption. R. Soc. Open Sci. 2018, 5, 171746. [Google Scholar] [CrossRef]
- Kong, J.; Sheng, X.; Zhou, Y.; Zhang, Y.; Zhou, S.; Zhang, Z. Ultrasound-Assisted Synthesis of Nanosized Hierarchical ZSM-5 and Its Catalytic Performance as the Support for Heteropolyacid. J. Porous Mater. 2014, 21, 241–249. [Google Scholar] [CrossRef]
- Klein, J.; Kumacheva, E. Confinement-Induced Phase Transitions in Simple Liquids. Science 1995, 269, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Langford, J.I.; Wilson, A.J.C. Scherrer After Sixty Years: A Survey and Some New Results in the Determination of Crystallite Size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Salem, M.M.K.; Braeuer, P.; Szombathely, M.V.; Heuchel, M.; Harting, A.P.; Quitzsch, K.; Jaroniec, M. Thermodynamics of High-Pressure Adsorption of Argon, Nitrogen, and Methane on Microporous Adsorbents. Langmuir 1998, 14, 3376–3389. [Google Scholar] [CrossRef]
- Brandani, S.; Mangano, E.; Sarkisov, L. Net, Excess and Absolute Adsorption and Adsorption of Helium. Adsorption 2016, 22, 261–276. [Google Scholar] [CrossRef]
- Pini, R. Interpretation of Net and Excess Adsorption Isotherms in Microporous Adsorbents. Microporous Mesoporous Mater. 2014, 187, 40–52. [Google Scholar] [CrossRef]
- Wang, J.-T.; Chen, C.; Wang, E.; Kawazoe, Y. A New Carbon Allotrope with Six-Fold Helical Chains in all-sp2 Bonding Networks. Sci. Rep. 2015, 4, 4339. [Google Scholar] [CrossRef]
- Li, Z.; Lu, C.; Xia, Z.; Zhou, Y.; Luo, Z. X-ray Diffraction Patterns of Graphite and Turbostratic Carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Stadie, N.; Purewal, J.J.; Ahn, C.C.; Fultz, B. Measurements of Hydrogen Spillover in Platinum Doped Superactivated Carbon. Langmuir 2010, 26, 15481–15485. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Chilev, C.; Lamari, F.D. Hydrogen Storage at Low Temperature and High Pressure for Application in Automobile Manufacturing. Int. J. Hydrog. Energy 2016, 41, 1744–1758. [Google Scholar] [CrossRef]
- Chilev, C.; Lamari, F.; Kirilova, E.; Pentchev, I. Comparison of Gas Excess Adsorption Models and High Pressure Experimental Validation. Chem. Eng. Res. Des. 2012, 90, 2002–2012. [Google Scholar] [CrossRef]
- Chilev, C.; Weinberger, B.; Passarello, J.P.; Lamari, F.; Pentchev, I. A New Approach to Describe High-Pressure Adsorption Isotherms in Subcritical and Supercritical Conditions. AIChE J. 2009, 55, 1793–1802. [Google Scholar] [CrossRef]
- Do, D.; Do, H. Adsorption of Supercritical Fluids in Non-Porous and Porous Carbons: Analysis of Adsorbed Phase Volume and Density. Carbon 2003, 41, 1777–1791. [Google Scholar] [CrossRef]
- Broom, D.P. Hydrogen Sorption Properties of Materials. In Smart and Sustainable Planning for Cities and Regions; Springer Science and Business Media LLC: Berlín, Alemania, 2011; pp. 61–115. [Google Scholar]
- Zubizarreta, L.; Gomez, E.I.; Arenillas, A.; Ania, C.O.; Parra, J.B.; Pis, J.J. H2 Storage in Carbon Materials. Adsorption 2008, 14, 557–566. [Google Scholar] [CrossRef]
- Blanco, A.A.G.; Vallone, A.; Gil, A.; Sapag, K. A Comparative Study of Various Microporous Materials to Store Hydrogen by Physical Adsorption. Int. J. Hydrog. Energy 2012, 37, 14870–14880. [Google Scholar] [CrossRef]
- Ströbel, R.; Garche, J.; Moseley, P.; Jörissen, L.; Wolf, G. Hydrogen Storage by Carbon Materials. J. Power Sour. 2006, 159, 781–801. [Google Scholar] [CrossRef]
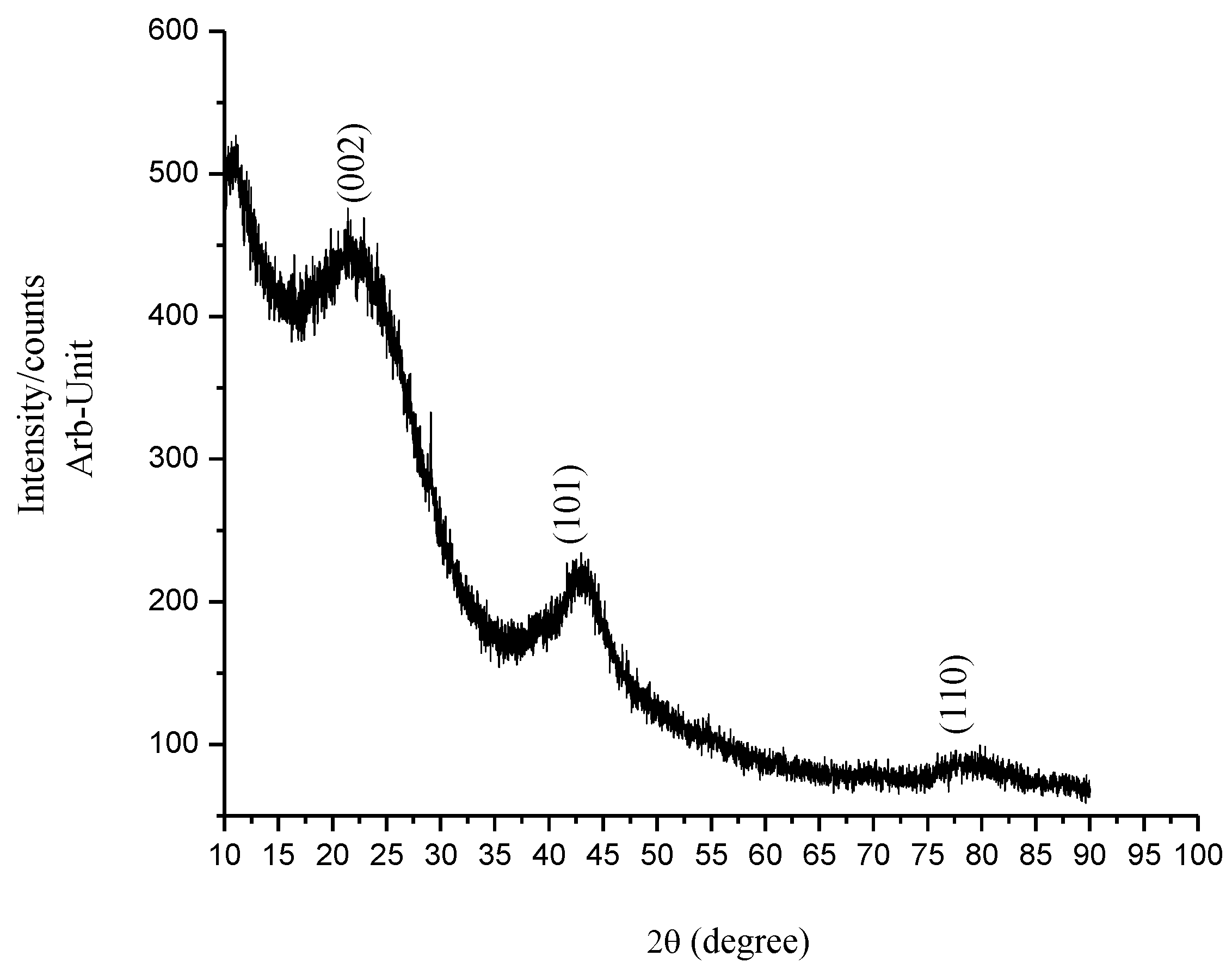



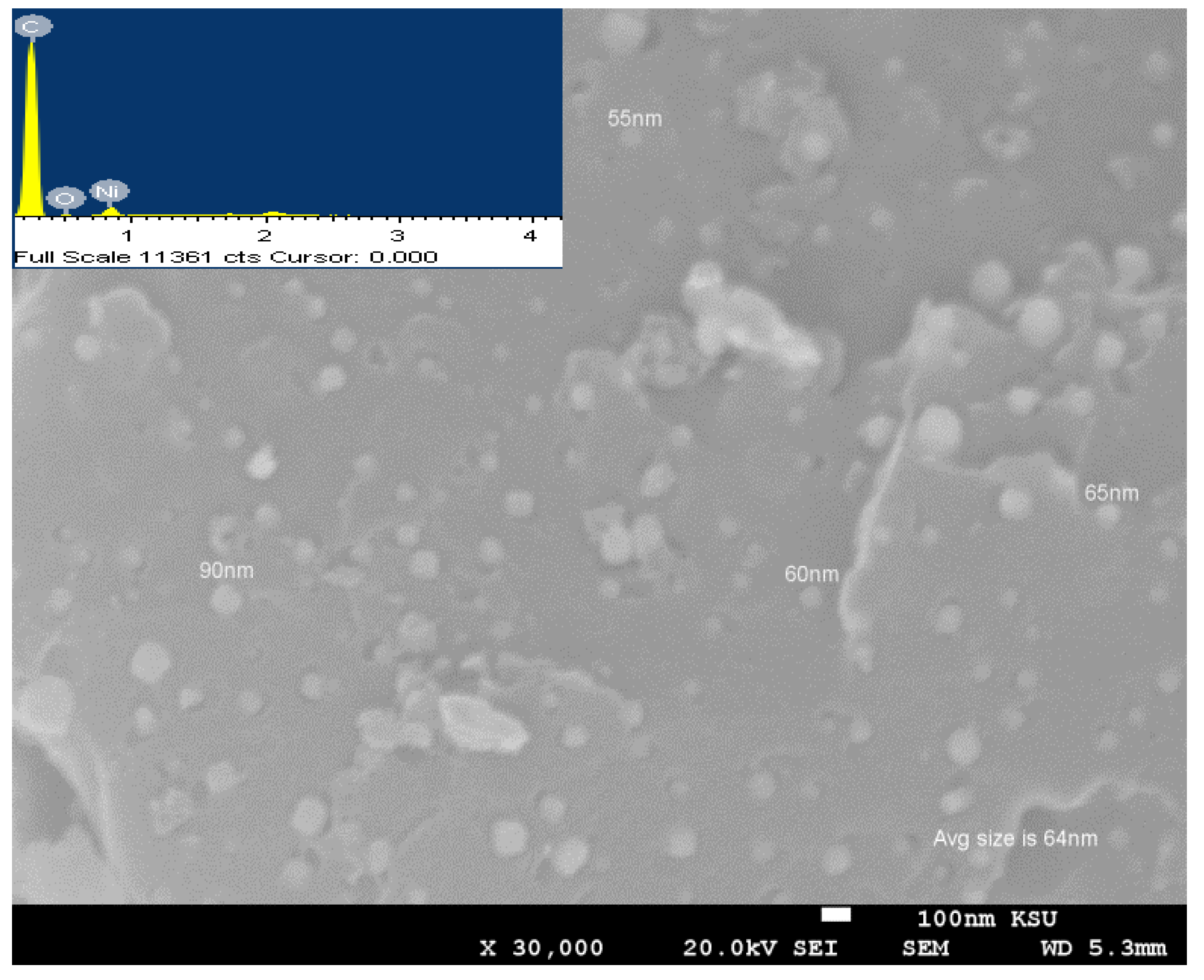
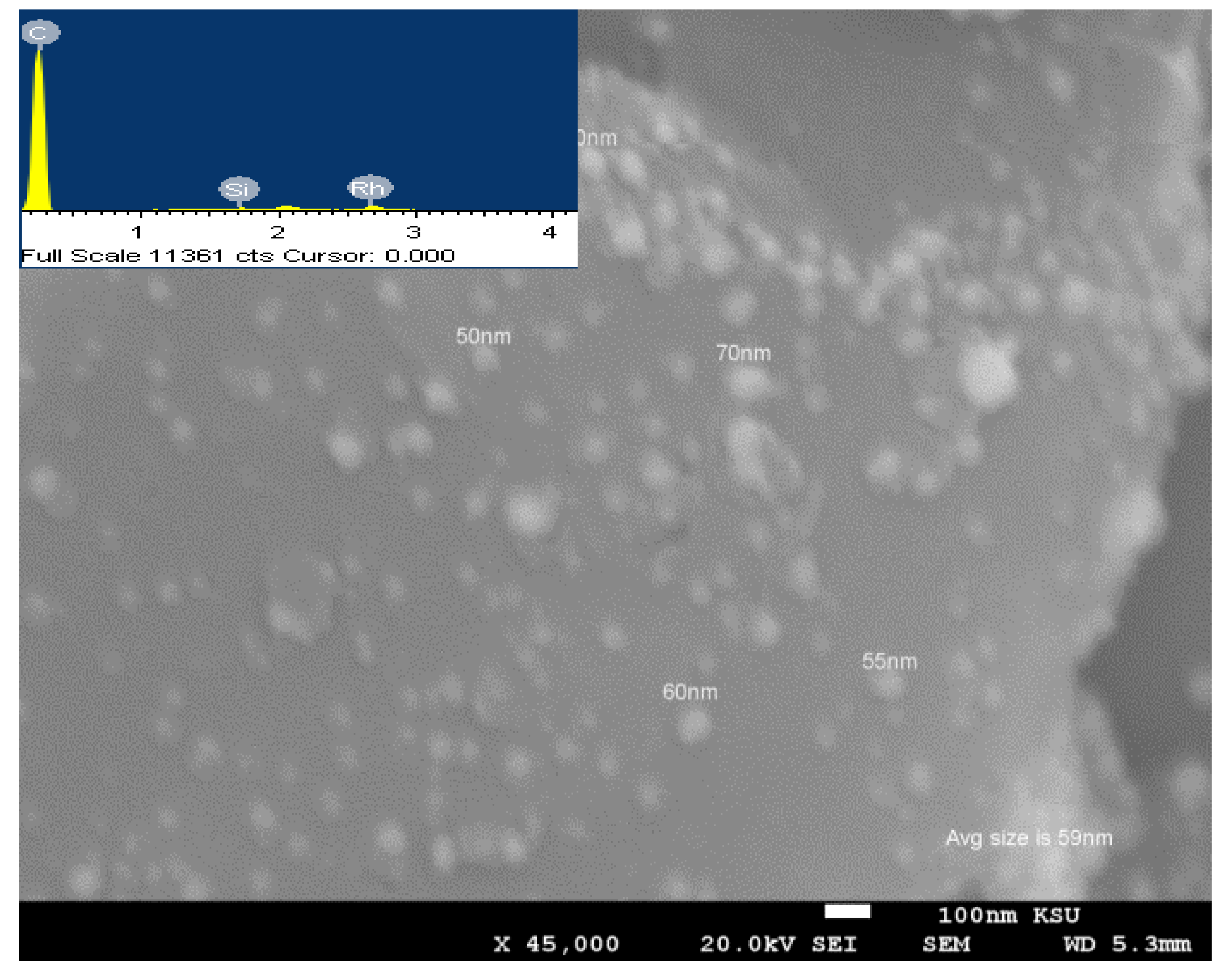
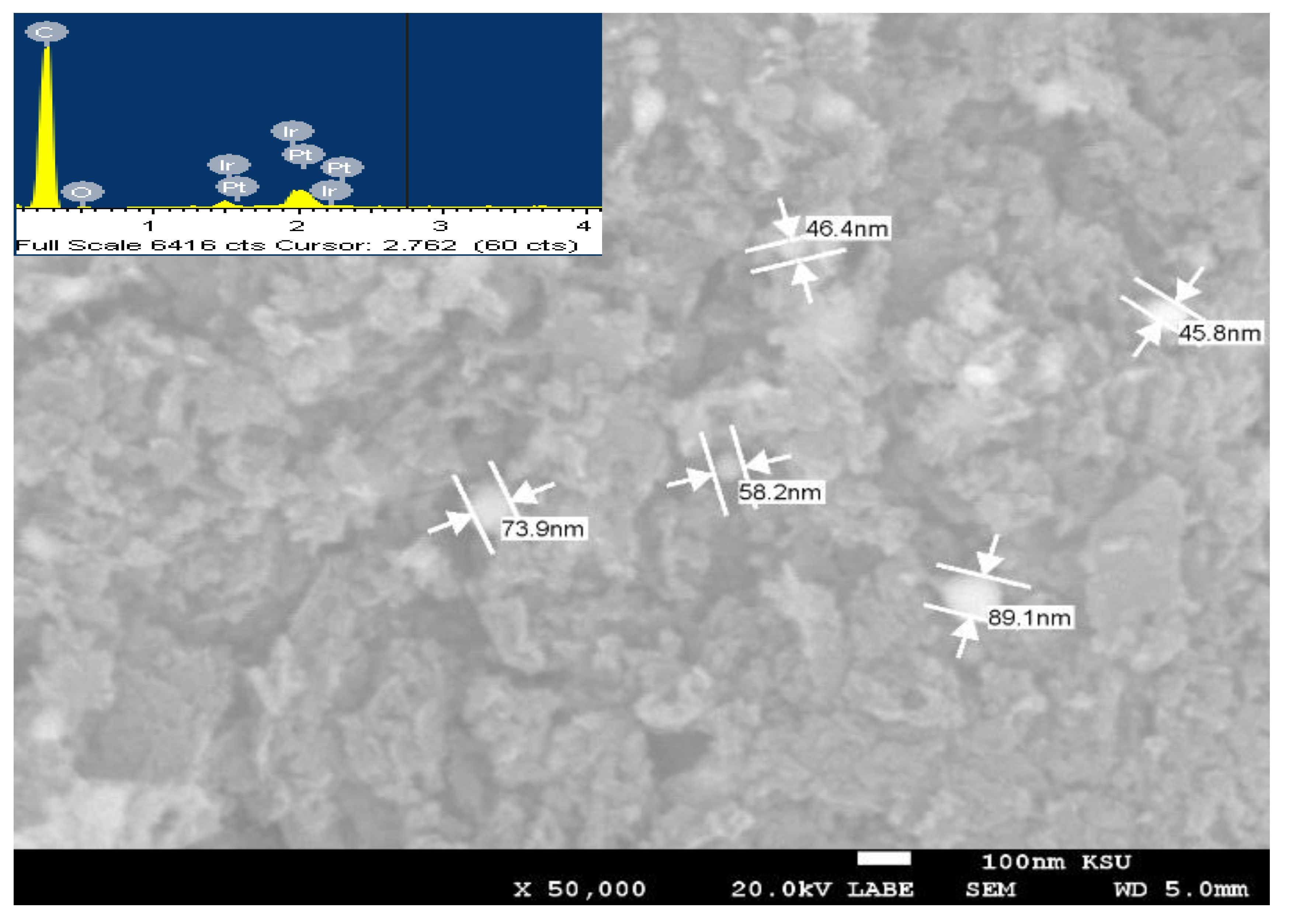
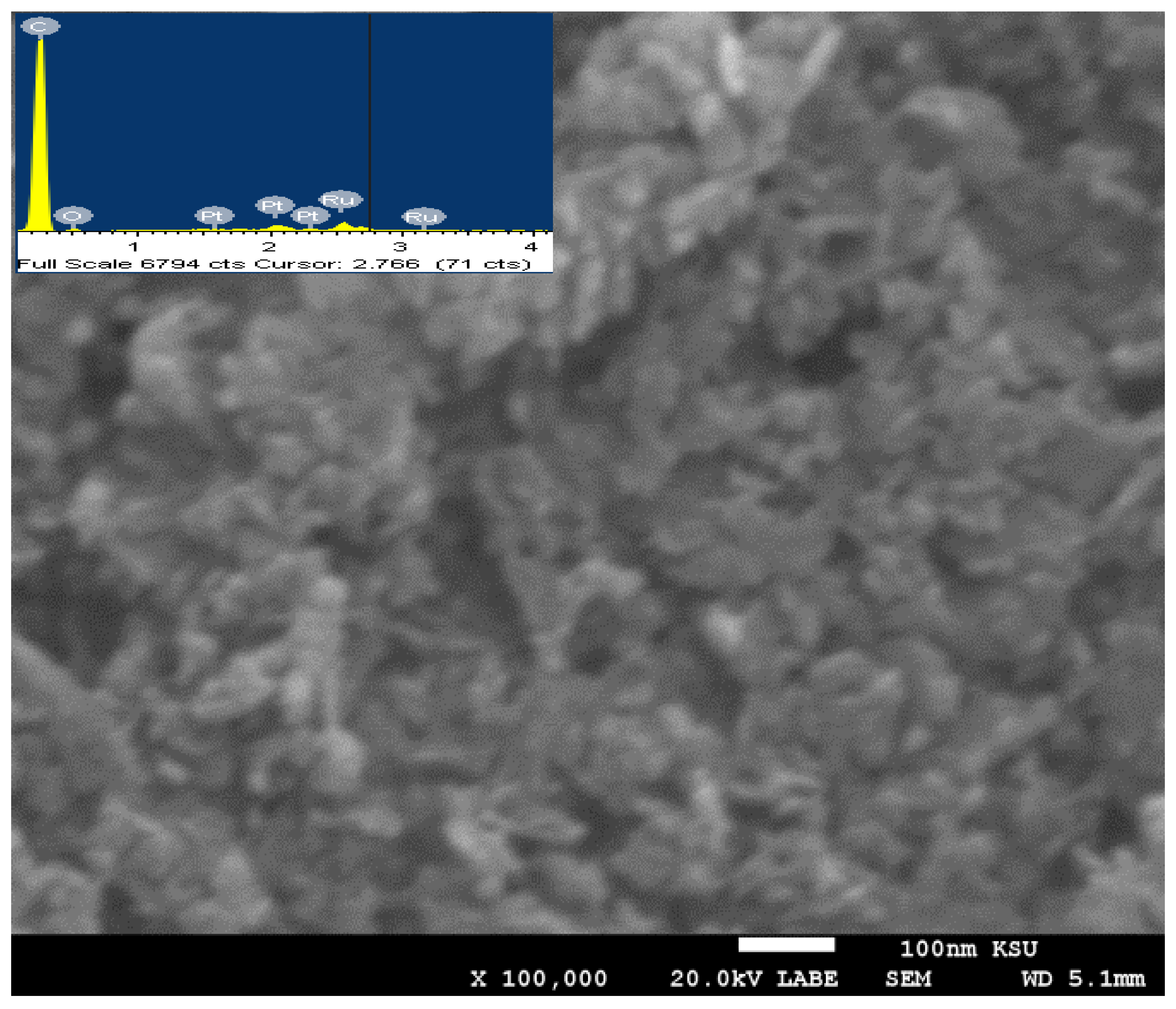
| Sample | Peak Position (2θ°), and Its Miller Index (hkl) | JCPDS Card No. | Corresponding Crystallite Size, nm | Ave. Crystallite Size from XRD, nm | Ave Particle Size from SEM, nm |
|---|---|---|---|---|---|
| Pd-decorated, NH3-treated AC | 40.6° (111), 47.2° (200), 68.6° (220), 82.5° (311), and 86.9° (222) | 03-065-2867 | 59.37, 54.36, 62.57, 59.72, and 63.37 | 59.87 | 61 |
| Ni-decorated, NH3-treated AC | 44.7° (111), 52.1° (200), 76.5° (220), 93.1° (311), and 98.5° (222) | 00-004-0850 | 65.96, 65.37, 74.18, 93.87, and 91.32 | 78.14 | 64 |
| Rh-decorated, NH3-treated AC | 41.1° (111), 47.8° (200), 69.9° (220), 84.4° (311), and 89.1° (222) | 00-005-0685 | 68.51, 62.59, 70.07, 73.14, and 72.99 | 69.46 | 59 |
| Ir-decorated, NH3-treated AC | 40.8° (111), 47.4° (200), 69.2° (220), 83.5° (311), and 88.2° (222) | 03-065-1686 | 58.16, 58.52, 70.94, 81.60, and 67.52 | 67.35 | 63 |
| Ru-decorated, NH3-treated AC | 38.5° (100), 42.2° (002), 44.1° (101), 58.3° (101), 69.4° (110), 78.4° (103), 84.5° (112), 85.99° (201), 91.98° (004), and 96.94° (202) | 01-089-3942 | 37.87, 33.33, 38.24, 45.51, 51.16, 43.43, 22.33, and 33.30 | 38.14 | 35 |
| Sample | Hydrogen (H2) Excess Capacity (wt.%) | ||||
|---|---|---|---|---|---|
| ~7 bar & 77 K | Incremental wt.% after Ammonia Treatment at 77 k | Incremental wt.% after Metal Decoration at 298 K | ~100 bar & 298 K | Incremental wt.% after Ammonia Treatment at 298 K | |
| AC | 2.5 ± 0.1 | ~4 | 0.21 ± 0.01 | ~57 | |
| Ammonia-treated AC | 2.6 ± 0.1 | 0.33 ± 0.01 | |||
| Pt-decorated AC | 2.3 ± 0.1 | ~4 | ~24 | 0.26 ± 0.01 | ~42 |
| Pt-decorated, ammonia-treated AC | 2.4 ± 0.1 | ~12 | 0.37 ± 0.01 | ||
| Pd-decorated AC | 2.5 ± 0.1 | ~4 | ~95 | 0.41 ± 0.01 | ~20 |
| Pd-decorated, ammonia-treated AC | 2.6 ± 0.1 | ~48 | 0.49 ± 0.01 | ||
| Ni-decorated AC | 2.4 ± 0.1 | ~4 | ~86 | 0.39 ± 0.01 | ~10 |
| Ni-decorated, ammonia-treated AC | 2.5 ± 0.1 | ~30 | 0.43 ± 0.01 | ||
| Rh-decorated AC | 2.3 ± 0.1 | ~4 | ~71 | 0.36 ± 0.01 | ~11 |
| Rh-decorated, ammonia-treated AC | 2.4 ± 0.1 | ~21 | 0.40 ± 0.01 | ||
| Ir-decorated AC | 2.3 ± 0.1 | ~4 | ~105 | 0.43 ± 0.01 | ~12 |
| Ir-decorated, ammonia-treated AC | 2.4 ± 0.1 | ~45 | 0.48 ± 0.01 | ||
| Ru-decorated AC | 2.3 ± 0.1 | ~4 | ~86 | 0.39 ± 0.01 | ~8 |
| Ru-decorated, ammonia-treated AC | 2.4 ± 0.1 | ~27 | 0.42 ± 0.01 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboud, M.F.A.; ALOthman, Z.A.; Bagabas, A.A. Hydrogen Storage in Untreated/Ammonia-Treated and Transition Metal-Decorated (Pt, Pd, Ni, Rh, Ir and Ru) Activated Carbons. Appl. Sci. 2021, 11, 6604. https://doi.org/10.3390/app11146604
Aboud MFA, ALOthman ZA, Bagabas AA. Hydrogen Storage in Untreated/Ammonia-Treated and Transition Metal-Decorated (Pt, Pd, Ni, Rh, Ir and Ru) Activated Carbons. Applied Sciences. 2021; 11(14):6604. https://doi.org/10.3390/app11146604
Chicago/Turabian StyleAboud, Mohamed F. Aly, Zeid A. ALOthman, and Abdulaziz A. Bagabas. 2021. "Hydrogen Storage in Untreated/Ammonia-Treated and Transition Metal-Decorated (Pt, Pd, Ni, Rh, Ir and Ru) Activated Carbons" Applied Sciences 11, no. 14: 6604. https://doi.org/10.3390/app11146604
APA StyleAboud, M. F. A., ALOthman, Z. A., & Bagabas, A. A. (2021). Hydrogen Storage in Untreated/Ammonia-Treated and Transition Metal-Decorated (Pt, Pd, Ni, Rh, Ir and Ru) Activated Carbons. Applied Sciences, 11(14), 6604. https://doi.org/10.3390/app11146604






