Adsorption and Desorption Characteristics of Total Flavonoids from Acanthopanax senticosus on Macroporous Adsorption Resins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Total Flavonoids Content
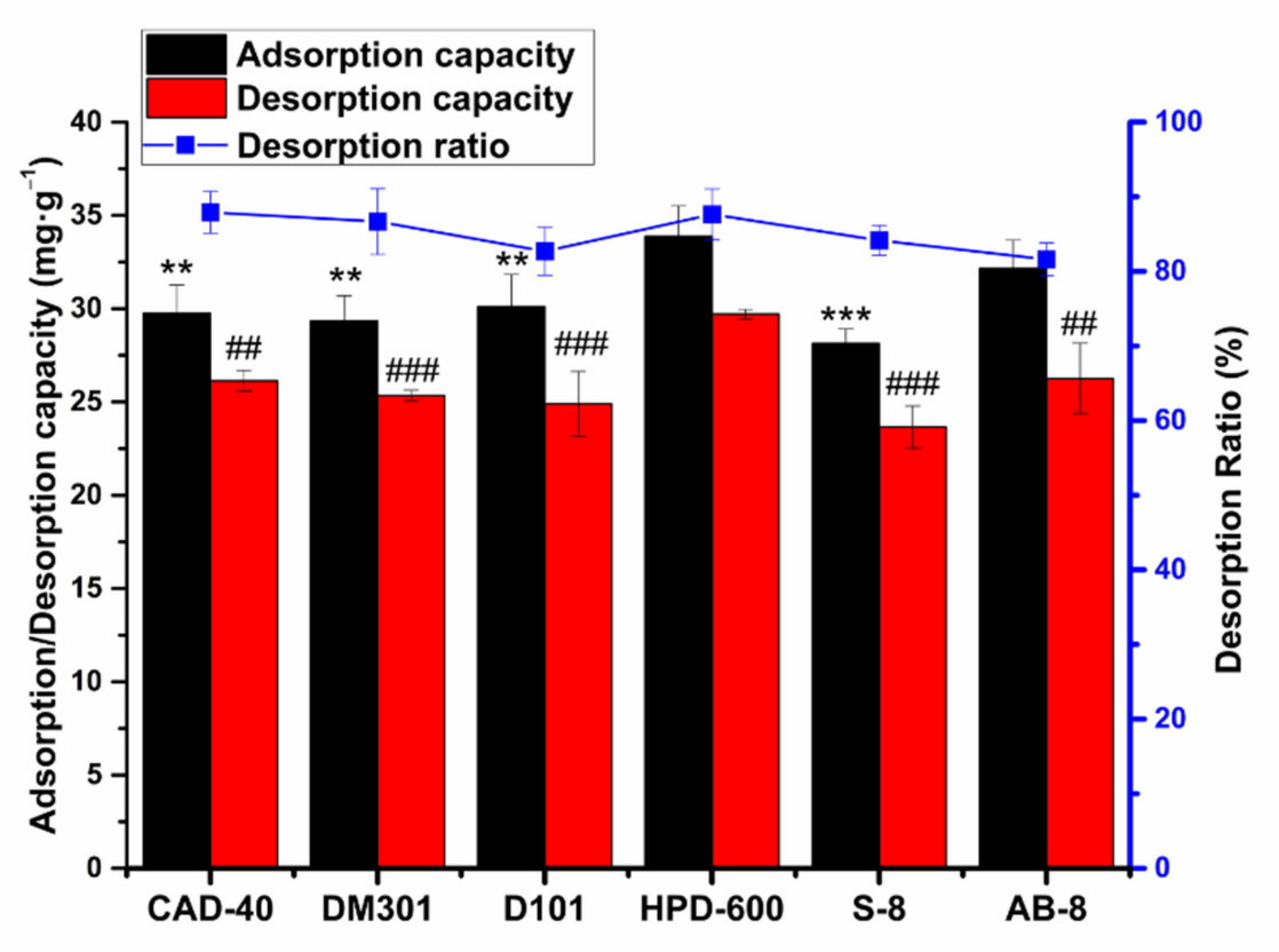
2.2. Selection of Macroporous Resins
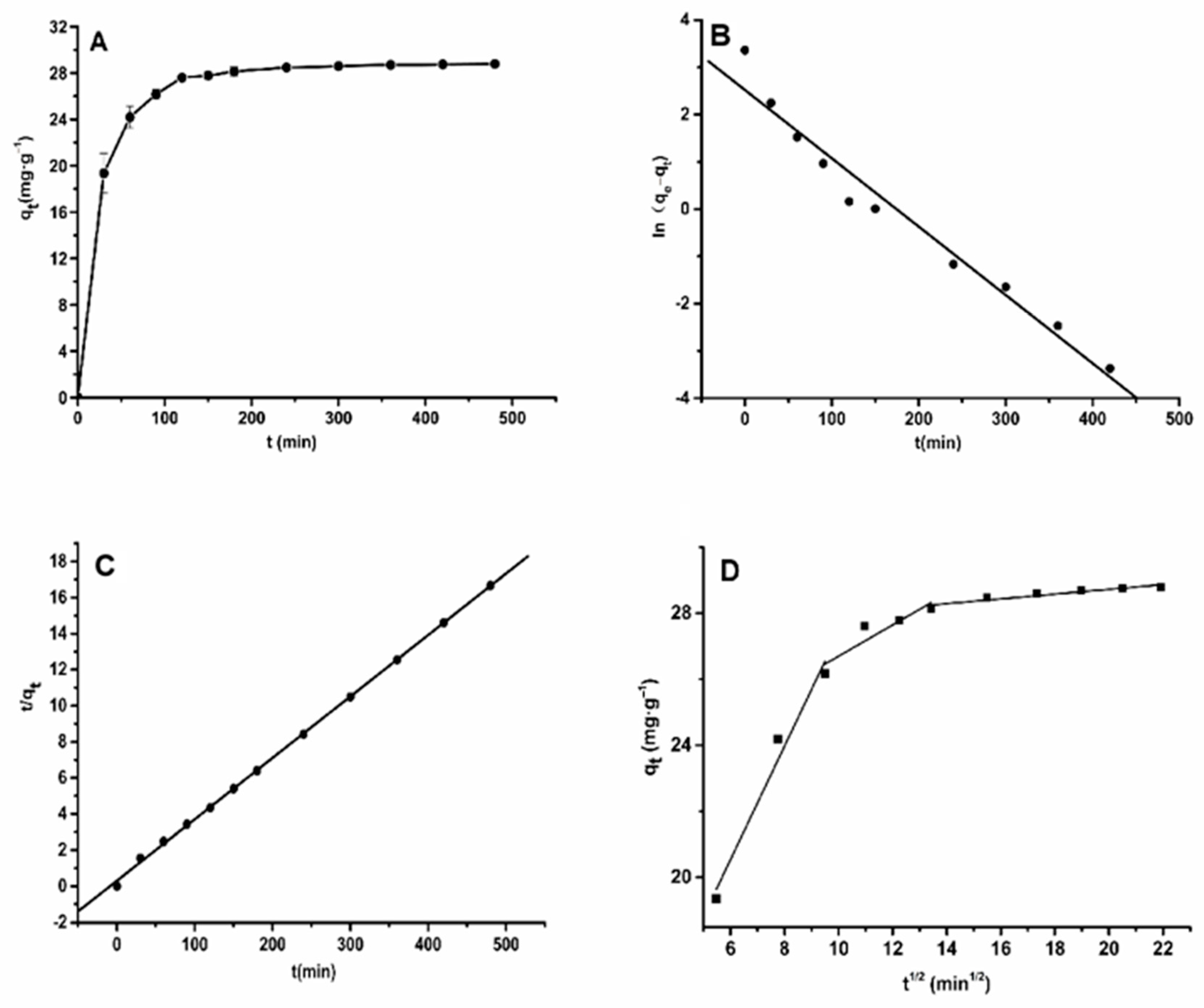
2.3. Adsorption Kinetics on HPD-600 Resin
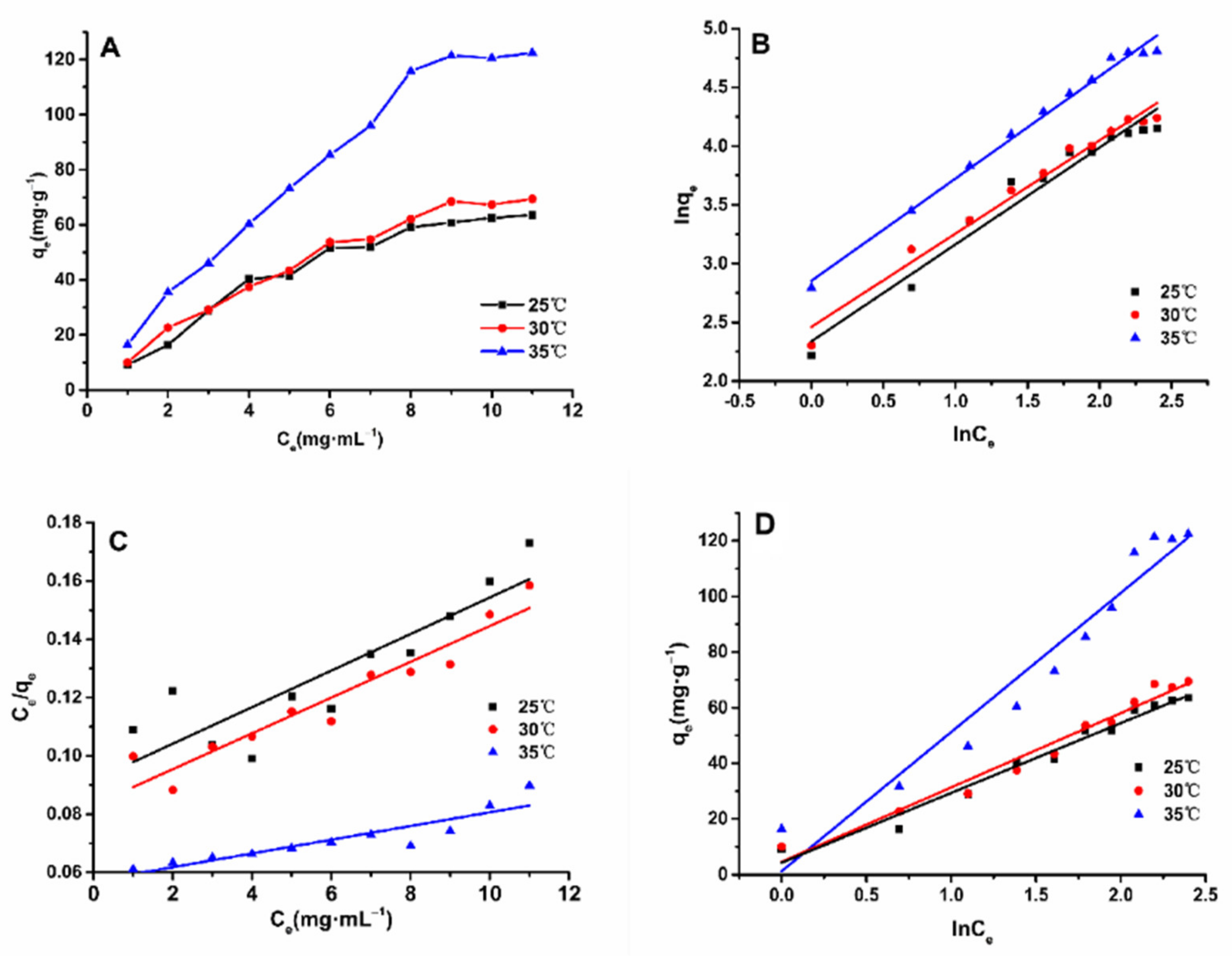
2.4. Adsorption Isotherms on HPD-600 Resin
2.5. Adsorption Thermodynamics on HPD-600 Resin
2.6. Static Adsorption and Desorption Experiments
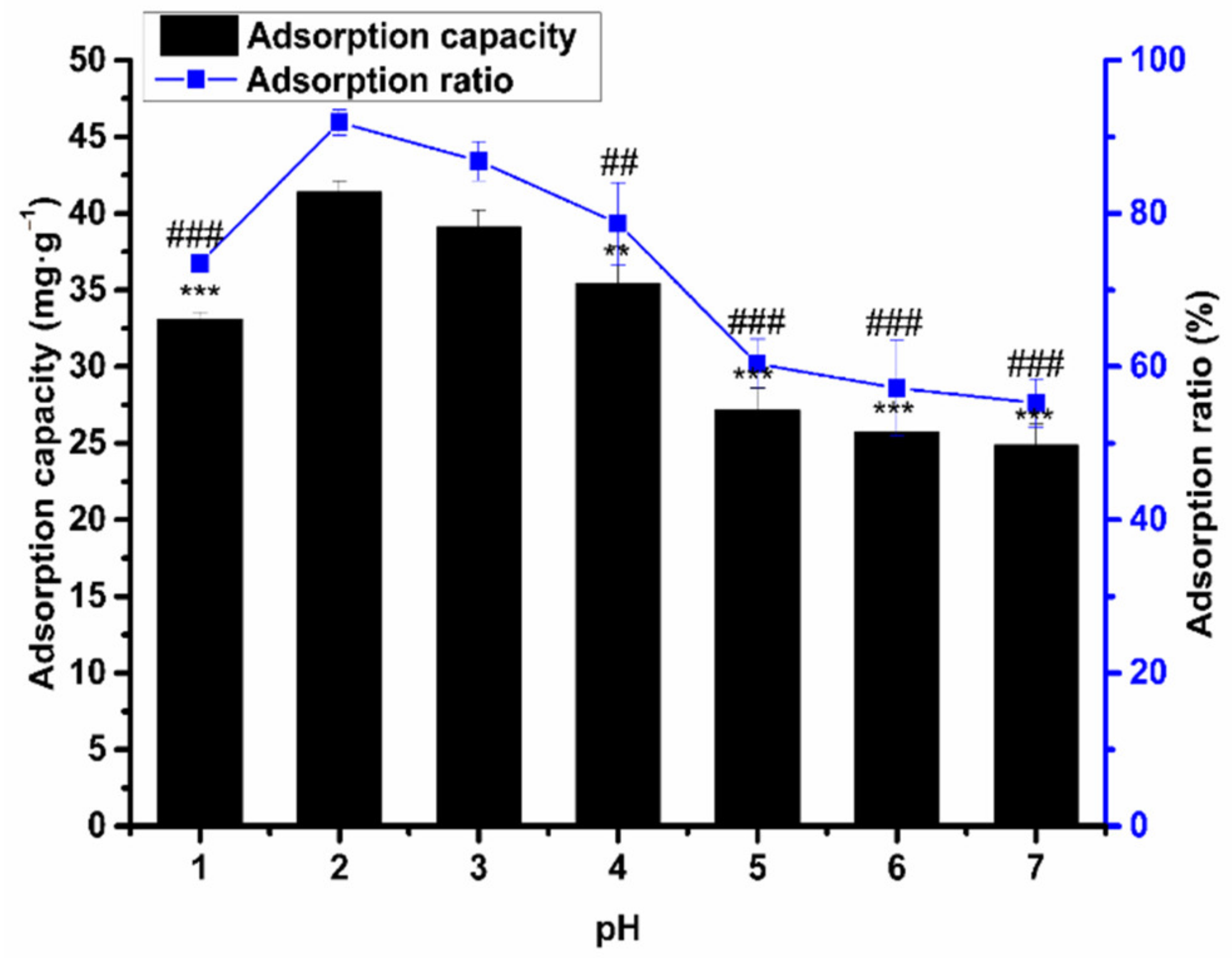
2.6.1. The Influence of pH on Adsorption Capacity and Ratio
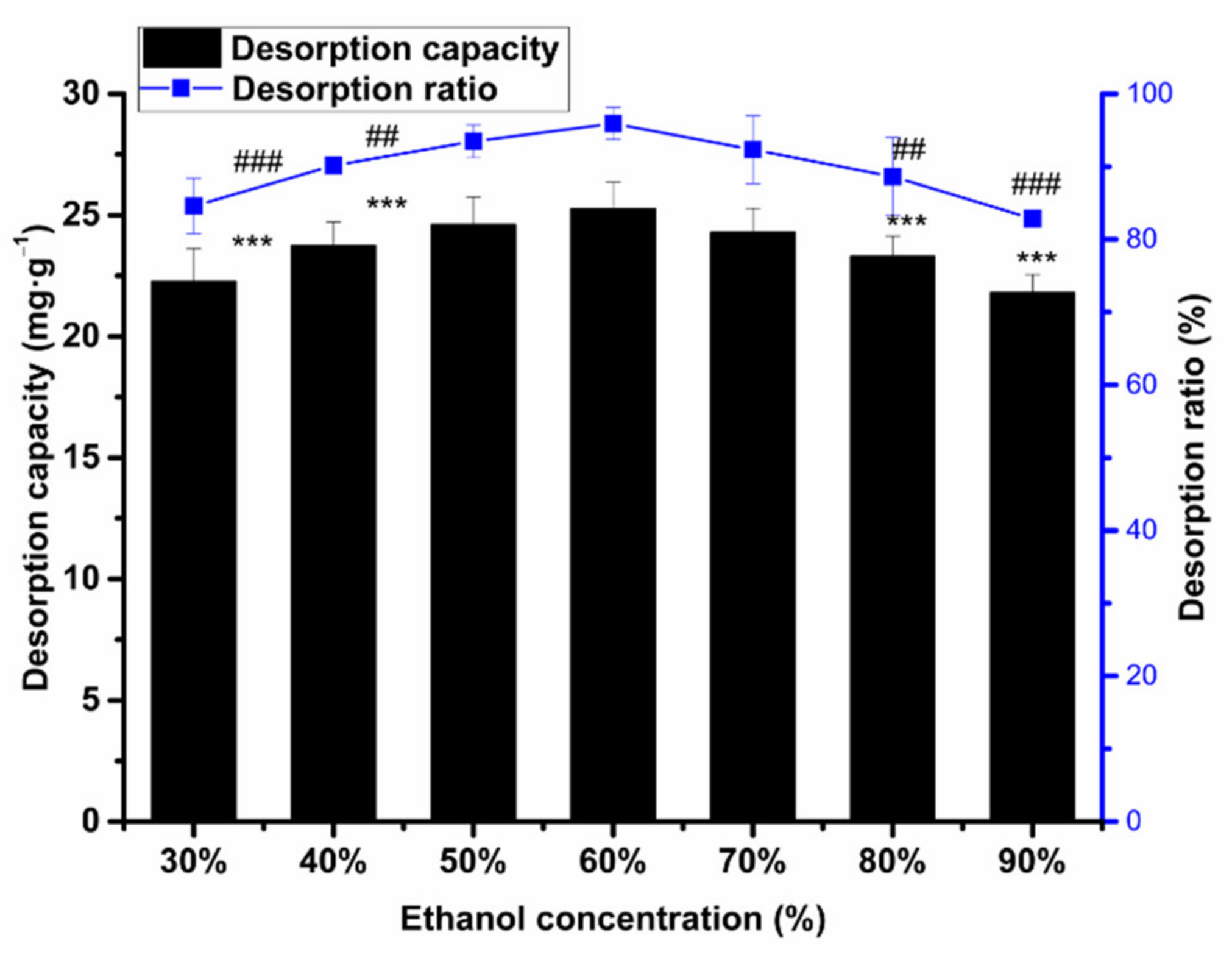
2.6.2. The Influence of Ethanol Concentration on Desorption Capacity and Ratio
2.7. Dynamic Adsorption and Desorption Experiments
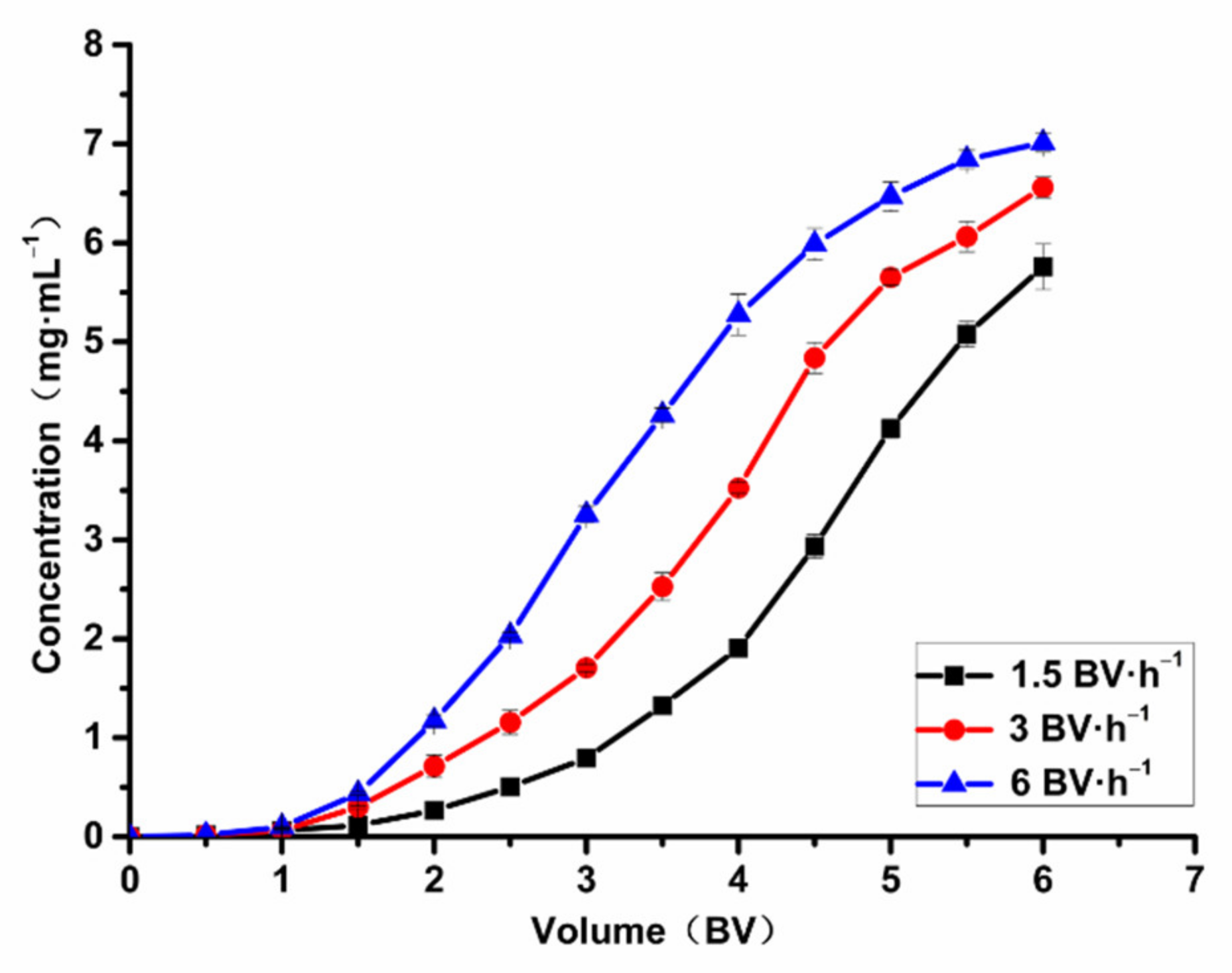
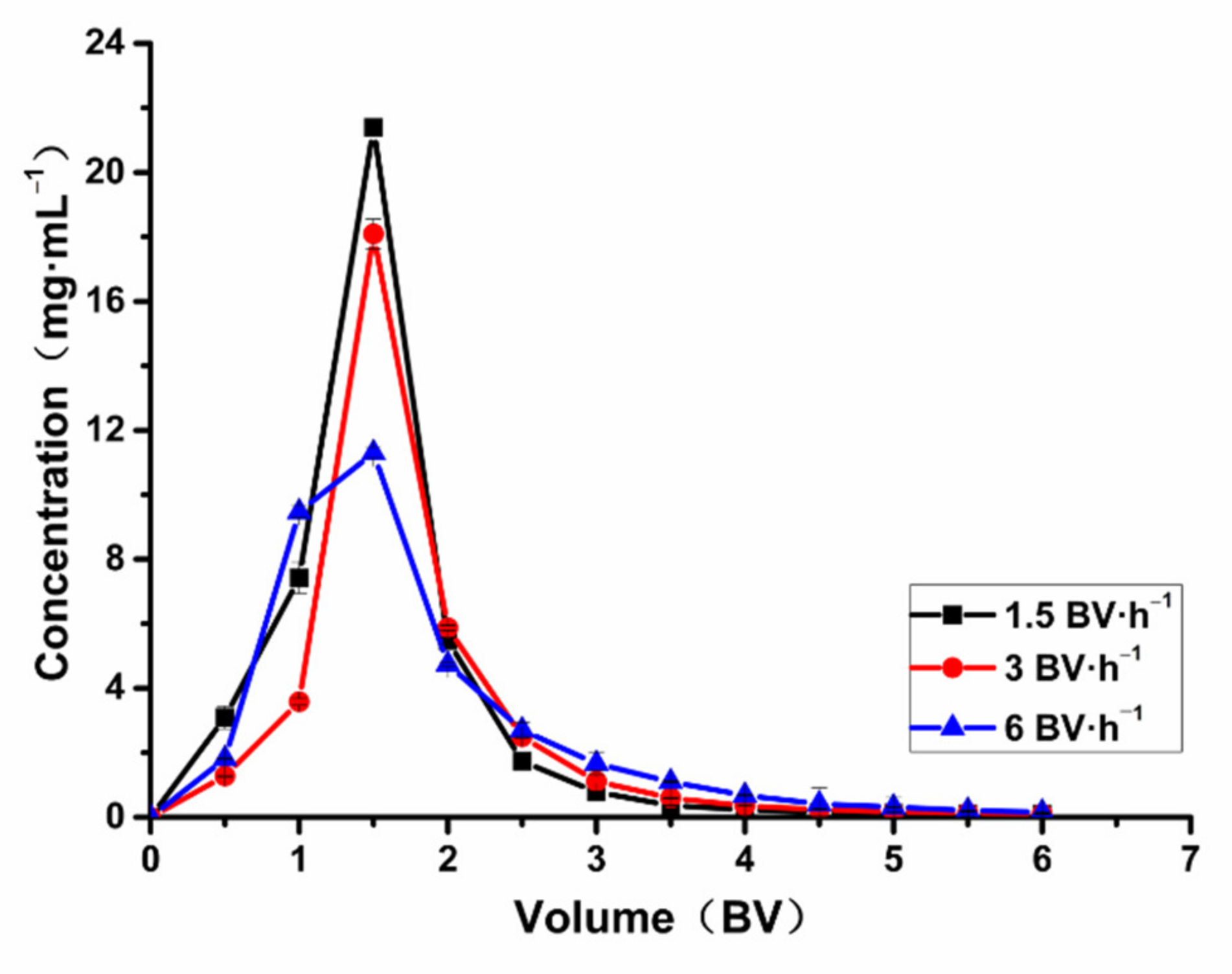
2.7.1. Effect of Adsorption Flow Rate on Adsorption Capacity
2.7.2. The Influence of Desorption Flow Rate on Desorption Capacity
2.8. Validation Experiments
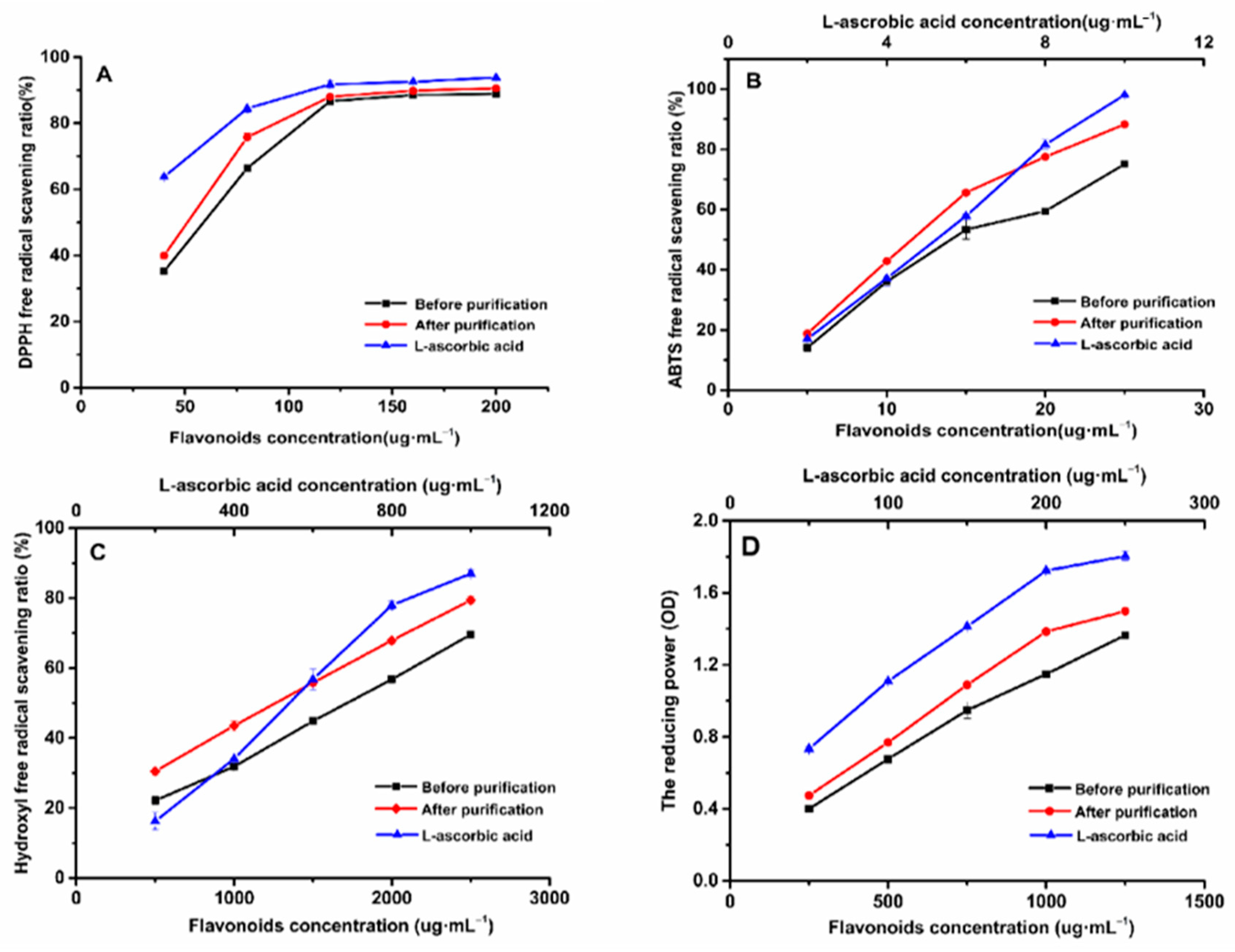
2.9. Antioxidant Activities Test
2.10. UHPLC–MS/MS Analysis of the Enriched Flavonoid and Crude Flavonoid Extract of AS
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Experimental Methods
3.2.1. Pretreatment of Total Flavonoids Extract from Acanthopanax senticosus
3.2.2. Determination of Total Flavonoid Content
3.2.3. Pretreatment of Macroporous Resin
3.2.4. Screening of Macroporous Resins
3.2.5. Adsorption Kinetics of ASTFs on HPD-600 Resins
3.2.6. Adsorption Isotherms of ASTFs on HPD-600 Resins
3.2.7. Adsorption Thermodynamics of ASTFs on HPD-600 Resins
3.2.8. Static Adsorption and Desorption Experiments
Sample Liquid pH Investigation
Ethanol Concentration on Desorption Capacity
3.2.9. Dynamic Adsorption and Desorption Experiments
Effect of Sample Condition
Effect of Elute Condition
3.3. Validation Test
3.4. Antioxidant Activities Test
3.5. UHPLC–MS/MS Analysis of Crude and Total Flavonoid-Enriched Extracts
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jia, A.; Zhang, Y.; Gao, H.; Zhang, Z.; Zhang, Y.; Wang, Z.; Zhang, J.; Deng, B.; Qiu, Z.; Fu, C. A review of Acanthopanax senticosus (Rupr and Maxim.) harms: From ethnopharmacological use to modern application. J. Ethnopharmacol. 2020, 268, 113586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Meng, Y.; Zhai, C.; Wang, M.; Avula, B.; Yuk, J.; Smith, K.M.; Isaac, G.; Khan, I.A. The Chemical Characterization of Eleutherococcus senticosus and Ci-wu-jia Tea using UHPLC-UV-QTOF/MS. Int. J. Mol. Sci. 2019, 20, 475. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Wang, B.; Qiao, A.; Ji, Y. Preparation of the particles of Acanthopanax senticosus. Saudi J. Biol. Sci. 2019, 26, 1932–1935. [Google Scholar] [CrossRef] [PubMed]
- Davydov, M.; Krikorian, A.D. Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Araliaceae) as an adaptogen: A closer look. J. Ethnopharmacol. 2000, 72, 345–393. [Google Scholar] [PubMed]
- Zhang, H.; Wang, S.; Jin, L.H. Acanthopanax senticosus polysaccharide regulates the intestinal homeostasis disruption induced by toxic chemicals in Drosophila. Phytother. Res. PTR 2020, 34, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, J.H.; Bai, G.; Shen, G.S.; Chen, J.; Liu, J.N.; Wang, S.; Liu, X.J. Acanthopanax senticosus polysaccharides-induced intestinal tight junction injury alleviation via inhibition of NF-κB/MLCK pathway in a mouse endotoxemia model. World J. Gastroenterol. 2017, 23, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Ma, J.; Tang, Y.; Wang, X.; Zhang, J.; Yao, X.; Jiang, W.; Duan, J.A. Optimization of Ultrasound-Assisted Extraction Followed by Macroporous Resin Purification for Maximal Recovery of Functional Components and Removal of Toxic Components from Ginkgo biloba Leaves. BioMed Res. Int. 2018, 2018, 4598067. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, J.; Li, Y.; Wang, C.; Hou, G.; Cong, W.; Zhao, F. Purification of spinosin from Ziziphi Spinosae Semen using macroporous resins followed by preparative high-performance liquid chromatography. J. Sep. Sci. 2019, 42, 3134–3140. [Google Scholar] [CrossRef]
- Wang, N.; Chen, T.; Yang, X.; Shen, C.; Li, H.; Wang, S.; Zhao, J.; Chen, J.; Chen, Z.; Li, Y. A practicable strategy for enrichment and separation of four minor flavonoids including two isomers from barley seedlings by macroporous resin column chromatography, medium-pressure LC, and high-speed countercurrent chromatography. J. Sep. Sci. 2019, 42, 1717–1724. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.; et al. Adsorption properties of macroporous adsorbent resins for separation of anthocyanins from mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.; Chen, Z.; Mao, R.; Xiao, Q.; Gao, H.; Wei, M. Separation and purification of epigallocatechin-3-gallate (EGCG) from green tea using combined macroporous resin and polyamide column chromatography. J. Chromatography. B Anal. Technol. Biomed. Life Sci. 2015, 1002, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Xin, H.; Su, Y.; Zheng, G.; Ling, C. Combined application of macroporous resin and high speed counter-current chromatography for preparative separation of three flavonoid triglycosides from the leaves of Actinidia valvata Dunn. J. Sep. Sci. 2012, 35, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Hu, W.; Xiu, Z.; Jiang, A.; Men, L.; Hao, K.; Sun, X.; Cao, D. Preparative Purification of Total Flavonoids from Sophora tonkinensis Gagnep. by Macroporous Resin Column Chromatography and Comparative Analysis of Flavonoid Profiles by HPLC-PAD. Molecules 2019, 24, 3200. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Ma, J.; Tang, Y.; Wang, X.; Zhang, J.; Duan, J.A. Multi-Response Optimization of Ultrasonic Assisted Enzymatic Extraction Followed by Macroporous Resin Purification for Maximal Recovery of Flavonoids and Ginkgolides from Waste Ginkgo biloba Fallen Leaves. Molecules 2018, 23, 1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zheng, D.; Zhang, Q.F. Purification of total flavonoids from Rhizoma Smilacis Glabrae through cyclodextrin-assisted extraction and resin adsorption. Food Sci. Nutr. 2019, 7, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Hou, M.; Hu, W.; Xiu, Z.; Shi, Y.; Hao, K.; Cao, D.; Guan, Y.; Yin, H. Efficient enrichment of total flavonoids from Pteris ensiformis Burm. extracts by macroporous adsorption resins and in vitro evaluation of antioxidant and antiproliferative activities. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1138, 121960. [Google Scholar] [CrossRef]
- Liu, N.; Song, M.; Wang, N.; Wang, Y.; Wang, R.; An, X.; Qi, J. The effects of solid-state fermentation on the content, composition and in vitro antioxidant activity of flavonoids from dandelion. PLoS ONE 2020, 15, e0239076. [Google Scholar] [CrossRef]
- Liu, B.; Dong, B.; Yuan, X.; Kuang, Q.; Zhao, Q.; Yang, M.; Liu, J.; Zhao, B. Enrichment and separation of chlorogenic acid from the extract of Eupatorium adenophorum Spreng by macroporous resin. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1008, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, Y.; Wang, X. Selective adsorption of tannin from flavonoids by organically modified attapulgite clay. J. Hazard. Mater. 2008, 160, 382–387. [Google Scholar] [CrossRef]
- Park, J.J.; Lee, W.Y. Adsorption and desorption characteristics of a phenolic compound from Ecklonia cava on macroporous resin. Food Chem. 2021, 338, 128150. [Google Scholar] [CrossRef]
- Guo, Z.; Li, J.; Guo, Z.; Guo, Q.; Zhu, B. Phosphorus removal from aqueous solution in parent and aluminum-modified eggshells: Thermodynamics and kinetics, adsorption mechanism, and diffusion process. Environ. Sci. Pollut. Res. Int. 2017, 24, 14525–14536. [Google Scholar] [CrossRef] [PubMed]
- Che Zain, M.S.; Lee, S.Y.; Teo, C.Y.; Shaari, K. Adsorption and Desorption Properties of Total Flavonoids from Oil Palm (Elaeis guineensis Jacq.) Mature Leaf on Macroporous Adsorption Resins. Molecules 2020, 25, 778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandro, M.M.V.; André, L.C.; Alessandro, C.M.; Juliana, C.G.M.; Edivaldo, E.G.; Gisele, F.G.; Willian, F.C.; Vitor, C.A. Kinetic and equilibrium studies: Adsorption of food dyes Acid Yellow 6, Acid Yellow 23, and Acid Red 18 on activated carbon from flamboyant pods. Chem. Eng. J. 2012, 181–182, 243–250. [Google Scholar]
- Dong, Y.; Zhao, M.; Sun-Waterhouse, D.; Zhuang, M.; Chen, H.; Feng, M.; Lin, L. Absorption and desorption behaviour of the flavonoids from Glycyrrhiza glabra L. leaf on macroporous adsorption resins. Food Chem. 2015, 168, 538–545. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Radhakrishnan, P.G. Kinetics, thermodynamics and surface heterogeneity assessment of uranium (VI) adsorption onto cation exchange resin derived from a lignocellulosic residue. Appl. Surf. Sci. 2009, 255, 4983–4991. [Google Scholar] [CrossRef]
- Aksu, Z.; Kabasakal, E. Batch adsorption of 2,4-dichlorophenoxy-acetic acid (2,4-D) from aqueous solution by granular activated carbon. Sep. Purif. Technol. 2004, 35, 223–240. [Google Scholar] [CrossRef]
- Liu, B.; Ouyang, J.; Yuan, X.; Wang, L.; Zhao, B. Adsorption properties and preparative separation of phenylethanoid glycosides from Cistanche deserticola by use of macroporous resins. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 937, 84–90. [Google Scholar] [CrossRef]
- Ge, X.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Han, L.; Yu, X.; Li, W. The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with different color. Food Chem. 2021, 335, 127655. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qian, Y.; Tian, Y.J.; Yuan, S.M.; Wei, W.; Wang, G. Optimization of Ionic Liquid-Assisted Extraction of Biflavonoids from Selaginella doederleinii and Evaluation of Its Antioxidant and Antitumor Activity. Molecules 2017, 22, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.C.; Lin, C.; Chen, M.H.; Chiang, P.Y. Stability and Quality of Anthocyanin in Purple Sweet Potato Extracts. Foods 2019, 8, 393. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Pan, G.; Xu, W.; Sun, Q.; Wang, B.; Zhang, Y.; Yang, T. Spectrum-effect relationship study between HPLC fingerprints and antioxidant activity of Sabia parviflora. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1140, 121970. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, Y.; Li, Q.; Yu, X.; Wang, J.; Zheng, J. The synthesis and evaluation of novel hydroxyl substituted chalcone analogs with in vitro anti-free radicals pharmacological activity and in vivo anti-oxidation activity in a free radical-injury Alzheimer’s model. Molecules 2013, 18, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Gao, Z.; Liu, W.; Li, H.; Zhang, Y.; Feng, Y.; Song, X.; Wang, W.; Zhang, J.; Huang, C.; et al. Characterization, Antioxidant, Anti-Aging and Organ Protective Effects of Sulfated Polysaccharides from Flammulina velutipes. Molecules 2019, 24, 3517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.P.; Fan, C.; Dong, W.M.; Gao, B.; Yuan, W.; Gong, J.S. Free radical scavenging and anti-oxidative activities of an ethanol-soluble pigment extract prepared from fermented Zijuan Pu-erh tea. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 59, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Gao, Y.; Wang, Y.; Wang, L.; Shang, Z.; Wang, S.; Pei, J. Protective effect of a polysaccharide from Rhizoma Atractylodis Macrocephalae on acute liver injury in mice. Int. J. Biol. Macromol. 2016, 87, 85–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Xu, X.; Ye, Q.; Dong, L. Ultrasound extraction optimization of Acanthopanax senticosus polysaccharides and its antioxidant activity. Int. J. Biol. Macromol. 2013, 59, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C.; Quan, Y. Extraction of polysaccharides from Phellinus nigricans mycelia and their antioxidant activities in vitro. Carbohydr. Polym. 2014, 99, 110–115. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Wang, L.; Zhao, Y. Total phenols, flavonoids, and procyanidins levels and total antioxidant activity of different Korean pine(Pinus koraiensis) varieties. J. For. Res. 2019, 30, 1743–1754. [Google Scholar] [CrossRef]
- Liang, L.; Liu, G.; Yu, G.; Song, Y.; Li, Q. Simultaneous decoloration and purification of crude oligosaccharides from pumpkin (Cucurbita moschata Duch) by macroporous adsorbent resin. Food Chem. 2019, 277, 744–752. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Huang, S.; Zhang, L.; Ge, Z.; Sun, L.; Zong, W. Purification of Polyphenols from Distiller’s Grains by Macroporous Resin and Analysis of the Polyphenolic Components. Molecules 2019, 24, 1284. [Google Scholar] [CrossRef] [Green Version]
- Fan, R. Separation of Glycyrrhizic Acid and Its Derivants from Hydrolyzation in Subcritical Water by Macroporous Resin. Molecules 2020, 25, 4305. [Google Scholar] [CrossRef] [PubMed]
- Yuanfeng, W.; Lei, Z.; Jianwei, M.; Shiwang, L.; Jun, H.; Yuru, Y.; Lehe, M. Kinetic and thermodynamic studies of sulforaphane adsorption on macroporous resin. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1028, 231–236. [Google Scholar] [CrossRef]
- Hu, X.; Du, X. Adsorption of Tea Polyphenols using Microporous Starch: A Study of Kinetics, Equilibrium and Thermodynamics. Molecules 2019, 24, 1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limwachiranon, J.; Huang, H.; Li, L.; Duan, Z.; Luo, Z. Recovery of lotus (Nelumbo nucifera Gaertn.) seedpod flavonoids using polar macroporous resins: The updated understanding on adsorption/desorption mechanisms and the involved intermolecular attractions and bonding. Food Chem. 2019, 299, 125108. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of bioactive compounds potential and antioxidant activity of brown, green and red propolis from Brazilian northeast region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef]
- Ullah, S.; Hussain, S.A.; Shaukat, F.; Hameed, A.; Yang, W.; Song, Y. Antioxidant Potential and the Characterization of Arachis hypogaea Roots. BioMed Res. Int. 2019, 2019, 7073456. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Huang, G.; Yang, Z.; Hou, Y. Antioxidant activity of Momordica charantia polysaccharide and its derivatives. Int. J. Biol. Macromol. 2019, 138, 673–680. [Google Scholar] [CrossRef]

| Polarity | Resin Type | Surface Area/m2·g−1 | Average Pore/A° |
|---|---|---|---|
| D101 | No-polar | 500~550 | 90~100 |
| AB-8 | Weak polar | 480~520 | 130~140 |
| CAD-40 | Middle polar | 450~500 | 70~80 |
| DM301 | Middle polar | 330~380 | 130~170 |
| HPD-600 | Polar | 550~600 | 80 |
| S-8 | Polar | 100~120 | 280~300 |
| Dynamics Model | Kinetic Equation | Parameters |
|---|---|---|
| The pseudo-first-order model | ln(qe − qt) = −0.0145t + 2.488 | k1 = 0.0145 |
| R2 = 0.9616 | ||
| qe = 12.037 mg·g−1 | ||
| The pseudo-second-order model | t/qt = 0.0341t + 0.3004 | k2 = 0.0031 g·(mg·min)−1 |
| R2 = 0.9995 | ||
| qe = 29.326 mg·g−1 | ||
| Intra-particle diffusion model | qt = 1.72t0.5 + 10.22 | kp = 1.0062 mg·(g·min)−1/2 |
| R2 = 0.9739 | ||
| I = 10.22 | ||
| qt = 0.47t0.5 + 21.96 | kp = 1.0062 mg·(g·min)−1/2 | |
| R2 = 0.8541 | ||
| I = 21.96 | ||
| qt = 0.07t0.5 + 27.28 | kp = 1.0062 mg·(g·min)−1/2 | |
| R2 = 0.8958 | ||
| I = 27.28 |
| Model | T/(°C) | Equations | Parameters | ||
|---|---|---|---|---|---|
| KF [(mg·g−1) (mL·mg−1)1/n] | n | R2 | |||
| Freundlich | 25 | lnqe = 0.8435lnCe + 2.295 | 9.926 | 1.1855 | 0.9772 |
| 30 | lnqe = 0.7898lnCe + 2.465 | 11.761 | 1.2661 | 0.9788 | |
| 35 | lnqe = 0.8708lnCe + 2.8538 | 17.354 | 1.1484 | 0.9908 | |
| KL (mL·mg−1) | Qm (mg·g−1) | R2 | |||
| Langmuir | 25 | Ce/qe = 0.0056Ce + 0.0969 | 0.0578 | 178.57 | 0.8017 |
| 30 | Ce/qe = 0.0063Ce + 0.0827 | 0.0762 | 158.73 | 0.9319 | |
| 35 | Ce/qe = 0.0024Ce + 0.0571 | 0.0434 | 416.67 | 0.8316 | |
| KT (L·mg−1) | BT (J·mol−1) | R2 | |||
| Temkin | 25 | qe = 25.788lnCe + 2.7832 | 1.1139 | 25.788 | 0.9667 |
| 30 | qe = 26.741lnCe + 4.5813 | 1.1869 | 26.741 | 0.9694 | |
| 35 | qe = 50.038lnCe + 1.2145 | 1.0246 | 50.038 | 0.9456 | |
| Adsorption Capacity qe/(mg·mL−1) | Enthalpy Change ΔH/(kJ·mol−1) | Free Energy Changes ΔG/(kJ·mol−1) | Entropy Change ΔS/J·(mol·K)−1 | ||||
|---|---|---|---|---|---|---|---|
| 25 °C | 30 °C | 35 °C | 25 °C | 30 °C | 35 °C | ||
| 20 | 58.2429 | 205.5695 | 202.5583 | 198.5412 | |||
| 30 | 59.3270 | 209.2122 | 206.136 | 202.0647 | |||
| 40 | 60.0961 | −2.9385 | −3.1384 | −2.8467 | 211.7962 | 208.6738 | 204.5641 |
| 50 | 60.6922 | 213.7992 | 210.641 | 206.5014 | |||
| 60 | 61.1802 | 215.4389 | 212.2515 | 208.0875 | |||
| Average Rt (min) | Adduct Type | Contents | Formula | SMILES | Class | Content (%) | |
|---|---|---|---|---|---|---|---|
| Before Purification | After Purification | ||||||
| 5.255 | [M − H]− | Protocatechuic acid | C7H6O4 | OC(=O)C1=CC(O)=C(O)C=C1 | Flavones | 0.0538 | 11.4892 |
| 6.346 | [M + H]+ | Isofraxidin | C11H10O5 | O=C1OC=2C(OC)=C(O)C(OC)=CC2C=C1 | 7-hydroxycoumarins | 0.1130 | 1.4791 |
| 7.839 | [M + H]+ | 6″,6″-Dimethylpyrano[2″,3″:7,8]flavone | C20H16O3 | O=C1C=C(OC=2C1=CC=C3OC(C=CC32)(C)C)C=4C=CC=CC4 | Pyranoflavonoids | 0.1686 | 0.2432 |
| 8.001 | [M − H]− | Umbelliferone | C9H6O3 | O=C1OC=2C=C(O)C=CC2C=C1 | Flavones | 0.0082 | 1.3348 |
| 9.454 | [M + H]+ | Luteolin | C15H10O6 | OC1=CC(O)=C2C(=O)C=C(OC2=C1)C1=CC(O)=C(O)C=C1 | Flavones | 0.0064 | 1.0353 |
| 10.163 | [M + H]+ | Baicalin | C21H18O11 | O=C(O)C4OC(OC1=CC=2OC(=CC(=O)C=2(C(O)=C1(O)))C3=C | Flavone O-glycosides | 0.0532 | 5.6938 |
| 15.43 | [M − H]− | Baicalein | C15H10O5 | O=C1C=C(OC2=CC(O)=C(O)C(O)=C12)C=3C=CC=CC3 | Flavones | 0.0870 | 2.4605 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Su, J.; Chu, X.; Zhang, X.; Kan, Q.; Liu, R.; Fu, X. Adsorption and Desorption Characteristics of Total Flavonoids from Acanthopanax senticosus on Macroporous Adsorption Resins. Molecules 2021, 26, 4162. https://doi.org/10.3390/molecules26144162
Wang X, Su J, Chu X, Zhang X, Kan Q, Liu R, Fu X. Adsorption and Desorption Characteristics of Total Flavonoids from Acanthopanax senticosus on Macroporous Adsorption Resins. Molecules. 2021; 26(14):4162. https://doi.org/10.3390/molecules26144162
Chicago/Turabian StyleWang, Xiaoya, Jianqing Su, Xiuling Chu, Xinyu Zhang, Qibin Kan, Ruixue Liu, and Xiang Fu. 2021. "Adsorption and Desorption Characteristics of Total Flavonoids from Acanthopanax senticosus on Macroporous Adsorption Resins" Molecules 26, no. 14: 4162. https://doi.org/10.3390/molecules26144162
APA StyleWang, X., Su, J., Chu, X., Zhang, X., Kan, Q., Liu, R., & Fu, X. (2021). Adsorption and Desorption Characteristics of Total Flavonoids from Acanthopanax senticosus on Macroporous Adsorption Resins. Molecules, 26(14), 4162. https://doi.org/10.3390/molecules26144162






