Disinfection of Neonatal Resuscitation Equipment in Low-Resource Settings: The Importance, the Reality, and Considerations for the Future
Abstract
:1. Introduction
2. A Brief History of Disinfection
3. The Importance of Equipment Reprocessing: From Spaulding to PATH
3.1. Disinfection of Equipment in High-Resource Settings
3.2. Disinfection of Equipment in Low-Resource Settings
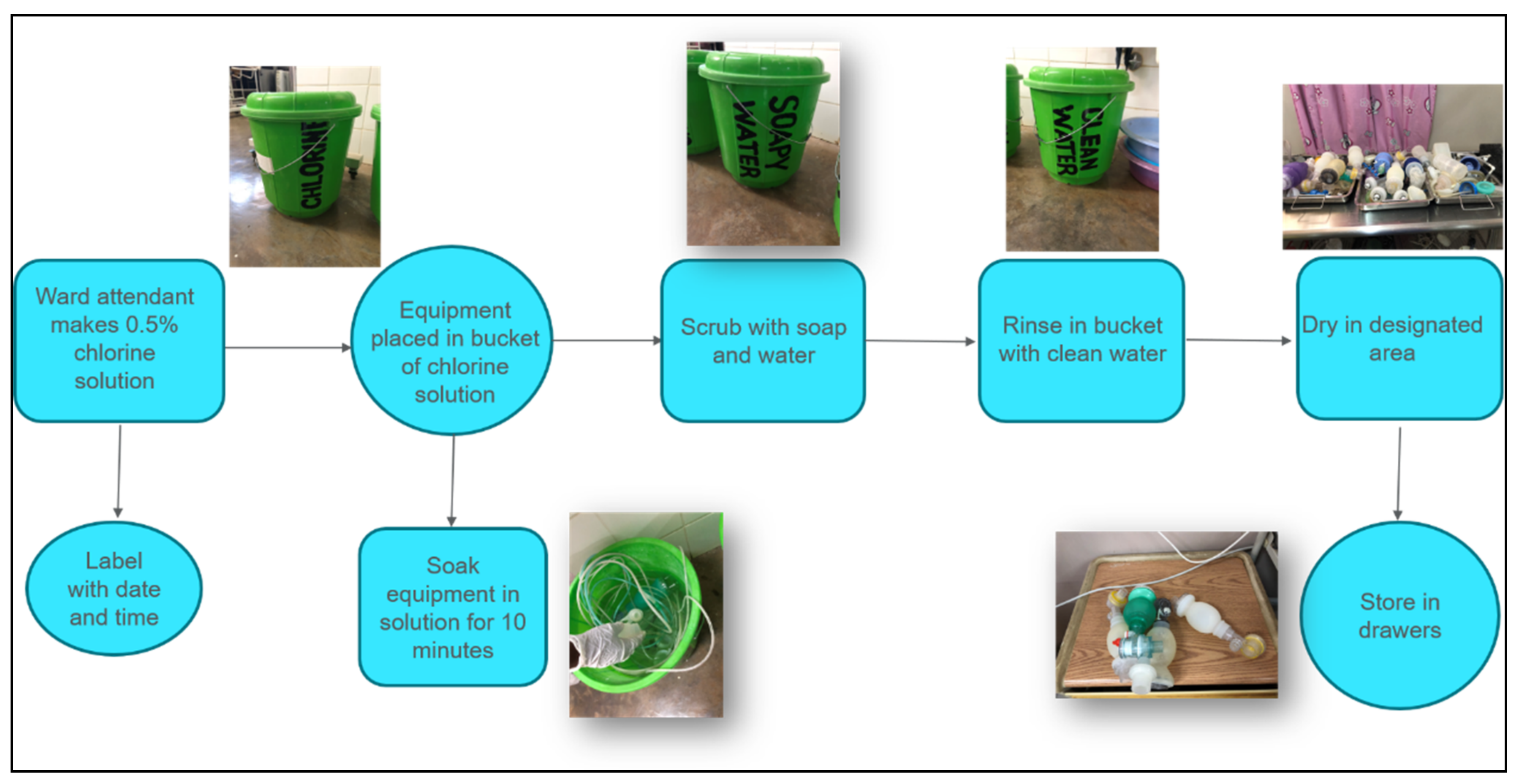
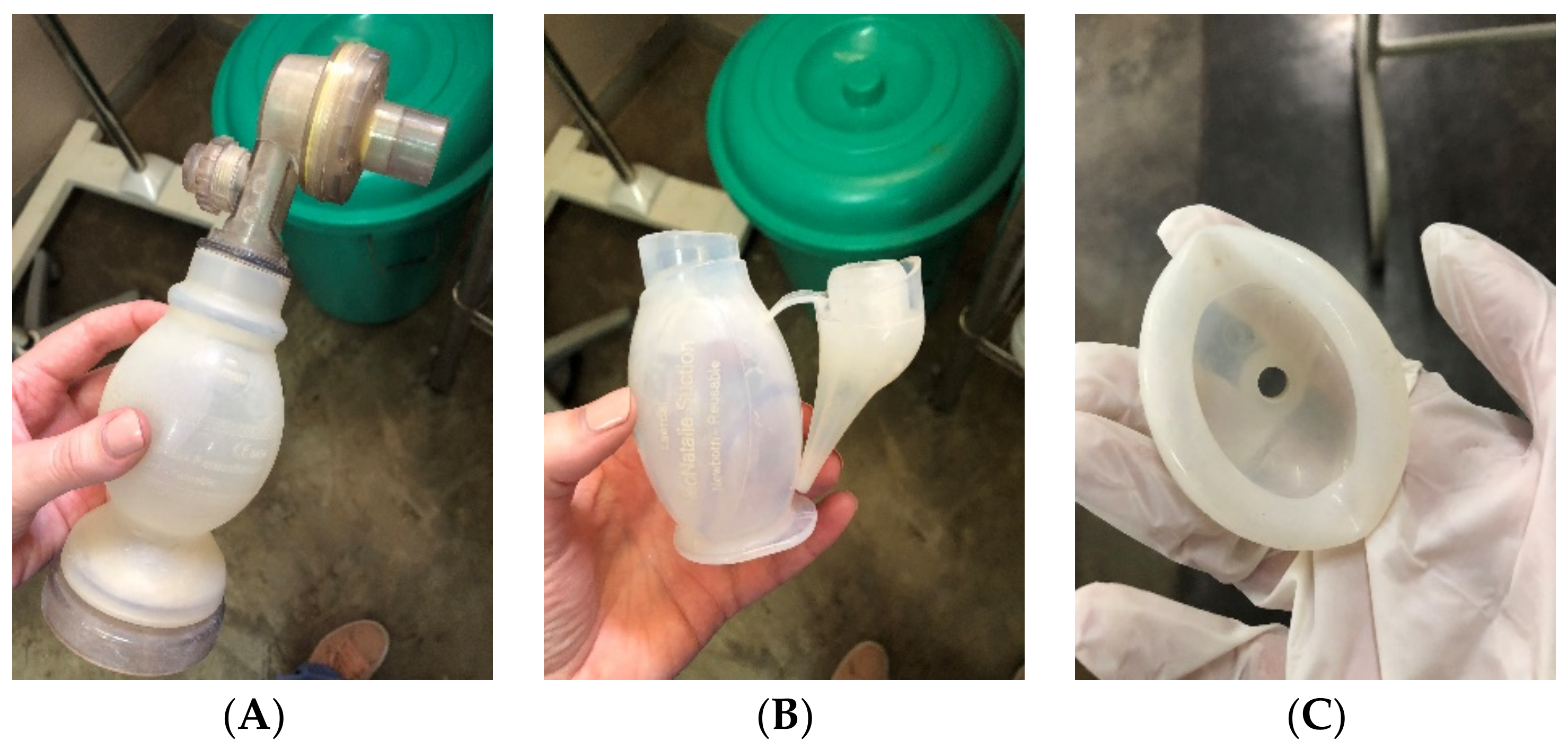
4. Reprocessing Realities in Kenya and Malawi
5. Considerations for the Future of Reprocessing in Resource-Constrained Settings
5.1. Engagement of Front-Line Providers for Front-Line Solutions
5.2. Simplification of the PATH Guidelines for Feasibility
5.3. Quality Assurance of Reprocessing in Resource-Constrained Settings
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Millennium Development Goals and Beyond 2015. Available online: https://www.un.org/millenniumgoals/childhealth.shtml (accessed on 24 April 2018).
- United Nations. The Sustainable Development Goals Report 2016; Organization of the United Nations: New York, NY, USA, 2016; Available online: https://unstats.un.org/sdgs/report/2016/The%20Sustainable%20Development%20Goals%20Report%202016.pdf (accessed on 24 April 2018).
- UNICEF. Every Child Alive: The Urgent Need to End Newborn Deaths. 2018. Available online: https://data.unicef.org/wp-content/uploads/2018/02/Every-Child-Alive-report_FINAL-1.pdf (accessed on 24 April 2018).
- Berkelhamer, S.K.; Kamath-Rayne, B.D.; Niermeyer, S. Neonatal Resuscitation in Low-Resource Settings. Clin. Perinatol. 2016, 43, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Kamath-Rayne, B.D.; Berkelhamer, S.K.; Kc, A.; Ersdal, H.L.; Niermeyer, S. Neonatal resuscitation in global health settings: An examination of the past to prepare for the future. Pediatr. Res. 2017, 82, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Niermeyer, S.; Kamath-Rayne, B.; Keenan, W.; Little, G.; Singhal, N.; Visick, M. Helping Babies Breathe: Facilitator Flip Chart. Helping Babies Survive, 2nd ed. 2016. Available online: http://internationalresources.aap.org/Resource/ShowFile?documentName=HBB_Flipbook_Second_Edition_20-00371_Rev_E.pdf (accessed on 10 January 2020).
- Survive & Thrive. Guiding The Way Forward: 5 Year Report. 2018. Available online: https://surviveandthrive.org/resources/Documents/Survive%20%20Trive%205%20year%20report%20FINAL.pdf (accessed on 24 April 2018).
- Msemo, G.; Massawe, A.; Mmbando, D.; Rusibamayila, N.; Manji, K.; Kidanto, H.L.; Mwizamuholya, D.; Ringia, P.; Ersdal, H.L.; Perlman, J. Newborn Mortality and Fresh Stillbirth Rates in Tanzania After Helping Babies Breathe Training. Pediatrics 2013, 131, e353–e360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rule, A.R.; Maina, E.; Cheruiyot, D.; Mueri, P.; Simmons, J.M.; Kamath-Rayne, B.D. Using quality improvement to decrease birth asphyxia rates after ‘Helping Babies Breathe’ training in Kenya. Acta Paediatr. 2017, 106, 1666–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Global Burden of Disease Child and Adolescent Health Collaboration. Child and Adolescent Health from 1990 to 2015: Findings From the Global Burden of Diseases, Injuries, and Risk Factors 2015 Study. JAMA Pediatr. 2017, 171, 573–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kissoon, N.; Uyeki, T.M. Sepsis and the Global Burden of Disease in Children. JAMA Pediatr. 2016, 170, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, E. The role of chemical disinfection in the prevention of nosocomial infections. In Proceedings of the International Conference on Nosocomial Infections, Chicago, IL, USA, 3–6 August 1970; American Hospital Association: Chicago, IL, USA, 1971; pp. 247–254. [Google Scholar]
- Ontario Agency for Health Protection and Promotion (Public Health Ontario); Provincial Infectious Diseases Advisory Committee. Best Practices for Cleaning, Disinfection and Sterilization of Medical Equipment/Devices, 3rd ed.; Queen’s Printer for Ontario: Toronto, ON, Canada, 2013. [Google Scholar]
- CDC. Glossary: Infection Prevention & Control in Dental Settings. Available online: https://www.cdc.gov/oralhealth/infectioncontrol/glossary.htm (accessed on 10 January 2020).
- PATH. Reprocessing Guidelines for Basic Neonatal Resuscitation Equipment in Resource-Limited Settings. 2016. Available online: http://www.path.org/publications/files/PATH_reprocessing_guidelines_basic_neo_resusc_equip3.pdf (accessed on 10 January 2020).
- Centers for Disease Control and Prevention. Reuse of Single-Use Medical Devices: Guideline for Disinfection and Sterilization in Healthcare Facilities. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/reuse-of-devices.html (accessed on 20 January 2019).
- Nelson, K.E.; Warren, D.; Tomasi, A.M.; Raju, T.N.; Vidyasagar, D. Transmission of Neonatal Listeriosis in a Delivery Room. Arch. Pediatr. Adolesc. Med. 1985, 139, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.; Thompson, S.; Page, B. Neonatal Infections with Pseudomonas Aeruginosa Associated with Contaminated Resuscitation Equipment. Lancet 1965, 285, 781–784. [Google Scholar] [CrossRef]
- Eslami, P.; Bucher, S.; Mungai, R. Improper Reprocessing of Neonatal Resuscitation Equipment in rural Kenya compromises function: Recommendations for more effective implementation of Helping Babies Breathe. Resusciation 2015, 91, e5–e6. [Google Scholar] [CrossRef]
- Zaidi, A.K.; Huskins, W.C.; Thaver, D.; Bhutta, Z.A.; Abbas, Z.; Goldmann, D.A. Hospital-acquired neonatal infections in developing countries. Lancet 2005, 365, 1175–1188. [Google Scholar] [CrossRef]
- National Public Radio. Rage against the Busted Medical Machines. Available online: https://www.npr.org/sections/goatsandsoda/2016/09/08/492842274/rage-against-the-busted-medical-machines (accessed on 8 September 2016).
- Kuupiel, D.; Bawontuo, V.; Mashamba-Thompson, T.P. Improving the Accessibility and Efficiency of Point-of-Care Diagnostics Services in Low- and Middle-Income Countries: Lean and Agile Supply Chain Management. Diagnostics 2017, 7, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunyoto, T.; Potet, J.; Boer, M.D.; Ritmeijer, K.; Postigo, J.A.R.; Ravinetto, R.; Alves, F.; Picado, A.; Boelaert, M. Exploring global and country-level barriers to an effective supply of leishmaniasis medicines and diagnostics in eastern Africa: A qualitative study. BMJ Open 2019, 9, e029141. [Google Scholar] [CrossRef] [PubMed]
- Shue, R. Partnering in a Pandemic: Advancing Global Access to Health During COVID-19. Devex. 2020. Available online: https://www.devex.com/news/partnering-in-a-pandemic-advancing-global-access-to-health-during-covid-19-98207 (accessed on 10 October 2020). Published September 29, 2020.
- Gilbertson, J.; Quintanar-Solares, M.; Liland, F.; Niermeyer, S. High-level disinfection of re-usable neonatal resuscitation equipment through boiling and steaming. J. Hosp. Infect. 2020, 106, 721–725. [Google Scholar] [CrossRef] [PubMed]
- White, A.M.; Mutai, D.; Cheruiyot, D.; Rule, A.R.L. Get Me a Mask! The Challenge of Equipment Supply Chains. Pediatrics 2021. Accepted for publication. [Google Scholar]
- White, A.M.; Rule, A.R.L.; Schaffzin, J.K.; Kamath-Rayne, B.D.; Mortensen, J.E. Laboratory Simulation to Improve Bag-Valve-Mask Reprocessing in Low-Resource Settings: A Pilot Study; Poster Presentation at Pediatric Academic Societies Meeting: Baltimore, MD, USA, 2019. [Google Scholar]
- World Health Organization. Decontamination and Reprocessing of Medical Devices for Health-Care Facilities. 2016. Available online: https://www.who.int/infection-prevention/publications/decontamination/en/ (accessed on 20 January 2019).
- Salsgiver, E.; Bernstein, D.; Simon, M.S.; Greendyke, W.; Jia, H.; Robertson, A.; Salter, S.; Schuetz, A.N.; Saiman, L.; Furuya, E.Y.; et al. Comparing the Bioburden Measured by Adenosine Triphosphate (ATP) Luminescence Technology to Contact Plate–Based Microbiologic Sampling to Assess the Cleanliness of the Patient Care Environment. Infect. Control. Hosp. Epidemiology 2018, 39, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.E.; Cooper, R.A.; Griffith, C.J. Use of audit tools to evaluate the efficacy of cleaning systems in hospitals. Am. J. Infect Control. 2003, 31, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Zemitis, S.; Harman, M.; Hargett, Z.; Weinbrenner, D. Single-Use Bag Valve Masks: Evaluation of Device Design and Residual Bioburden Analytical Methods. J. Biomed. Sci. Eng. 2018, 11, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frickmann, H.; Bachert, S.; Warnke, P.; Podbielski, A. Validated measurements of microbial loads on environmental surfaces in intensive care units before and after disinfecting cleaning. J. Appl. Microbiol. 2018, 124, 874–880. [Google Scholar] [CrossRef] [PubMed]




| Classification | Definition | Level of Reprocessing | Examples |
|---|---|---|---|
| Critical | Enters sterile tissue | Sterilization | Surgical instruments |
| Semi-critical | In contact with nonintact skin or mucous membranes but does not penetrate them | Minimum high-level disinfection Sterilization preferred | Respiratory equipment Anesthesia equipment |
| Noncritical | Touches only intact skin, or does not directly touch patient | Low-level disinfection | Electrocardiogram machine Oximeter Bedpan |
| Stage | Steps |
|---|---|
| Precleaning | 1. Rinse with water 2. Clean with enzymatic solution 3. Rinse with distilled water |
| Cleaning | 1. Ultrasonic cleaner 2. Automatic washer |
| Inspection and Test of Function | 1. Assemble equipment 2. Package 3. Test function 4. Place indicator for sterilization |
| Sterilization | Steam preferred |
| Storage | Store in clean, dry place |
| Stage | Steps |
|---|---|
| Preparation | 1. Wear complete personal protective equipment 2. Clean reprocessing area 3. Prepare reprocessing materials 4. Label containers |
| Predisinfection | 1. Preclean 2. Disassemble 3. Clean 4. Rinse (Remove limescale if needed) 5. Dry before sterilization or chemical disinfection |
| Disinfection | 1. Disinfect by: a. Chemical HLD b. Heat HLD c. Sterilization 2. Dry |
| Postdisinfection | 1. Inspect 2. Reassemble 3. Test function 4. Store |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, A.M.; Mutai, D.; Cheruiyot, D.; Rule, A.R.L.; Mortensen, J.E.; Schaffzin, J.K.; Kamath-Rayne, B.D. Disinfection of Neonatal Resuscitation Equipment in Low-Resource Settings: The Importance, the Reality, and Considerations for the Future. Int. J. Environ. Res. Public Health 2021, 18, 7065. https://doi.org/10.3390/ijerph18137065
White AM, Mutai D, Cheruiyot D, Rule ARL, Mortensen JE, Schaffzin JK, Kamath-Rayne BD. Disinfection of Neonatal Resuscitation Equipment in Low-Resource Settings: The Importance, the Reality, and Considerations for the Future. International Journal of Environmental Research and Public Health. 2021; 18(13):7065. https://doi.org/10.3390/ijerph18137065
Chicago/Turabian StyleWhite, Anne M., Dominic Mutai, David Cheruiyot, Amy R. L. Rule, Joel E. Mortensen, Joshua K. Schaffzin, and Beena D. Kamath-Rayne. 2021. "Disinfection of Neonatal Resuscitation Equipment in Low-Resource Settings: The Importance, the Reality, and Considerations for the Future" International Journal of Environmental Research and Public Health 18, no. 13: 7065. https://doi.org/10.3390/ijerph18137065
APA StyleWhite, A. M., Mutai, D., Cheruiyot, D., Rule, A. R. L., Mortensen, J. E., Schaffzin, J. K., & Kamath-Rayne, B. D. (2021). Disinfection of Neonatal Resuscitation Equipment in Low-Resource Settings: The Importance, the Reality, and Considerations for the Future. International Journal of Environmental Research and Public Health, 18(13), 7065. https://doi.org/10.3390/ijerph18137065





