Abstract
A two-stage continuous process was developed for improved silica extraction from rice husk. The two-stage continuous process consists of attrition ball milling and alkaline leaching methods. To find the optimum conditions for the continuous process, the effects of alkaline leaching parameters, such as the alkaline solution type and reaction conditions, on the silica extraction yield were investigated in a batch process. The use of NaOH showed a slightly higher silica yield than KOH. The optimum reaction conditions were found to be 0.2 M, 80 °C, 3 h, and 6% (w/v) for the reaction concentration, temperature, duration time, and solid content, respectively. Attrition ball milling was used to make micron-sized rice husk particles and to improve the fluidity of the rice husk slurry. The two-stage continuous process was performed using optimum conditions as determined based on the results of the batch experiment. The two-stage continuous extraction was stably operated for 80 h with an 89% silica yield. During the operation, the solid content remained consistent at 6% (w/v). The obtained silica was characterized using inductively coupled plasma–optical emission spectrometry (ICP–OES), X-ray diffraction (XRD), and the Brunauer–Emmett–Teller (BET) method.
1. Introduction
Rice is a major agricultural product across the world, and its annual production was approximately 996 million tons in 2018 [1]. Rice husk accounts for 20% of rice byproducts [2] and has various applications in different industries, e.g., (a) as an industrial fuel for paddy processing and in the generation of process steam in power plants; (b) as a fertilizer and substrate or pet food fiber; (c) as an ingredient for the preparation of activated carbon or substrate for silica and silicon compound production, and (d) as raw material for brick production [3,4]. Rice husk is composed of approximately 70–80% organic substances such as cellulose, hemicellulose, and lignin, and the remaining 20–30% comprises inorganic compounds [5,6]. A major inorganic component is silica, which accounts for approximately 95% of the inorganic compounds. The silica in rice husk is amorphous and has a colloidal state in water. Silica is an industrial material that is highly utilized as an additive for catalysts, insulation, toothpaste [7], coating solutions [8,9], and cosmetics [10]. The use of “biosilica” (rice husk-derived silica) as an alternative for silica in various industrial applications would mitigate high energy consumption, natural resource depletion, and greenhouse gas emissions [3].
Two approaches are used to extract silica from rice husk: combustion and chemical treatment. Direct combustion is the most popular method and is conducted in open fire stoves or boilers. During burning, rice husk is oxidized, resulting in ash products. This is the simplest method to obtain inorganic compounds from rice husk. The inorganic compounds, so-called rice husk ash, can be converted into soluble sodium silicate by reacting with aqueous sodium hydroxide [11]. Lee et al. used sulfuric acid to remove organic compounds from rice husk before combustion [5]. Sulfuric acid dissolves most celluloses and hemicelluloses, which are discarded by separating liquids from solids. The acid treatment improved the purity of silica finally obtained. Hincapié-Rojas et al. obtained submicron silica particles from rice husk by subsequent treatments of combustion, acid leaching, and mechanical ball milling [12]. Souza et al. compared the use of hot organic acid and boiling water before combustion to obtain high-quality silica [13]. Chemical extraction is adopted for environmentally friendly extraction. The chemical extraction method consists of acid treatment and an alkaline leaching step [14]. In the chemical extraction method, several chemical routes are used to achieve highly efficient silica extraction [14,15,16,17]. Zulkifli et al. combusted rice husk to obtain rice husk ash, followed by acid and alkaline leaching [17]. Chun et al. directly treated rice husk with sulfuric acid to leach metallic impurities, followed by combustion to remove organic residual compounds; afterward, high-purity silica was dissolved in sodium hydroxide to control the size and pores in silica particles [14]. In their study, 99.8% silica purity was obtained. Costa and Paranhos converted rice husk to rice husk ash (RHA) by combustion. The rice husk ash was dissolved in concentrated sulfuric acid, followed by treatment with alkaline solution to obtain sodium silicate. Nanosilica particles were synthesized by precipitation using phosphoric acid [15]. Song et al. employed the Taguchi method to obtain surfactant-free synthesis of high surface area silica nanoparticles from rice husk [16]. The Taguchi method was efficient for designing factorial experiments with a minimum number of experiments. Regardless of the specific chemical route used, the alkaline leaching step is critical for obtaining high-purity silica from rice husk [6].
This study developed a two-stage continuous silica extraction process from rice husk using attrition ball milling and alkaline leaching methods. A continuous process has several advantages over a batch process, namely production of a narrow specification product, reduced production cost, and increased productivity. Rice husk has a very low density, within the range 90–150 kg/m3 [18], and conveying is usually conducted by a pneumatic conveying system [19]. In this study, rice husk was ground into micron-sized particles and mixed with a sodium hydroxide solution to make a rice husk slurry. The rice husk slurry can be easily conveyed by a fluid pump and continuously reacted to leach silica from rice husk. In addition, alkaline leaching was performed under an atmospheric pressure, which is safe to apply in a rice mill where rice husk is generated. Therefore, this study is an initial step toward the field application of a silica extraction process using rice husk. The circular bioeconomy has gained attention as a key concept for sustainable technical cycles. The circular bioeconomy focuses on the valorization of biomass in integrated production chains and making use of residues [20]. Currently, biomass valorization focuses on valorizing the organic fraction of biomass [21]. However, the valorization of ash content is also important and has the potential to extract more value from biomass. In this respect, this study is worthwhile to extend the area of biomass valorization and, ultimately, promote the facilitation of circular bioeconomy.
2. Materials and Methods
2.1. Materials
Rice husk was kindly supplied by a rice processing facility in the Chungbuk region, Rep. Korea, which was harvested in 2019. Sodium hydroxide powder (97%), acetic acid (99.5%), and potassium hydroxide (93.0%) were purchased from Daejung Chemicals & Metals Inc. (Goryeong, Korea). Sodium hydroxide powders were dissolved in distilled water and used in the experiments; the others were used as received without further purification.
2.2. Alkaline Leaching Process
Before using the rice husk, it was washed with deionized water three times and dried at 80 °C overnight. After drying, the rice husk was immersed in an alkaline solution (sodium hydroxide or potassium hydroxide) and thoroughly mixed to allow sufficient soaking in the solution. The sample was moved to a heating oven (ThermoStableTM “OF-105”, Daihan Scientific, Wonju, Korea) set at a specific temperature for reaction over a given reaction time. After reaction, the solids were separated from the solution using vacuum filtration (Circulating Aspirator (WJ-15, SIBATA, Saitama, Japan)) and filter paper (Whatman No. 41, 20~25 μm, Maidstone, UK). To measure the leached ash, acetic acid was added to the solution to adjust the pH to 7.0, which was stirred at 300 rpm overnight. The precipitation was washed three times with deionized water at 4000 rpm for 10 min. The washed precipitation was dried at 80 °C overnight. The organics such as hemicellulose and lignin were leached during the alkaline leaching process and contained in the precipitation. Therefore, the washed precipitation was calcined at 900 °C for 6 h to remove the organics in the precipitation. The silica yield was calculated using Equation (1) below:
To find the optimum alkaline leaching conditions, four experimental parameters—the solid content, alkaline reaction concentration, temperature, and duration—were optimized.
2.3. Attrition Ball Mill
An attrition pulverizer (Korea powder system Co., Ltd., Incheon, Korea) previously developed for lignocellulosic pretreatment [22] was used to prepare micron-sized rice husk particles. One-third of the inner space was filled with rice husk, while another third was filled with grinding steel balls (10 mm in diameter). Alkaline solvent was added to the grinding jar. The rice husk was pulverized under wet-grinding conditions at 300 rpm for 20 or 30 min. After milling, the rice husk was transferred to 1 mm shaking sieve (Aanlysette3, Fritsch GmbH, Idar-Oberstein, Germany) and shaken for 1 min to separate the pulverized rice husk particles from the grinding balls.
2.4. Two-Stage Continuous Silica Extraction Process
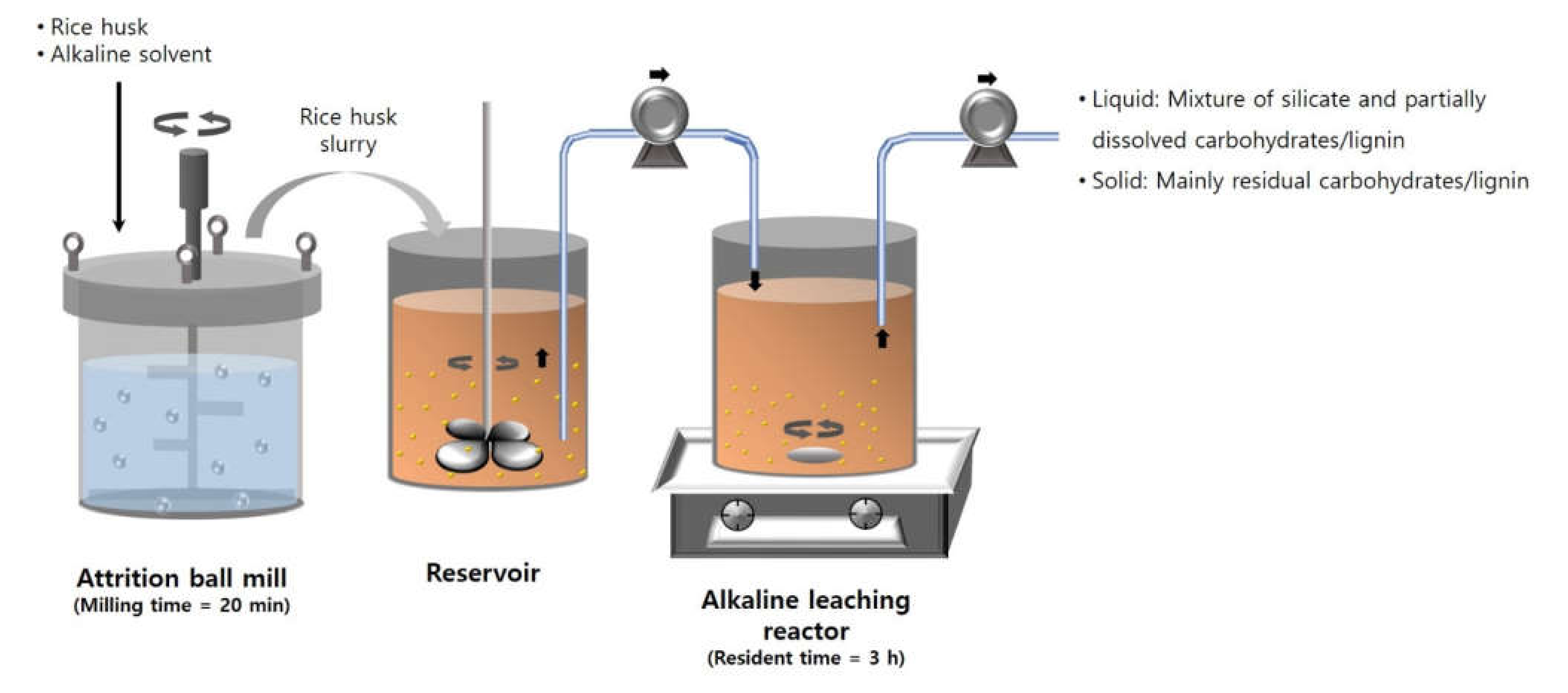
A schematic diagram of the continuous silica extraction process is shown in Figure 1. The continuous extraction process consists of two steps: pulverization and alkaline reaction. At the pulverization step, rice husk was pulverized to make fine rice husk particles and to increase the fluidity. In the continuous process, rice husk was pulverized in an alkaline solvent at 300 rpm for 20 min and stored in a reservoir after separating from grinding balls. The reservoir was stirred at 600 rpm using an electronic overhead stirrer (MS 3060D, MTOPS, Yangju, Korea) to prevent the rice husk particles from settling down. The rice husk slurry in the reservoir was continuously fed into a reactor using a peristaltic pump (BT100S, Lead Fluid Technology, Co., Ltd., Baoding, China). The reactor was stirred at 400 rpm and 80 °C. The outlet sample was collected and separated using a vacuum filter and filter paper (Whatman No. 41, 20–25 μm, Maidstone, UK) for calculating the silica yield, which was calculated as described in Section 2.2. For measuring the solid content, 10 g of the rice husk slurry was sampled at the outlet of the reservoir every 8 h. The sample was kept in a heating oven (ThermoStableTM “OF-105”, Daihan Scientific, Wonju, Korea) set to 105 °C for 24 h. The solid content was calculated by using the weight difference before and after drying.
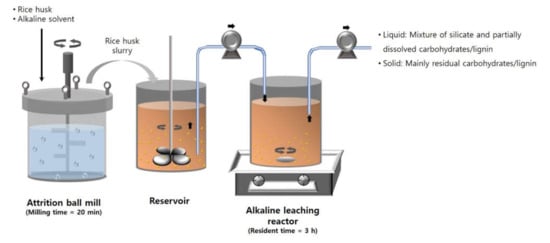
Figure 1.
Scheme of the continuous silica extraction process.
2.5. Analytical Methods
The compositions of rice husk, carbohydrates and lignin, were measured according to the standard procedure provided by National Renewable Energy Laboratory (NREL) [23]. The compositions of rice husk were compared between before and after extracting to calculate the quantities of extracted carbohydrates and lignin. The inorganic chemical composition was determined by using inductively coupled plasma–optical emission spectrometry (ICP–OES; Optima 5300DV, PerkinElmer, MA, USA). X-ray diffraction patterns were obtained using X-ray diffraction (XRD; D/Max 2500/PC, Rigaku, Tokyo, Japan). The surface areas of the obtained silica were calculated from the measured isotherms according to the Brunauer–Emmett–Teller (BET) method, and the pore volumes were taken at the P/P0 = 0.995 single point using a Micromeritics Tristar 3200 system (Micromeritics Inc., Norcross, GA, USA). The pore size distributions of the silica were calculated using the Barrett–Joyner–Halenda (BJH) method from the adsorption branches of the isotherms. The rheological properties of the pulverized rice husk slurry were analyzed using a stress-controlled rotational rheometer (MCR 702, Anton Paar, Graz, Austria) with a C-PTD200 (Cup-Peltier Temperature Device).
3. Results and Discussion
3.1. Optimization of Alkaline Leaching Conditions
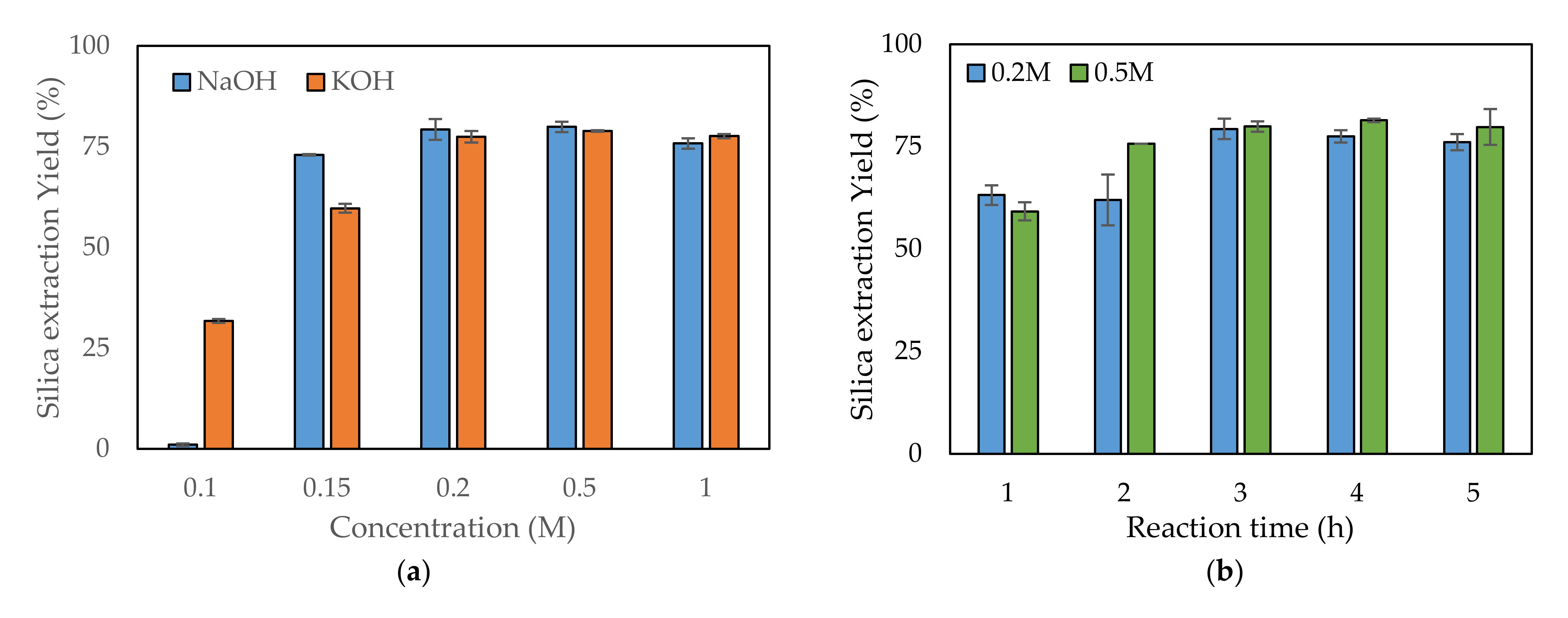
For extracting silica from rice husk, two alkaline solutions, NaOH and KOH, were used and their performance compared. Both are popularly used alkaline solutions and have similar properties. The performances of various concentrations of NaOH and KOH solutions were compared to assess which would be optimal for leaching. Figure 2a presents a comparison of the performances of the two alkaline solutions regarding silica leaching from rice husk depending on their concentrations. Both showed similar extracting yields, but there was a slight difference. At 0.1 M concentration, the silica extraction yields were 1% and 30% for NaOH and KOH, respectively. The use of KOH showed a higher extraction yield at 0.1 M concentration. As the concentration of the alkaline solution increased, the extracting yields increased up to a certain point. Over this point, the extracting yields were saturated and did not increase further, even when the alkaline concentration increased. When NaOH was used, the silica yield became saturated starting from 0.2 M, with an approximately 79% yield. When KOH was used, saturation of the extracting yield was approximately 77%, starting from 0.5 M KOH. Therefore, NaOH can be used at a lower concentration than KOH while obtaining a slightly higher yield.
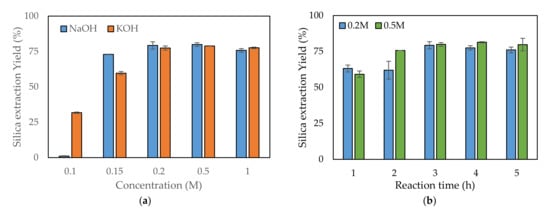
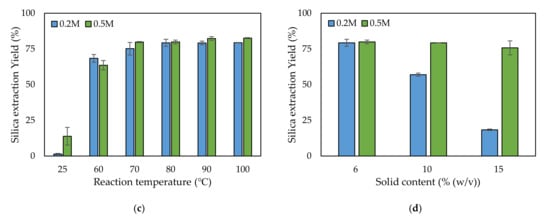
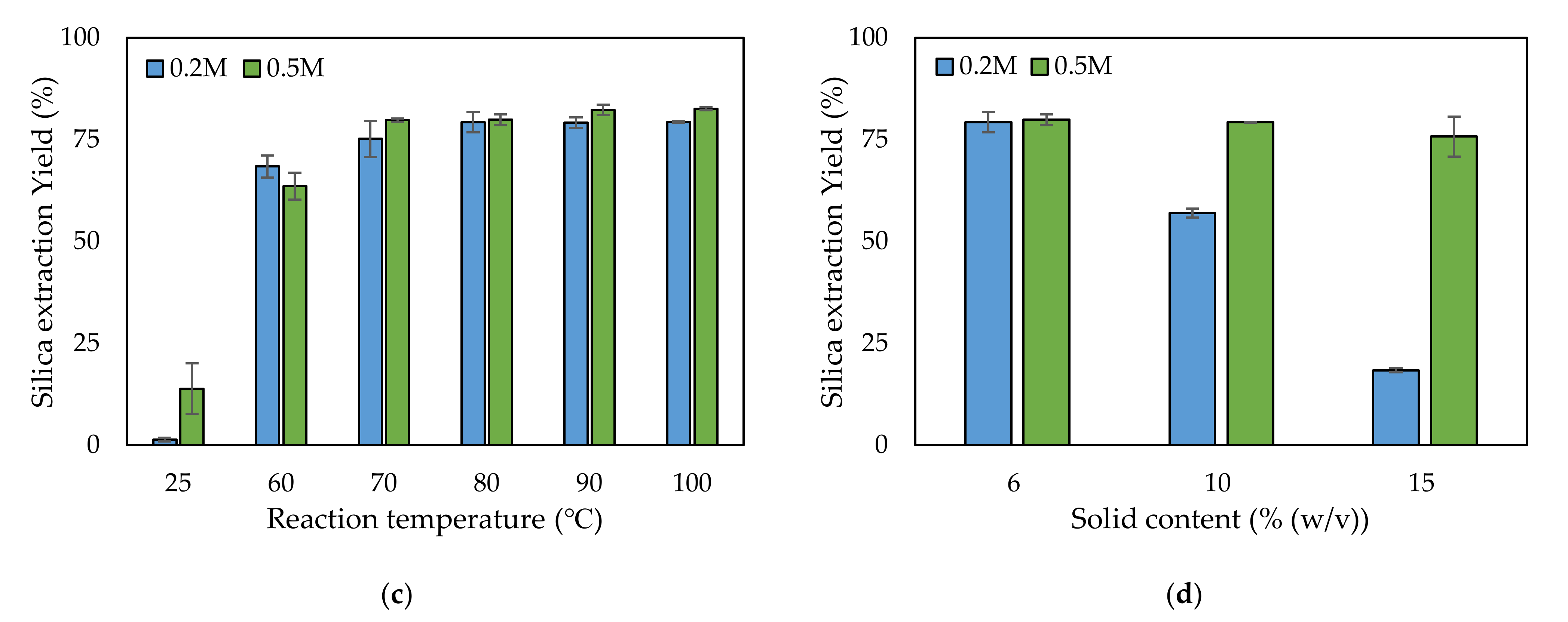
Figure 2.
Comparison of the silica extraction yield depending on (a) the type of alkaline solution, (b) the alkaline leaching reaction time, (c) the temperature, and (d) the solid content.
When using NaOH, similar silica extraction yields were found for 0.2 and 0.5 M—79.3% and 79.9%, respectively. Therefore, both concentrations were tested for optimizing the reaction time, temperature, and solid content. Under the conditions of 80 °C reaction temperature and 6% (w/v) solid content, five reaction times—1, 2, 3, 4, and 5 h—were investigated to determine the optimum reaction time. As the reaction time increased, the silica yield increased up to 3 h (Figure 2b). At reaction times over 3 h, the yield did not increase further (Figure 2b). At 3 h reaction time, no significant difference in yield was observed between use of 0.2 and 0.5 M concentrations. The optimum reaction temperature was determined among six chosen temperatures. At the temperature of 25 °C, only a small quantity of silica leached from the rice husk into the NaOH solution: 1.4% and 14% for 0.2 and 0.5 M, respectively (Figure 2c). At 60 °C, the silica yields increased to 68.4% and 63.6% for 0.2 and 0.5 M, respectively. Over 70 °C, the silica yield did not significantly increase, even when the reaction temperature was increased. The yield over 70 °C was approximately 80%. In the reaction temperature tests, there was no significant difference between 0.2 and 0.5 M. The solid content is related to reaction volume, which determines the reactor size. As the solid content increased, the silica yield decreased because of a lack of NaOH compared to Si (Figure 2d). Typically, there was tendency for a higher decrease in silica yield for 0.2 M compared with 0.5 M. The highest silica yield was found at 6% (w/v): 79.3% and 79.9% for 0.2 and 0.5 M, respectively.
3.2. Preparation of Rice Husk Slurry for Continuous Process
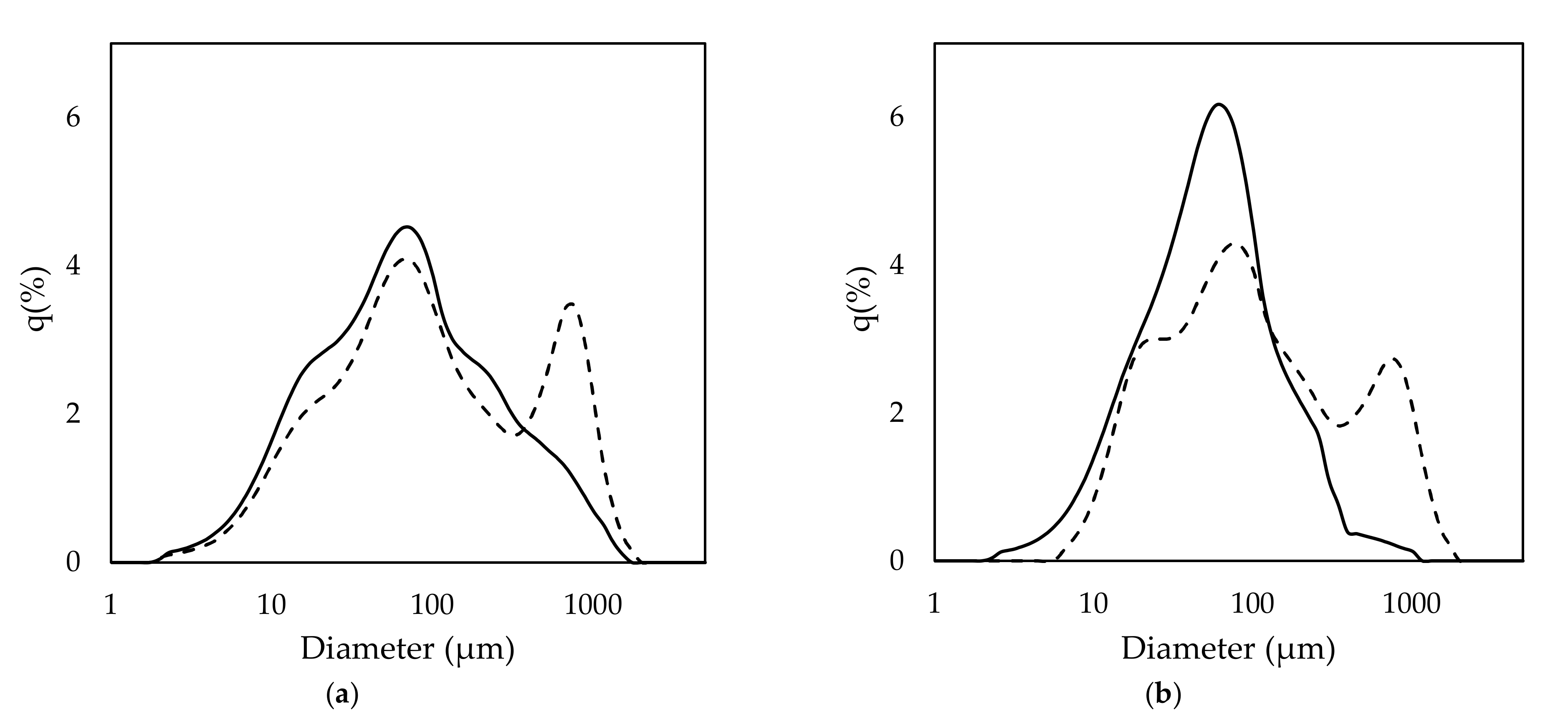
For application in a continuous process, rice husk should be continuously supplied to a reactor. Rice husk has a very low density and is usually conveyed by a pneumatic conveying system. In this study, rice husk slurry was prepared to easily convey the sample by a fluid pump. To prepare the rice husk slurry, rice husk was pulverized in NaOH solution by attrition ball milling. The rice husk had a diameter of approximately 6–7 mm before milling. After ball milling, the size of rice husk was drastically reduced, the extent of which was mainly related to milling time. However, the alkaline concentration also slightly affected the size reduction. The mean diameter of the rice husk particles was 228.9 and 139.0 µm for 20 and 30 min of milling, respectively, when it was treated with 0.2 M NaOH. When 0.5 M NaOH was used, the mean diameter was 218 and 78.1 µm for 20 and 30 min of milling, respectively. The size distribution of the rice husk after 20 min of milling showed a bimodal curve, with peaks being observed at approximately 77 and 777 µm (Figure 3). However, the peak around 777 µm reduced and shifted to 77 µm as the milling time was increased to 30 min. This result indicates that the size of rice husk particles became more homogenous as the milling time increased. The increase of milling time effectively reduced the portion of larger particle sizes. The concentration of NaOH also affected particle size distribution, but not as much as milling time. When comparing 0.2 and 0.5 M NaOH with 20 min milling time, 0.5 M NaOH showed a lower peak on 777 µm and a higher shoulder on 23 µm when compared to 0.2 M NaOH. In the 30 min milling condition, both samples of 0.2 and 0.5 M NaOH showed a monomodal curve. However, the graph of 0.5 M NaOH treatment showed a higher peak than 0.2 M NaOH. This result indicates that the size distribution of the rice husk particles was affected mainly by milling time and partially by NaOH concentration.
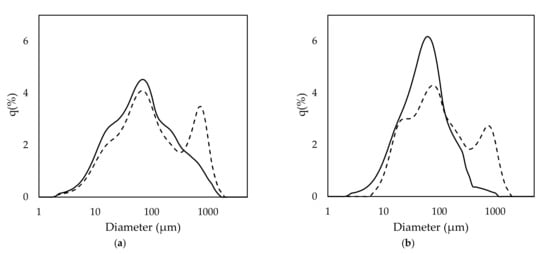
Figure 3.
Size distribution of the pretreated rice husk under the conditions of (a) 0.2 M NaOH and (b) 0.5 M NaOH. Dash and line indicate 20 and 30 min milling, respectively.
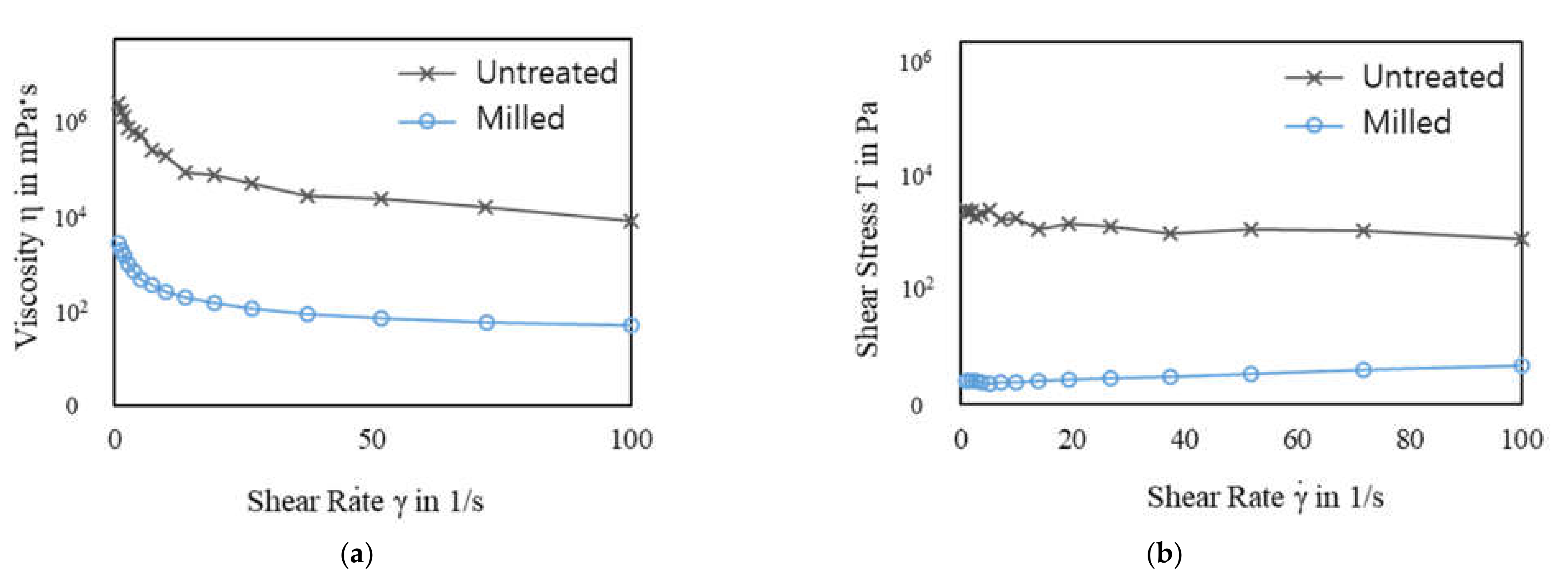
Figure 4 shows the rheological properties of the pulverized rice husk slurry with a 6% (w/v) solid content. The viscosity of the slurry solutions decreased as the shear rates increased. The viscosities of all samples decreased as the shear rate increased, indicating shear thinning. The untreated sample showed higher shear stress and viscosity than the treated samples (Figure 4). The shear stress and viscosity of a slurry are closely related to the particle size [24,25,26]. In general, the shear stress and viscosity of the slurry increased as the particle size increased, especially at low shear rates. In this study, the untreated slurry contained 6–7 mm rice husk particles. The rice husk particles were soaked with alkaline solution, meaning the larger particles could have been heavier than the smaller particles because of soaking up more of the alkaline solution and, thus, needed stronger force to be moved. Therefore, the untreated rice husk slurry showed higher shear stress and viscosity through the range of shear rate (Figure 4). As the particle size decreased, the shear stress and viscosity reduced. In Figure 4, the ball-milled samples show drastically reduced shear stress and viscosity. This indicates that the ball-milled rice husk slurries needed less force to be moved than the untreated slurry. The ball mill used in this study improved the fluidity of the rice husk slurry and made conveying rice husk easy.
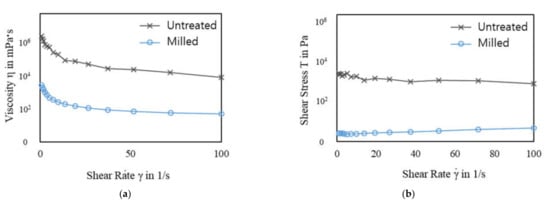
Figure 4.
Rheological properties of the rice husk slurry before/after ball milling and shown as a function of shear rate: (a) viscosity and (b) shear stress. The ball-milled rice husk slurry was prepared by ball milling for 20 min with 0.2 M NaOH.
3.3. Continuous Silica Extraction Process
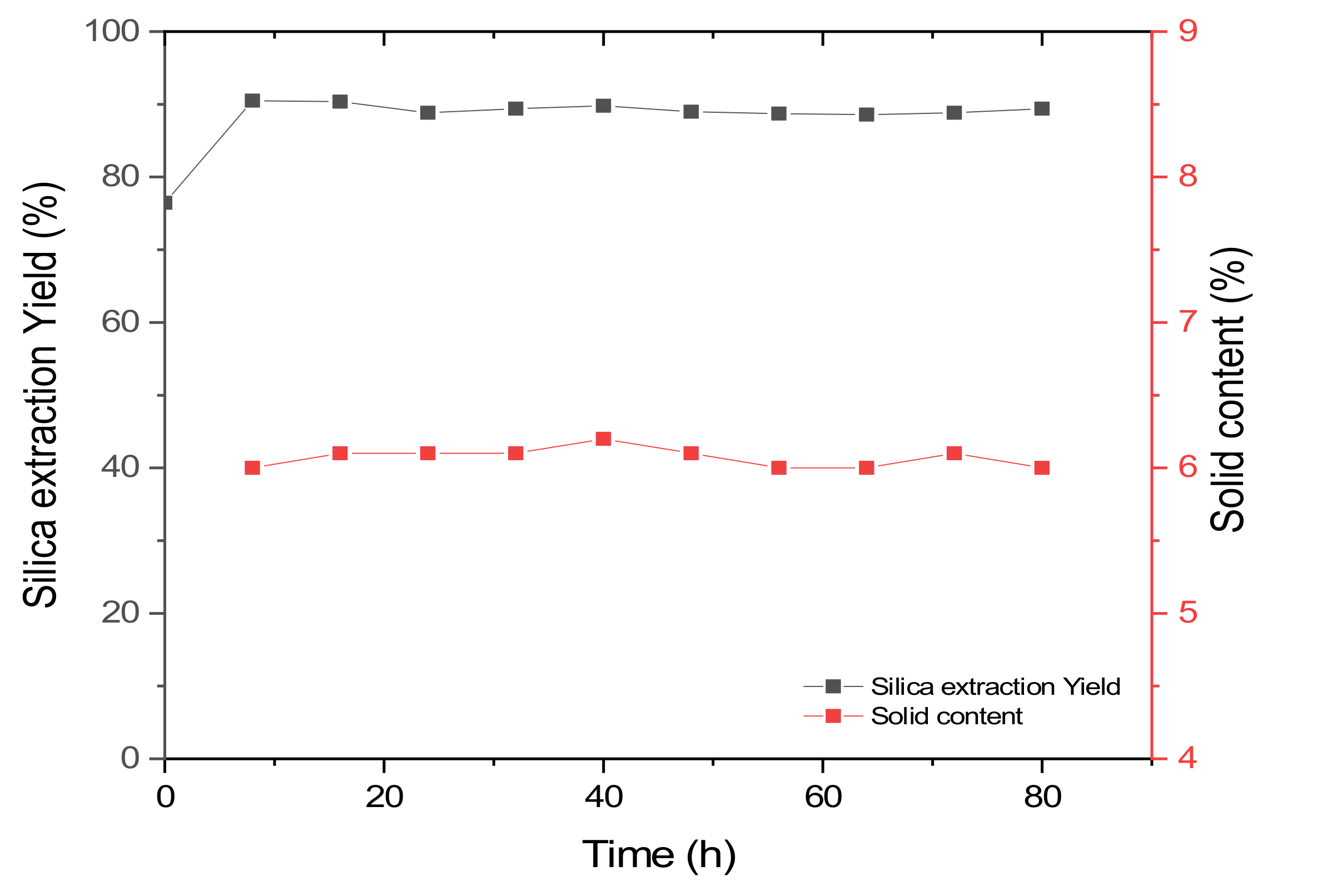
The continuous silica extraction process was performed using conditions based on the results of the batch experiments. The milled rice husk slurry with 0.2 M NaOH solution was stored in a reservoir and continuously supplied to a reactor. For stable operation in a continuous process, the solid content should be steadily supplied to the reactor because the quantity of raw material for silica should be constant during the process. Initially, a separate set of experiments was performed to measure the solid content. At every 8 h, samples were collected from the outlet of the reservoir. The first sample, obtained 8 h after starting the continuous process, showed a 6% (w/v) solid content. During the period of the continuous process, the solid content was steady, approximately 6% (w/v) (Figure 5). As previously mentioned, the ball mill used in this study improved the fluidity and enabled a steady supply of the rice husk slurry.
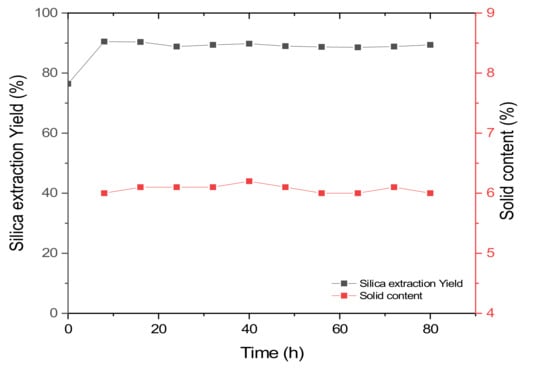
Figure 5.
Silica extraction yield and solid content in the continuous process depending on process time.
Initially, the rice husk slurry was reacted for 3 h before starting the continuous process. After the reaction, the yield of the silica extraction was 77% (Figure 5). After starting the continuous supply of the rice husk slurry, the silica yield increased to 90%. This could be due to extended reaction time for the initially filled sample. After starting the continuous process, the silica extraction yield slightly decreased, but reached a steady state after 24 h. After reaching a steady state, the silica yield was constant, indicating that the process was stable. In this study, the continuous silica extraction was performed for 80 h and the process was stable during the operation, with 89% silica yield.
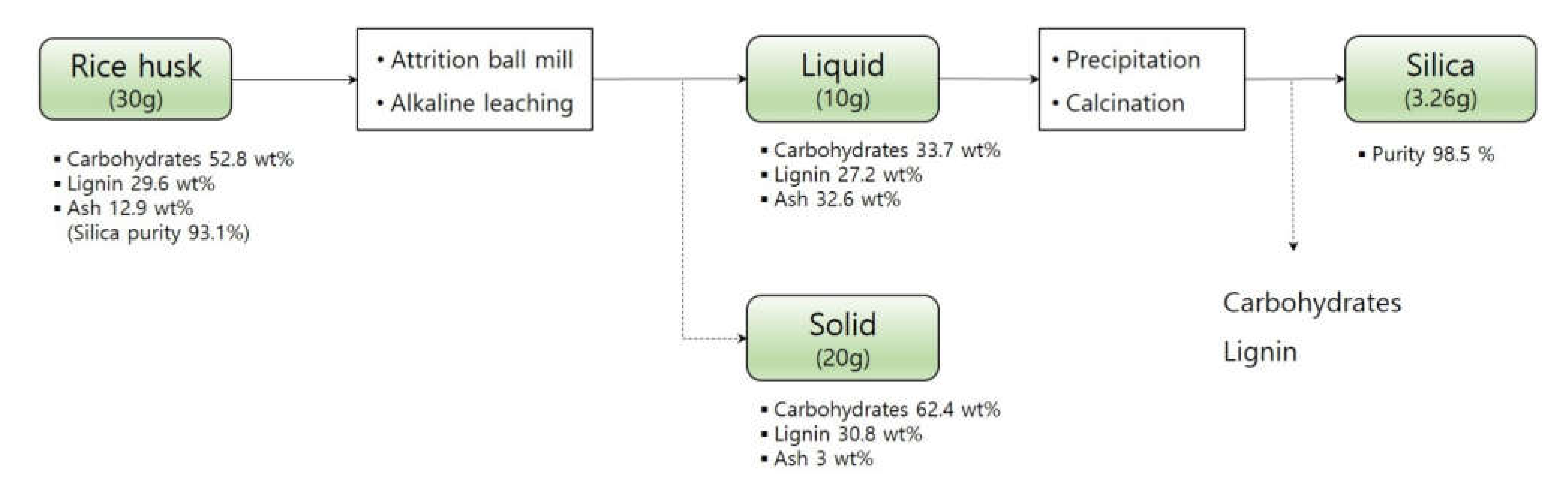
Usually, the alkaline pretreatment was performed for delignification. Therefore, the continuous process used in this study could remove carbohydrates and lignin. The compositions of rice husk before the NaOH leaching were 52.8 wt% carbohydrates, 29.6 wt% lignin, and 12.9 wt% ash (Figure 6). The continuous silica extraction reaction used in this study leached 21.3% carbohydrates, 30.6% lignin, and 84.2% ash into the NaOH solution. After precipitation and calcination, about 89% of silica in the rice husk was recovered. This two-stage alkaline leach strategy is capable to produce a de-ashed rice husk slurry which is more suitable for further biorefinery, a silica-rich by-product for high-value applications, and an extracted carbohydrates/lignin mixture for further valorization.
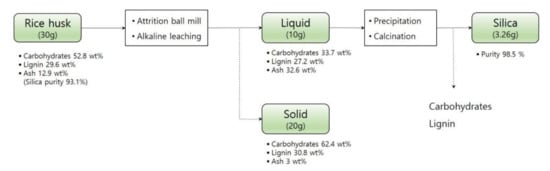
Figure 6.
Mass balance of the continuous silica extraction process used in this study.
3.4. Characterization of the Silica Obtained from the Continuous Extraction Process
The extracted silica from the continuous process was characterized after precipitation. Originally, the purity of silica in the rice husk ash was only 93.1% (Table 1). The rice husk ash contained high impurities such as CaO, MaO, and K2O. After alkaline leaching, the silica purity increased to 98.5%. The main impurity was Na2O, which increased from 0.08% to 0.96% after the NaOH leaching. Considering the increase in Na2O content after the NaOH leaching, it is possible that the sodium in the NaOH solution was precipitated and that it could be reduced by applying stringent washing steps.

Table 1.
Inorganic composition of the raw material ash and the extracted ash.
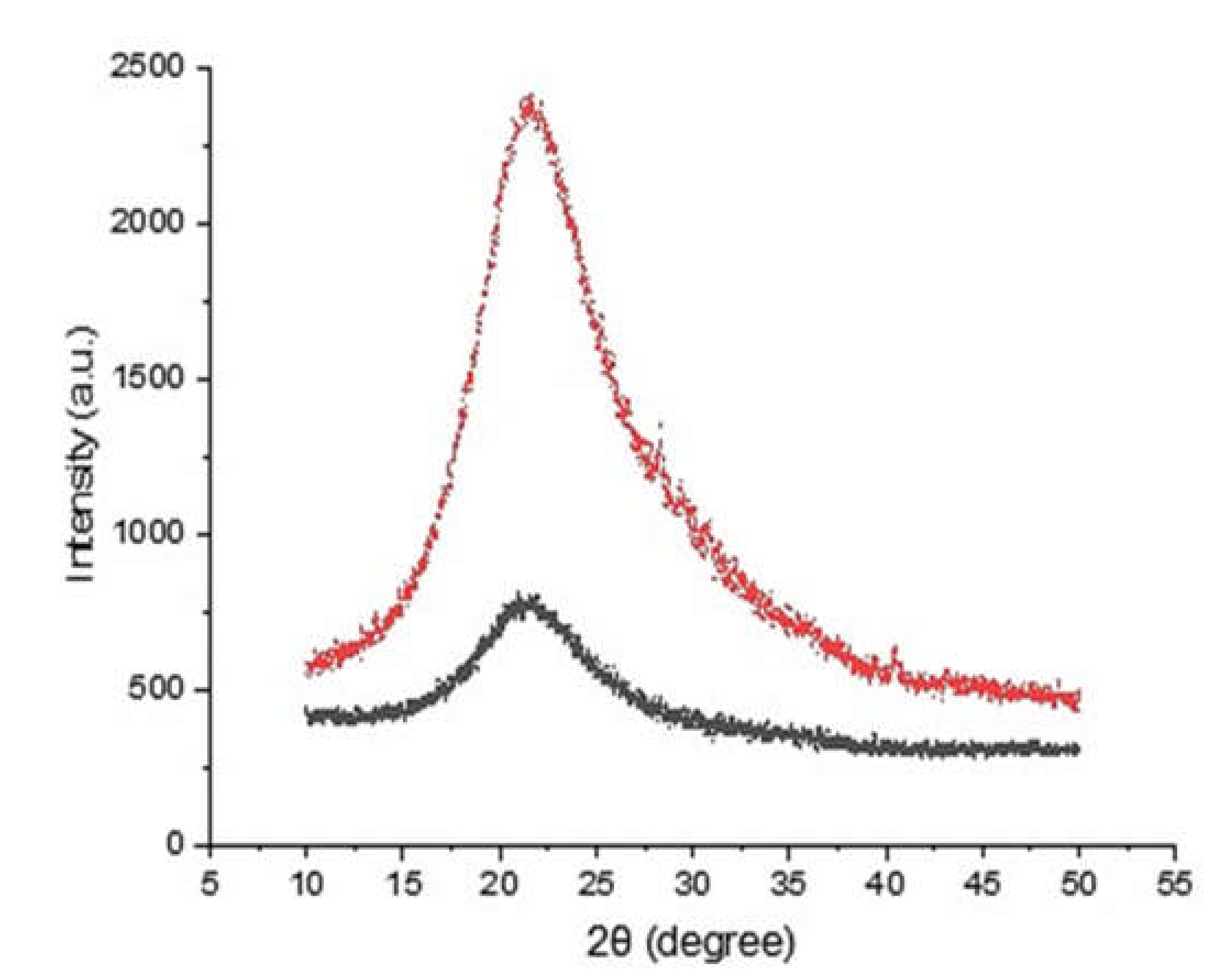
The crystallinity of the obtained silica was investigated using XRD. The XRD patterns of the silica samples obtained from both the rice husk ash and the continuous processes showed a broad diffraction near to 20°, which is typical for amorphous silica (Figure 7). In the XRD pattern, no crystalline structure of silica was found, and continuous alkaline leaching did not cause any crystallinity changes. The phase of silica is determined by the combustion temperature [27]. Over certain temperature, the phase transformation starts but the crystallization temperature varied depending on the composition of rice husk ash. In this study, the calcination was performed at 900 °C for 6 h to remove residual moisture and volatile compounds. The calcination did not cause any crystallinity changes of rice husk silica.
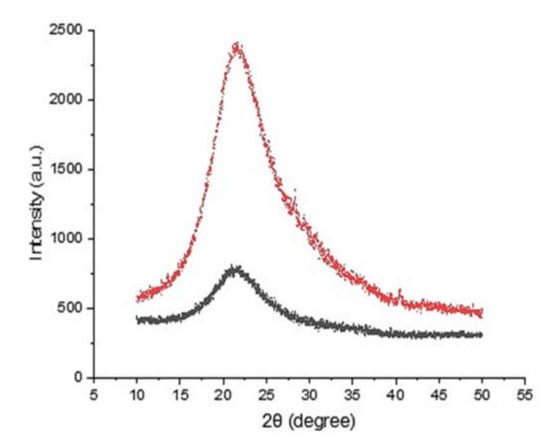
Figure 7.
X-ray patterns of silica samples obtained from rice husk ash (black) and the continuous process (red).
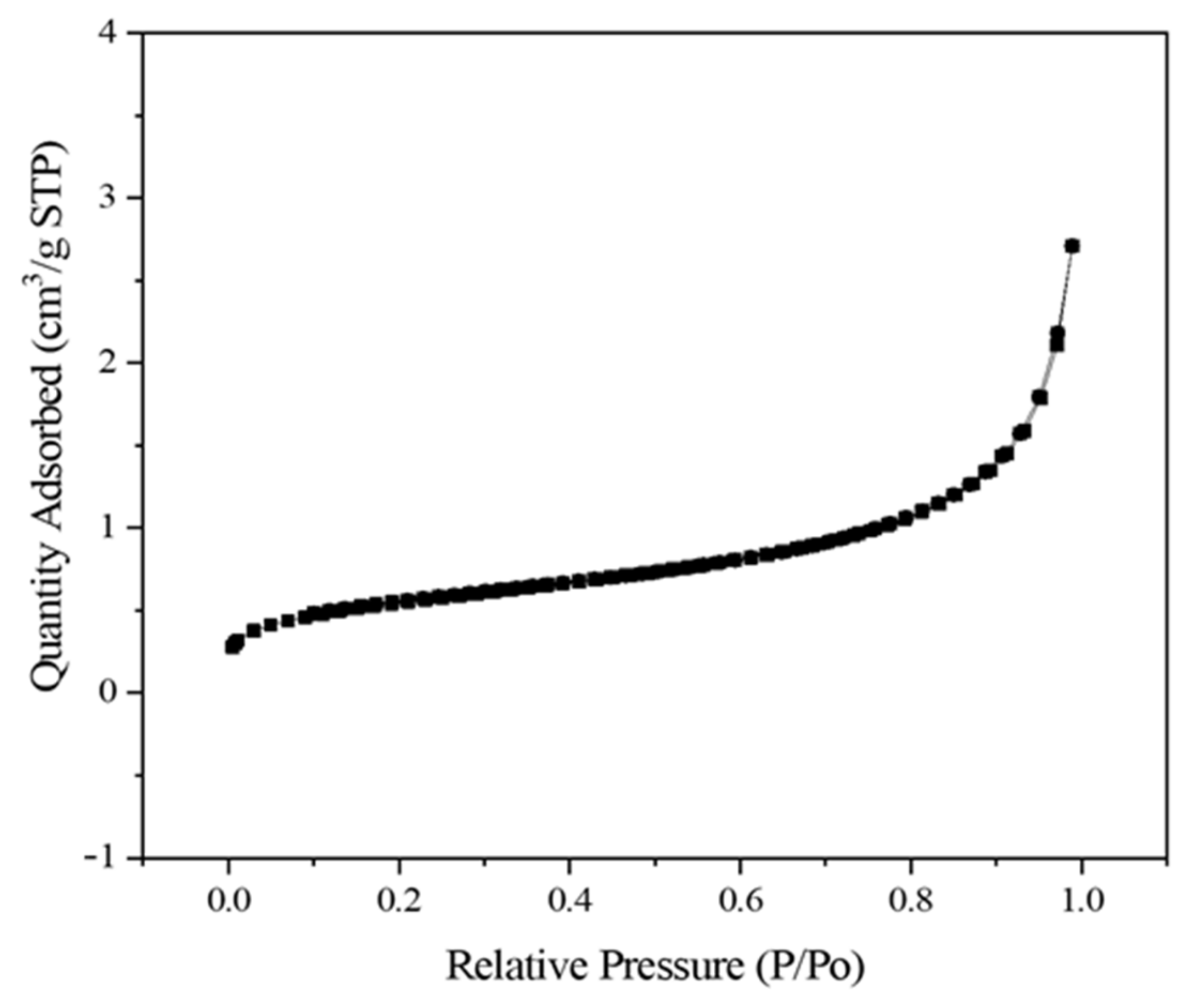
The surface area and pore volume of the silica obtained from the continuous process were 1.973 m2/g and 0.004 cm3/g, respectively (Figure 8). A previous study reported that the presence of metal impurities, such as Na and K, causes surface melting and agglomeration in the particles during combustion [28]. The surface melting and agglomeration led to reduced surface area and pore volume. In this study, the content of Na2O in the silica obtained increased due to the NaOH leaching and it could be the reason for low surface area and pore volume. This study did not control the structure of silica. Therefore, an additional process is required to obtain a highly specific surface area or well-defined nanostructures.
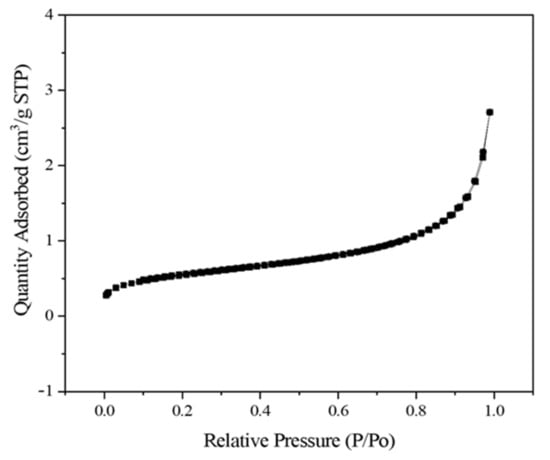
Figure 8.
N2 physisorption isotherms of silica obtained from the continuous process.
4. Conclusions
A process for two-stage continuous silica extraction from rice husk was successfully developed using alkaline leaching and attrition ball mill methods. The alkaline leaching conditions obtained in batch experiments were employed in the continuous process. The attrition ball mill treatment was used to obtain a rice husk slurry, which improved fluidity of the sample. By applying alkaline leaching conditions and the ball mill-treated rice husk slurry, continuous silica extraction from rice husk was stably operated, with 89% silica yield and 6% (w/v) solid content for 80 h. An improvement in silica purity was obtained from the continuous process, which increased to 98.5% when compared to rice husk ash. The continuous process did not change the crystallinity or surface properties of the silica.
The continuous extraction process developed in this study would be beneficial for product uniformity and process capacity. It is very easy to operate once the system has been set up. Therefore, we expect that this method can be used in the field for the mass production of rice husk silica.
Author Contributions
Conceptualization, J.H.L. and B.-I.S.; methodology, J.H.L. and J.C.; validation, E.T.H. and B.-I.S.; formal analysis, J.H.L. and J.C.; investigation, J.Y.P., S.Y.P. and Y.M.G.; data curation, J.Y.P.; writing—original draft preparation, J.Y.P. and J.H.L.; writing—review and editing, J.H.L. and J.C.; project administration, J.H.L.; funding acquisition, J.H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry (IPET) through the Agro and Livestock Products Safety Flow Management Technology Development Program, funded by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (319109-02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 12 November 2020).
- Esa, N.M.; Ling, T.B.; Peng, L.S. By-products of rice processing: An overview of health benefits and applications. J. Rice Res. 2013, 1, 107. [Google Scholar] [CrossRef] [Green Version]
- Pode, R. Potential applications of rice husk ash waste from rice husk biomass power plant. Renew. Sustain. Energy Rev. 2016, 53, 1468–1485. [Google Scholar] [CrossRef]
- Babaso, P.N.; Sharanagouda, H. Rice Husk and Its Applications: Review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1144–1156. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, J.H.; Lee, J.-W.; Lee, H.-S.; Chang, J.H.; Sang, B.-I. Preparation of high purity silica originated from rice husks by chemically removing metallic impurities. J. Ind. Eng. Chem. 2017, 50, 79–85. [Google Scholar] [CrossRef]
- Hossain, S.S.; Mathur, L.; Roy, P. Rice husk/rice husk ash as an alternative source of silica in ceramics: A review. J. Asian Ceram. Soc. 2018, 6, 299–313. [Google Scholar] [CrossRef]
- Mason, S.; Young, S.; Araga, M.; Butler, A.; Lucas, R.; Milleman, J.L.; Milleman, K.R. Stain control with two experimental dentin hypersensitivity toothpastes containing spherical silica: A randomised, early-phase development study. BDJ Open 2019, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Caldona, E.B.; Sibaen, J.W.; Tactay, C.B.; Mendiola, S.L.D.; Abance, C.B.; Añes, M.P.; Serrano, F.D.D.; De Guzman, M.M.S. Preparation of spray-coated surfaces from green-formulated superhydrophobic coatings. SN Appl. Sci. 2019, 1, 1657. [Google Scholar] [CrossRef] [Green Version]
- Mozumder, M.S.; Mourad, A.-H.I.; Pervez, H.; Surkatti, R. Recent developments in multifunctional coatings for solar panel applications: A review. Sol. Energy Mater. Sol. Cells 2019, 189, 75–102. [Google Scholar] [CrossRef]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef]
- Foletto, E.L.; Gratieri, E.; De Oliveira, L.H.; Jahn, S.L. Conversion of rice hull ash into soluble sodium silica. Mater. Res. 2006, 9, 335–338. [Google Scholar] [CrossRef] [Green Version]
- Rojas, D.F.H.; Gomez, P.P.; Rivera, A.R. Synthesis and characterisation of submicron silica particles from rice husk. Green Mater. 2018, 6, 15–22. [Google Scholar] [CrossRef] [Green Version]
- De Souza, M.; Magalhães, W.; Persegil, M. Silica Derived from Burned Rice Hulls. Mater. Res. 2002, 5, 467–474. [Google Scholar] [CrossRef]
- Chun, J.; Gu, Y.M.; Hwang, J.; Oh, K.K.; Lee, J.H. Synthesis of ordered mesoporous silica with various pore structures using high-purity silica extracted from rice husk. J. Ind. Eng. Chem. 2020, 81, 135–143. [Google Scholar] [CrossRef]
- Costa, J.A.; Paranhos, C.M. Systematic evaluation of amorphous silica production from rice husk ashes. J. Clean. Prod. 2018, 192, 688–697. [Google Scholar] [CrossRef]
- Song, S.; Cho, H.-B.; Kim, H.T. Surfactant-free synthesis of high surface area silica nanoparticles derived from rice husks by employing the Taguchi approach. J. Ind. Eng. Chem. 2018, 61, 281–287. [Google Scholar] [CrossRef]
- Zulkifli, N.S.C.; Ab Rahman, I.; Mohamad, D.; Husein, A. A green sol–gel route for the synthesis of structurally controlled silica particles from rice husk for dental composite filler. Ceram. Int. 2013, 39, 4559–4567. [Google Scholar] [CrossRef]
- Singh, B. Rice husk ash. In Waste and Supplementary Cementitious Materials in Concrete; Woodhead Publishing: Cambridge, UK, 2018; pp. 417–460. [Google Scholar]
- He, C.; Chen, X.; Wang, J.; Ni, H.; Xu, Y.; Zhou, H.; Xiong, Y.; Shen, X. Conveying characteristics and resistance characteris-tics in dense phase pneumatic conveying of rice husk and blendings of rice husk and coal at high pressure. Powder Technol. 2012, 227, 51–60. [Google Scholar] [CrossRef]
- Stegmann, P.; Londo, M.; Junginger, M. The circular bioeconomy: Its elements and role in European bioeconomy clusters. Resour. Conserv. Recycl. X 2020, 6, 100029. [Google Scholar] [CrossRef]
- Vea, E.B.; Romeo, D.; Thomsen, M. Biowaste valorization in a future circular bioeconomy. Procedia CIRP 2018, 69, 591–596. [Google Scholar] [CrossRef]
- Gu, Y.M.; Byun, H.R.; Kim, Y.-H.; Park, D.-Y.; Lee, J.H. Assessing the potential of facile biofuel production from corn stover using attrition mill treatment. Water-Energy Nexus 2019, 2, 46–49. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- He, M.; Wang, Y.; Forssberg, E. Slurry rheology in wet ultrafine grinding of industrial minerals: A review. Powder Technol. 2004, 147, 94–112. [Google Scholar] [CrossRef]
- Senapati, P.K.; Panda, D.; Parida, A. Predicting Viscosity of Limestone–Water Slurry. J. Miner. Mater. Charact. Eng. 2009, 8, 203–221. [Google Scholar] [CrossRef]
- Tangsathitkulchai, C.; Austin, L. Rheology of concentrated slurries of particles of natural size distribution produced by grinding. Powder Technol. 1988, 56, 293–299. [Google Scholar] [CrossRef]
- Chun, J.; Lee, J.H. Recent Progress on the Development of Engineered Silica Particles Derived from Rice Husk. Sustainability 2020, 12, 10683. [Google Scholar] [CrossRef]
- Zareihassangheshlaghi, A.; Dizaji, H.B.; Zeng, T.; Huth, P.; Ruf, T.; Denecke, R.; Enke, D. Behavior of Metal Impurities on Surface and Bulk of Biogenic Silica from Rice Husk Combustion and the Impact on Ash-Melting Tendency. ACS Sustain. Chem. Eng. 2020, 8, 10369–10379. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).