Impacts of Space Restriction on the Microstructure of Calcium Silicate Hydrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Analytical Methods
3. Results
3.1. Raman
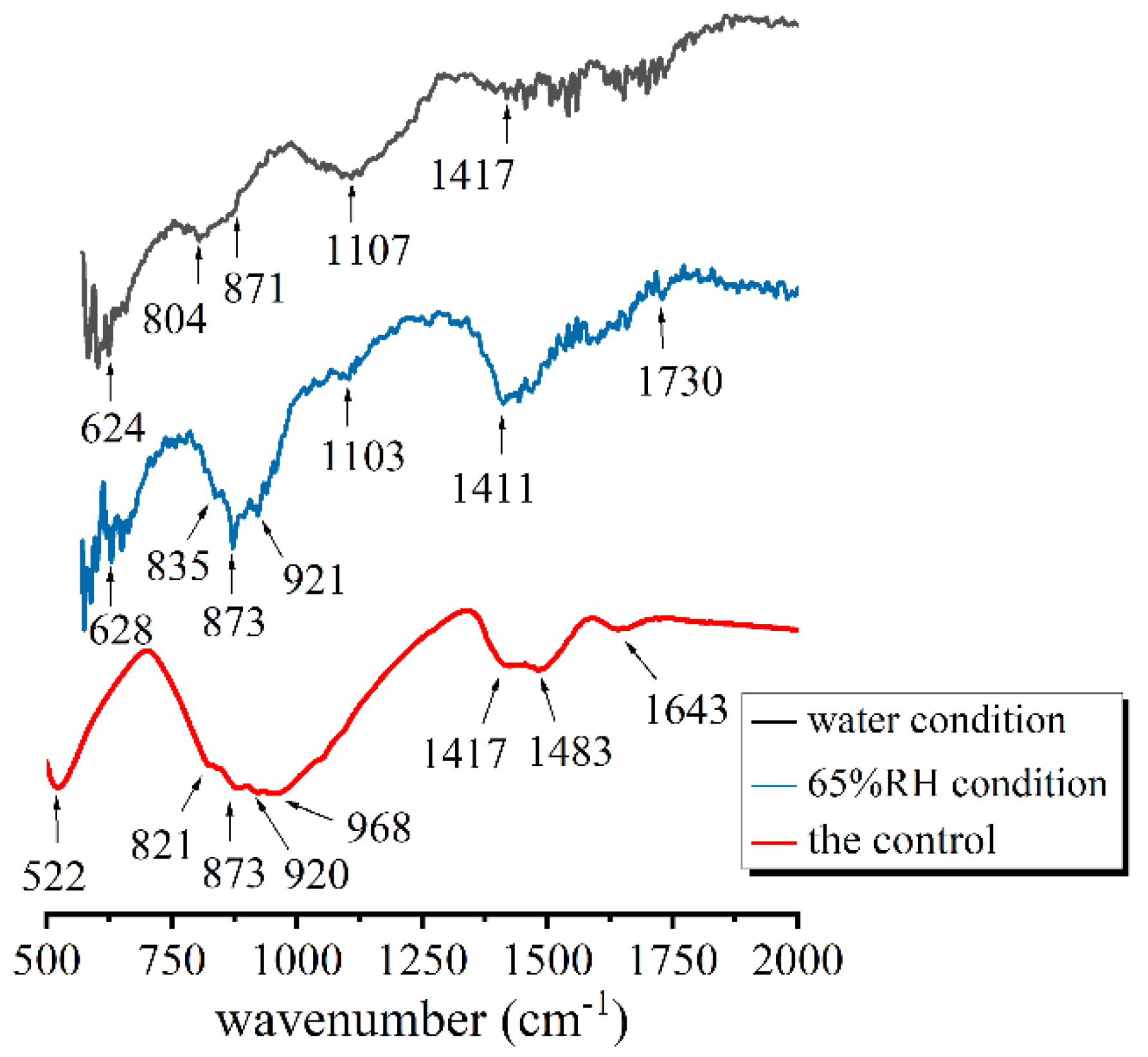
3.2. FT-IR
3.3. SEM/EDS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, A.J.; Thomas, J.J.; Jennings, H.M. Composition and density of nanoscale calcium-silicate-hydrate in cement. Nat. Mater. 2007, 6, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Scrivener, K.; Ouzia, A.; Juilland, P.; Mohamed, A.K. Advances in understanding cement hydration mechanisms. Cem. Concr. Res. 2019, 124, 105823. [Google Scholar] [CrossRef]
- Sato, M.; Umemura, Y.; Koizumi, K. Effect of water-binder ratio on silicate structure and hydration of silicafume cement. Cem. Sci. Concr. Technol. 2011, 65, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Saeki, T.; Sasaki, K.; Suda, Y. Fundamental study on density of C-S-H. Cem. Sci. Concr. Technol. 2009, 63, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Gallucci, E.; Zhang, X.; Scrivener, K.L. Effect of temperature on the microstructure of calcium silicate hydrate (C-S-H). Cem. Concr. Res. 2013, 53, 185–195. [Google Scholar] [CrossRef]
- Okada, Y.; Ishida, H.; Sasaki, K.; Young, J.; Mitsuda, T. Characterization of C-S-H from highly reactive β-dicalcium silicate prepared from hillebrandite. J. Am. Ceram. Soc. 1994, 77, 1313–1318. [Google Scholar] [CrossRef]
- Martínez-Ramírez, S.; Frías, M. The effect of curing temperature on white cement hydration. Constr. Build. Mater. 2009, 23, 1344–1348. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Tang, C.; Li, H.; Wu, K.; Zhang, Y.; Yang, Z. Hydration characteristics assessment of a binary calcium sulfoaluminate-anhydrite cement related with environment temperature. J. Therm. Anal. Calorim. 2021. [Google Scholar] [CrossRef]
- Narattha, C.; Chaipanich, A. Effect of curing time on the hydration and material properties of cold-bonded high-calcium fly ash–Portland cement lightweight aggregate. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Wu, K.; Han, H.; Xu, L.; Gao, Y.; Yang, Z.; Jiang, Z.; De Schutter, G. The improvement of freezing–thawing resistance of concrete by cellulose/polyvinyl alcohol hydrogel. Constr. Build. Mater. 2021, 291, 123274. [Google Scholar] [CrossRef]
- Schönlein, M.; Plank, J. A TEM study on the very early crystallization of C-S-H in the presence of polycarboxylate superplasticizers: Transformation from initial C-S-H globules to nanofoils. Cem. Concr. Res. 2018, 106, 33–39. [Google Scholar] [CrossRef]
- Bazzoni, A. Study of Early Hydration Mechanisms of Cement by Means of Electron Microscopy. Ph.D. Thesis, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2014. [Google Scholar]
- Ouzia, A.R.C.W.C. Modeling the Kinetics of the Main Peak and Later Age of Alite Hydration. Ph.D. Thesis, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2019. [Google Scholar]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Berodier, E.; Scrivener, K. Evolution of pore structure in blended systems. Cem. Concr. Res. 2015, 73, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Ou, Y.; Hecker, A.; Rößler, C.; Ludwig, H.M.; Yang, Z.; Wu, K. State of water in calcium sulfoaluminate cement paste modified by hydroxyethyl methyl cellulose ether. J. Build. Eng. 2021, 102894. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Wenzel, O.; Schwotzer, M.; Müller, E.; Chakravadhanula, V.S.K.; Scherer, T.; Gerdes, A. Investigating the pore structure of the calcium silicate hydrate phase. Mater. Charact. 2017, 133, 133–137. [Google Scholar] [CrossRef]
- Li, H.; Xue, Z.; Liang, G.; Wu, K.; Dong, B.; Wang, W. Effect of C-S-Hs-PCE and sodium sulfate on the hydration kinetics and mechanical properties of cement paste. Constr. Build. Mater. 2021, 266, 121096. [Google Scholar] [CrossRef]
- Jennings, H.M. A model for the microstructure of calcium silicate hydrate in cement paste. Cem. Concr. Res. 2000, 30, 101–116. [Google Scholar] [CrossRef]
- Jennings, H.M. Refinements to colloid model of C-S-H in cement: CM-II. Cem. Concr. Res. 2008, 38, 275–289. [Google Scholar] [CrossRef]
- S˘milauer, V.; Bittnar, Z.k. Microstructure-based micromechanical prediction of elastic properties in hydrating cement paste. Cem. Concr. Res. 2006, 36, 1708–1718. [Google Scholar] [CrossRef]
- Königsberger, M.; Hellmich, C.; Pichler, B. Densification of C-S-H is mainly driven by available precipitation space, as quantified through an analytical cement hydration model based on NMR data. Cem. Concr. Res. 2016, 88, 170–183. [Google Scholar] [CrossRef]
- Constantinides, G.; Ulm, F.-J. The effect of two types of C-S-H on the elasticity of cement-based materials: Results from nanoindentation and micromechanical modeling. Cem. Concr. Res. 2004, 34, 67–80. [Google Scholar] [CrossRef]
- Farhadi, N.; Peyvandi, A.; Holmes, D.; Soroushian, P.; Balachandra, A.M. Effects of Graphite Nanoplatelets on the Structure of Cementitious Materials. Iran. J. Sci. Technol. Trans. Civ. Eng. 2019, 43, 403–411. [Google Scholar] [CrossRef]
- Richardson, I.G. The nature of the hydration products in hardened cement pastes. Cem. Concr. Compos. 2000, 22, 97–113. [Google Scholar] [CrossRef]
- Kirkpatrick, R.J.; Yarger, J.L.; McMillan, P.F.; Ping, Y.; Cong, X. Raman spectroscopy of C-S-H, tobermorite, and jennite. Adv. Cem. Based Mater. 1997, 5, 93–99. [Google Scholar] [CrossRef]
- Cong, X.; Kirkpatrick, R.J. 29Si MAS NMR study of the structure of calcium silicate hydrate. Adv. Cem. Based Mater. 1996, 3, 144–156. [Google Scholar] [CrossRef]
- Rodriguez, E.T.; Richardson, I.G.; Black, L.; Boehm-Courjault, E.; Nonat, A.; Skibsted, J. Composition, silicate anion structure and morphology of calcium silicate hydrates (C-S-H) synthesised by silica-lime reaction and by controlled hydration of tricalcium silicate (C3S). Adv. Appl. Ceram. 2015, 114, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.J.; Jennings, H.M. A colloidal interpretation of chemical aging of the C-S-H gel and its effects on the properties of cement paste. Cem. Concr. Res. 2006, 36, 30–38. [Google Scholar] [CrossRef]
- Jennings, H.M. Colloid model of C−S−H and implications to the problem of creep and shrinkage. Mater. Struct. 2004, 37, 59–70. [Google Scholar] [CrossRef]
- Ortaboy, S.; Li, J.; Geng, G.; Myers, R.J.; Monteiro, P.J.M.; Maboudian, R.; Carraro, C. Effects of CO2 and temperature on the structure and chemistry of C–(A–)S–H investigated by Raman spectroscopy. RSC Adv. 2017, 7, 48925–48933. [Google Scholar] [CrossRef] [Green Version]
- Potgieter-Vermaak, S.S.; Potgieter, J.H.; Belleil, M.; Deweerdt, F.; Grieken, R.V. The application of Raman spectrometry to the investigation of cement: Part II: A micro-Raman study of OPC, slag and fly ash. Cem. Concr. Res. 2006, 36, 663–670. [Google Scholar] [CrossRef]
- Bai, Y.; Guo, X.-j.; Li, Y.-z.; Huang, T. Experimental and visual research on the microbial induced carbonate precipitation by Pseudomonas aeruginosa. Amb Express 2017, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Podborodnikov, I.; Shatskiy, A.; Arefiev, A.; Rashchenko, S.; Chanyshev, A.; Litasov, K. The system Na2CO3–CaCO3 at 3 GPa. Phys. Chem. Miner. 2018, 45, 773–787. [Google Scholar] [CrossRef]
- Liu, F.; Sun, Z.; Qi, C. Raman spectroscopy study on the hydration behaviors of Portland cement pastes during setting. J. Mater. Civ. Eng. 2014, 27, 04014233. [Google Scholar] [CrossRef]
- Tarrida, M.; Madon, M.; Le Rolland, B.; Colombet, P. An in-situ Raman spectroscopy study of the hydration of tricalcium silicate. Adv. Cem. Based Mater. 1995, 2, 15–20. [Google Scholar] [CrossRef]
- Garbev, K.; Stemmermann, P.; Black, L.; Breen, C.; Yarwood, J.; Gasharova, B. Structural features of C-S-H(I) and its carbonation in air—A Raman spectroscopic study. Part I: Fresh phases. J. Am. Ceram. Soc. 2007, 90, 900–907. [Google Scholar] [CrossRef]
- Liu, F.J.; Sun, Z.H. Chemical mapping of cement pastes by using confocal Raman spectroscopy. Front. Struct. Civ. Eng. 2016, 10, 168–173. [Google Scholar] [CrossRef]
- del Bosque, I.F.S.; Martinez-Ramirez, S.; Blanco-Varela, M.T. FTIR study of the effect of temperature and nanosilica on the nanostructure of C-S-H gel formed by hydrating tricalcium silicate. Constr. Build. Mater. 2014, 52, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, H.K.; Anupama, A.V.; Kumar, R.; Panzi, M.E.; Matteppanavar, S.; Sherikar, B.N.; Sahoo, B. Observation of phase transformations in cement during hydration. Constr. Build. Mater. 2015, 101, 122–129. [Google Scholar] [CrossRef]
- Al-Maliki, F. Detection of Random Laser Action from Silica Xerogel Matrices Containing Rhodamine 610 Dye and Titanium Dioxide Nanoparticles. Adv. Mater. Phys. Chem. 2012, 2, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Ramalla, I.; Gupta, R.; Bansal, K. Effect on superhydrophobic surfaces on electrical porcelain insulator, improved technique at polluted areas for longer life and reliability. Int. J. Eng. Technol. 2015, 4, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Darmakkolla, S.R.; Tran, H.; Gupta, A.; Rananavare, S.B. A method to derivatize surface silanol groups to Si-alkyl groups in carbon-doped silicon oxides. RSC Adv. 2016, 6, 93219–93230. [Google Scholar] [CrossRef]
- Grill, A.; Neumayer, D.A. Structure of low dielectric constant to extreme low dielectric constant SiCOH films: Fourier transform infrared spectroscopy characterization. J. Appl. Phys. 2003, 94, 6697–6707. [Google Scholar] [CrossRef]
- Fernandez, L.; Alonso, C.; Hidalgo, A.; Andrade, C. The role of magnesium during the hydration of C3S and C-S-H formation. Scanning electron microscopy and mid-infrared studies. Adv. Cem. Res. 2005, 17, 9–21. [Google Scholar] [CrossRef]
- Bonnier, F.; Blasco, H.; Wasselet, C.; Brachet, G.; Respaud, R.; Carvalho, L.F.C.S.; Bertrand, D.; Baker, M.J.; Byrne, H.J.; Chourpa, I. Ultra-filtration of human serum for improved quantitative analysis of low molecular weight biomarkers using ATR-IR spectroscopy. Analyst 2017, 142, 1285–1298. [Google Scholar] [CrossRef] [Green Version]
- Das, I.; Mishra, M.K.; Medda, S.K.; De, G. Durable superhydrophobic ZnO-SiO2 films: A new approach to enhance the abrasion resistant property of trimethylsilyl functionalized SiO2 nanoparticles on glass. RSC Adv. 2014, 4, 54989–54997. [Google Scholar] [CrossRef]
- Kalkan, E.; Nadaroglu, H.; Celebi, N.; Tozsin, G. Removal of textile dye Reactive Black 5 from aqueous solution by adsorption on laccase-modified silica fume. Desalin. Water Treat. 2014, 52, 6122–6134. [Google Scholar] [CrossRef]
- He, Y.; Zhao, X.; Lu, L.; Struble, L.J.; Hu, S. Effect of C/S ratio on morphology and structure of hydrothermally synthesized calcium silicate hydrate. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 770–773. [Google Scholar] [CrossRef]
- He, Y.; Lu, L.; Struble, L.J.; Rapp, J.L.; Mondal, P.; Hu, S. Effect of calcium–silicon ratio on microstructure and nanostructure of calcium silicate hydrate synthesized by reaction of fumed silica and calcium oxide at room temperature. Mater. Struct. 2014, 47, 311–322. [Google Scholar] [CrossRef]
- Hu, Q.N.; Aboustait, M.; Kim, T.; Ley, M.T.; Hanan, J.C.; Bullard, J.; Winarski, R.; Rose, V. Direct three-dimensional observation of the microstructure and chemistry of C3S hydration. Cem. Concr. Res 2016, 88, 157–169. [Google Scholar] [CrossRef] [Green Version]
- García Carmona, J.; Gómez Morales, J.; Rodríguez Clemente, R. Rhombohedral–scalenohedral calcite transition produced by adjusting the solution electrical conductivity in the system Ca(OH)2–CO2–H2O. J. Colloid Interface Sci. 2003, 261, 434–440. [Google Scholar] [CrossRef]
- Masmoudi, R.; Kupwade-Patil, K.; Bumajdad, A.; Buyukozturk, O. In situ Raman studies on cement paste prepared with natural pozzolanic volcanic ash and Ordinary Portland Cement. Constr. Build. Mater. 2017, 148, 444–454. [Google Scholar] [CrossRef]
- Dariz, P.; Schmid, T. Ferruginous phases in 19th century lime and cement mortars: A Raman microspectroscopic study. Mater. Charact. 2017, 129, 9–17. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Lothenbach, B.; Mohsen, B.H. Late hydration kinetics: Indications from thermodynamic analysis of pore solution data. Cem. Concr. Res. 2020, 129, 105975. [Google Scholar] [CrossRef]
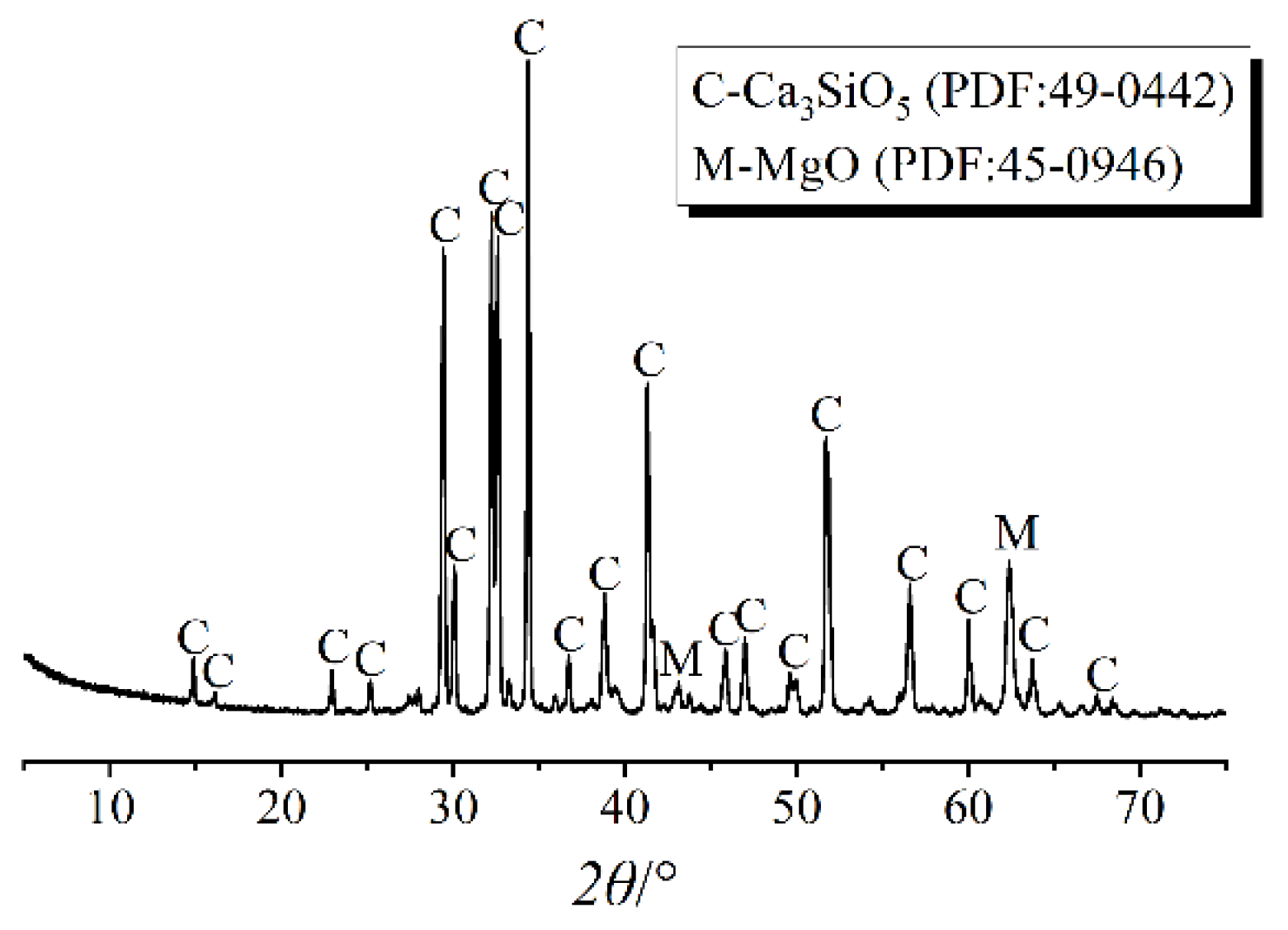

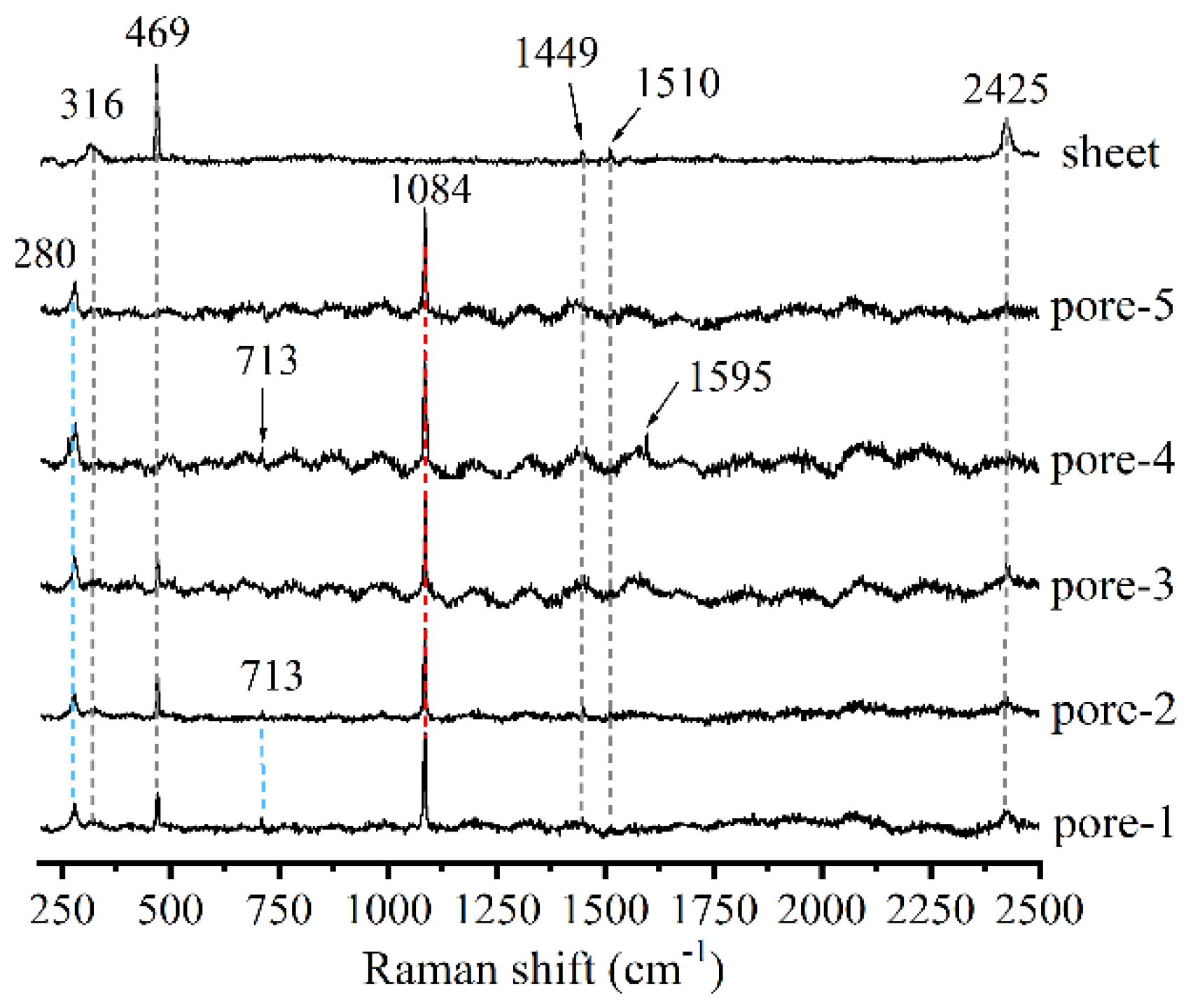
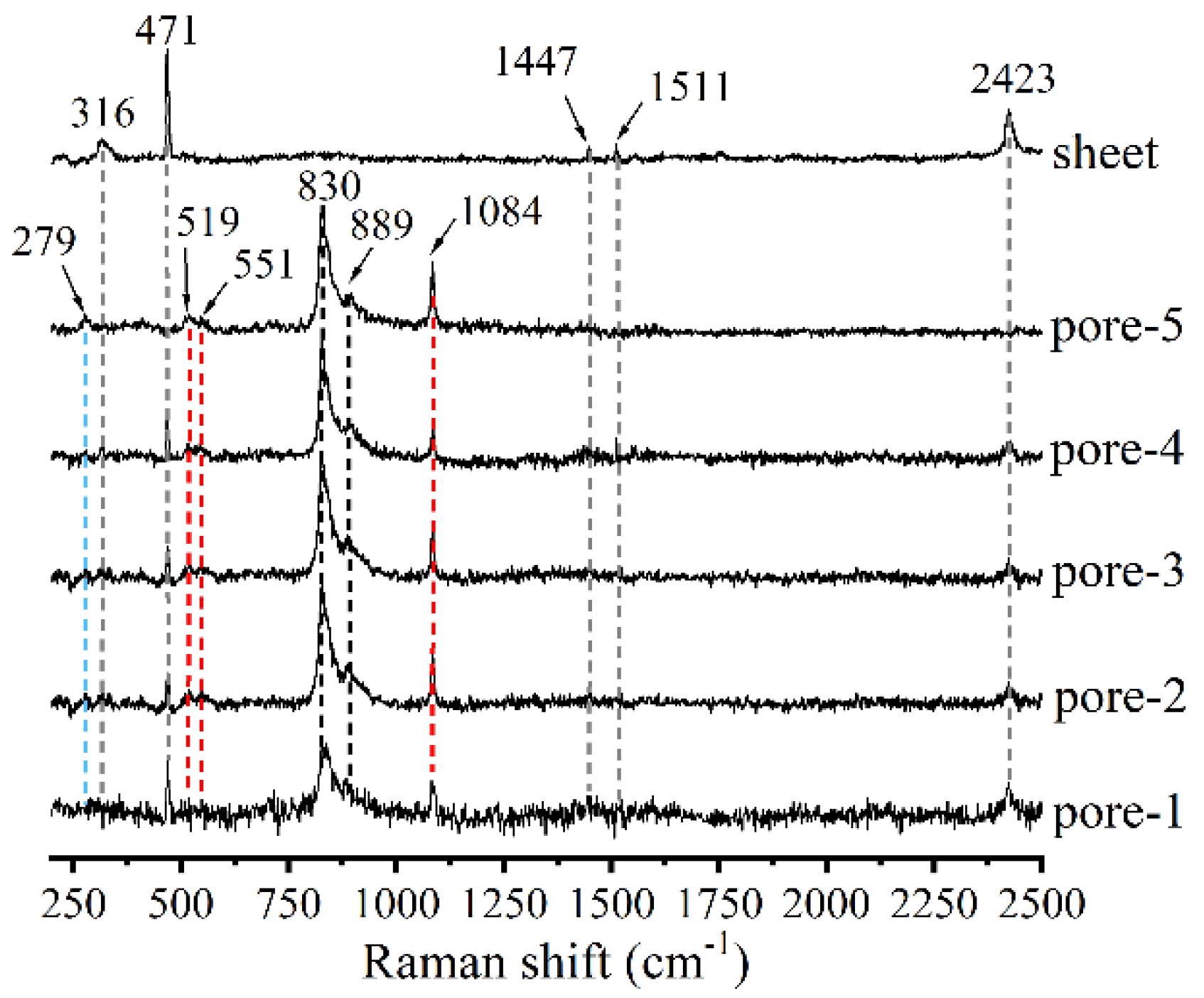
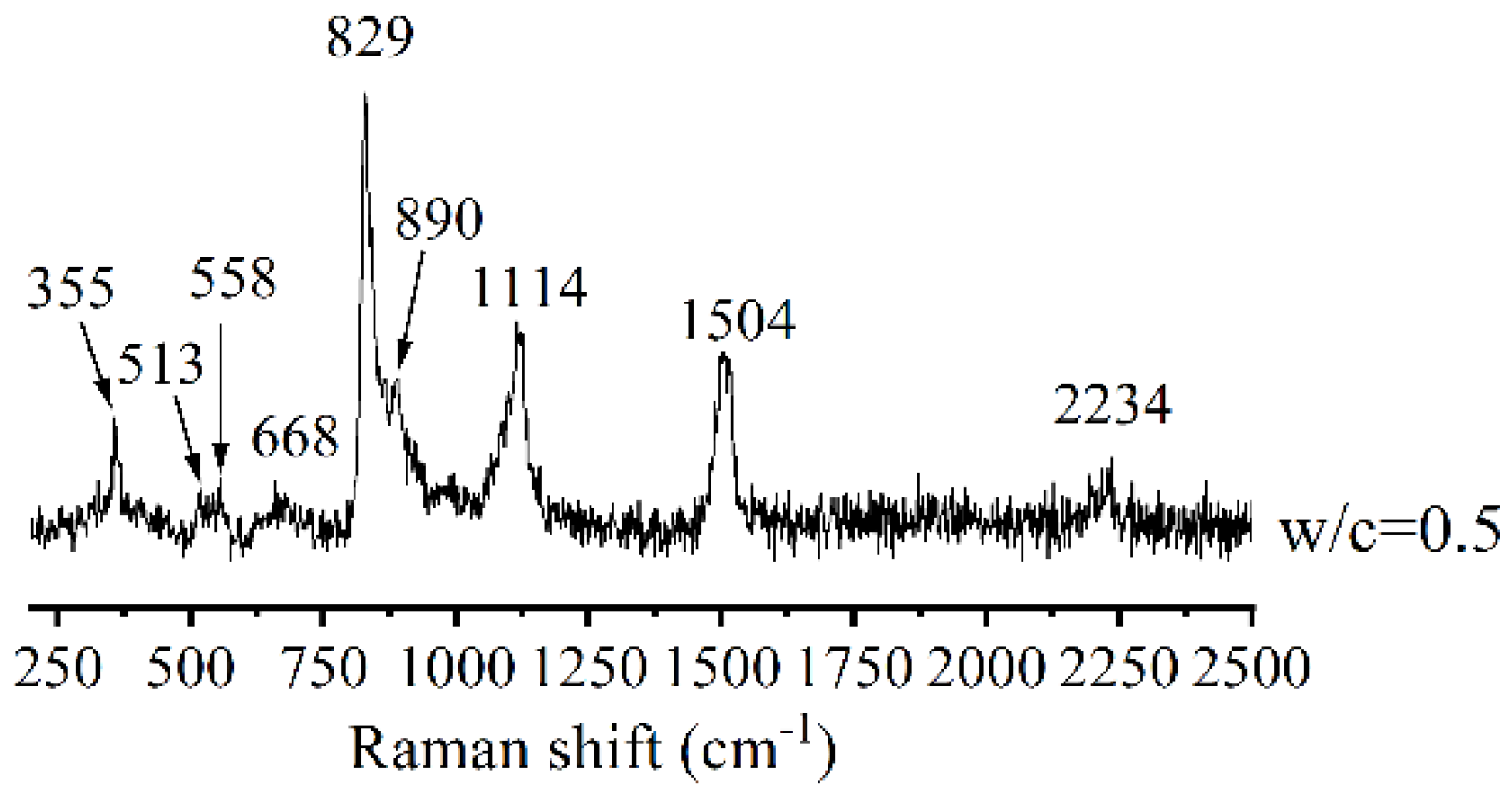
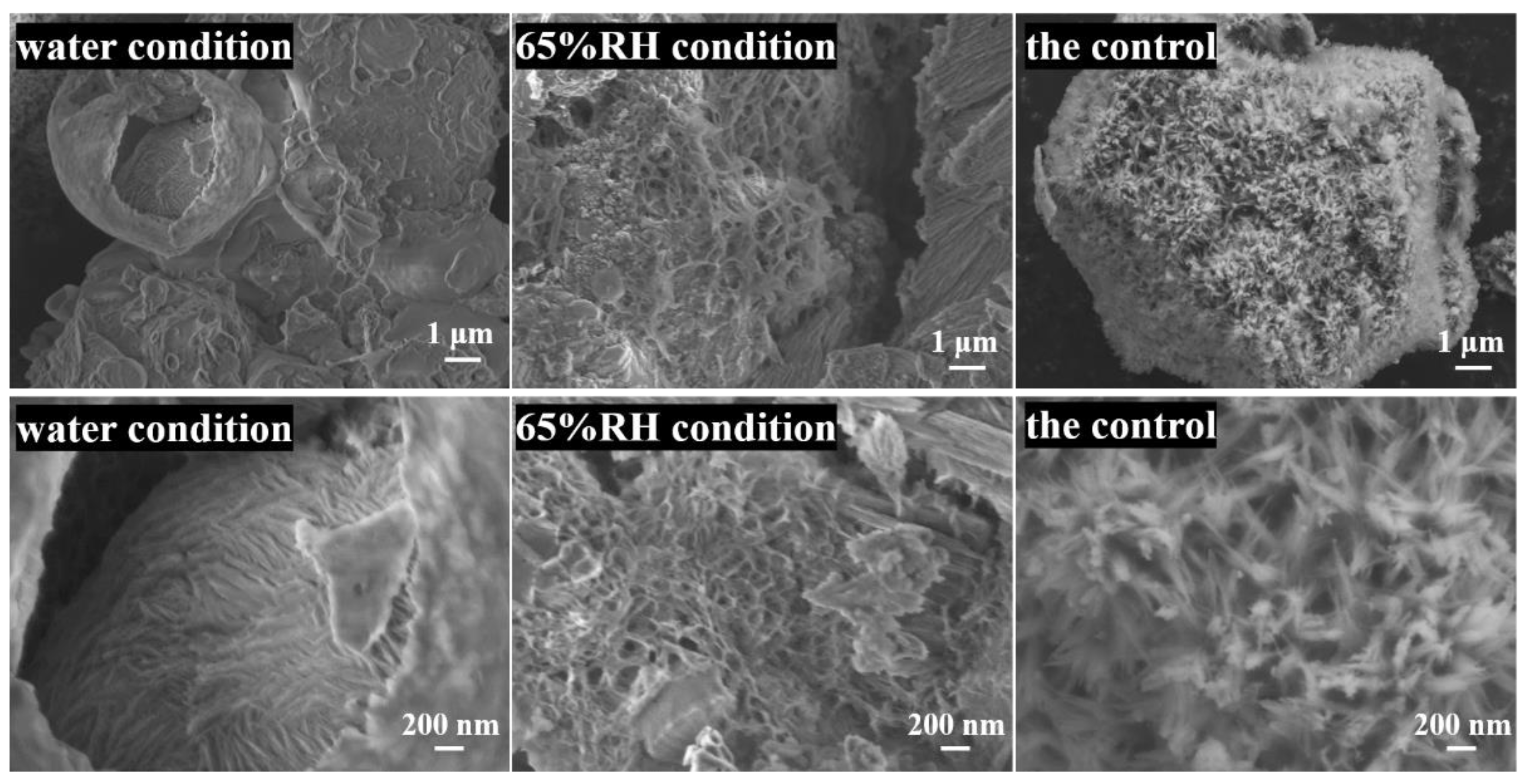
| Elements | Ca | Si | O | C | Mg | Al | Ti | Ca/Si |
|---|---|---|---|---|---|---|---|---|
| water | 22.8 | 8.6 | 60.9 | 5.9 | 0.9 | 0.3 | 0.6 | 2.65 |
| 65% RH | 33.6 | 21.2 | 25.3 | 8.1 | 1.1 | 0.3 | 9.0 | 1.58 |
| w/c = 0.5 | 20.1 | 10.6 | 58.8 | 9.7 | 0.7 | 0 | 0 | 1.89 |
| Conditions | Raman Shift (cm−1) | ||||
|---|---|---|---|---|---|
| C3S | C-S-H | CaCO3 | Ca(OH)2 | Stainless Steel Sheet | |
| water condition (pores) | —— | 1084 (Q3) | 280, 713, 1595, 1581 and 1084 | —— | 316, 469, 1449, 1510 and 2425 |
| 65% RH condition (pores) | 830 and 889 | 519; 551 and 1084 (Q3) | 279 and 1084 | —— | 316, 471, 1417, 1511 and 2423 |
| the control (w/c = 0.5) | 513, 558, 829 and 890 | 668 (Q2); and 1114 | —— | 355, 1504 and 2234 | —— |
| Conditions | FT-IR Spectra (cm−1) | |||
|---|---|---|---|---|
| C3S | C-S-H | CaCO3 | Water | |
| water condition (pores) | 804 | 624; 1107 (Q3) | 871 and 1417 | —— |
| 65% RH condition (pores) | 873, 921 and 835 | 628; 1103 (Q3) | 1411 and 1730 | —— |
| the control (w/c = 0.5) | 522, 821, 873 and 920 | 968 (Q2) | 1417 and 1483 | 1643 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wang, Z.; Zhu, Z.; Chen, Y.; Xu, L.; Wu, K. Impacts of Space Restriction on the Microstructure of Calcium Silicate Hydrate. Materials 2021, 14, 3645. https://doi.org/10.3390/ma14133645
Zhou Y, Wang Z, Zhu Z, Chen Y, Xu L, Wu K. Impacts of Space Restriction on the Microstructure of Calcium Silicate Hydrate. Materials. 2021; 14(13):3645. https://doi.org/10.3390/ma14133645
Chicago/Turabian StyleZhou, Yue, Zhongping Wang, Zheyu Zhu, Yuting Chen, Linglin Xu, and Kai Wu. 2021. "Impacts of Space Restriction on the Microstructure of Calcium Silicate Hydrate" Materials 14, no. 13: 3645. https://doi.org/10.3390/ma14133645






