A Comparative Study of Thermal Aging Effect on the Properties of Silicone-Based and Silicone-Free Thermal Gap Filler Materials
Abstract
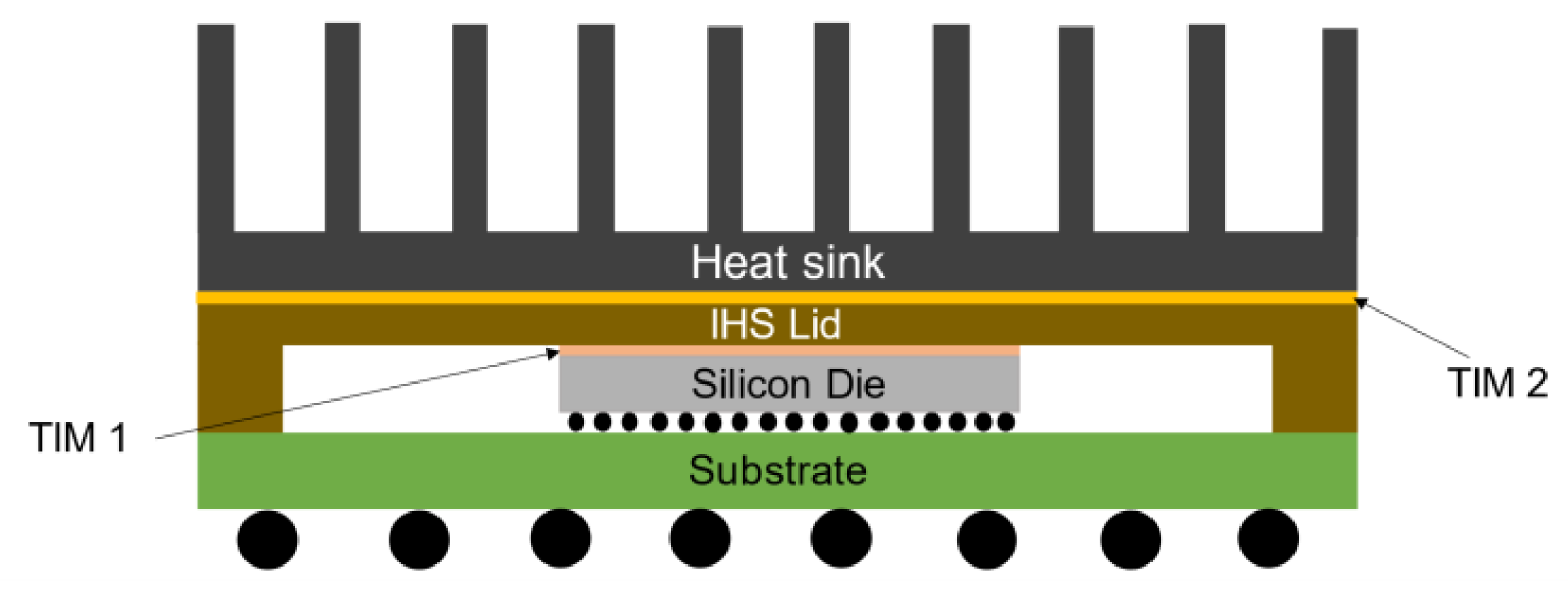
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
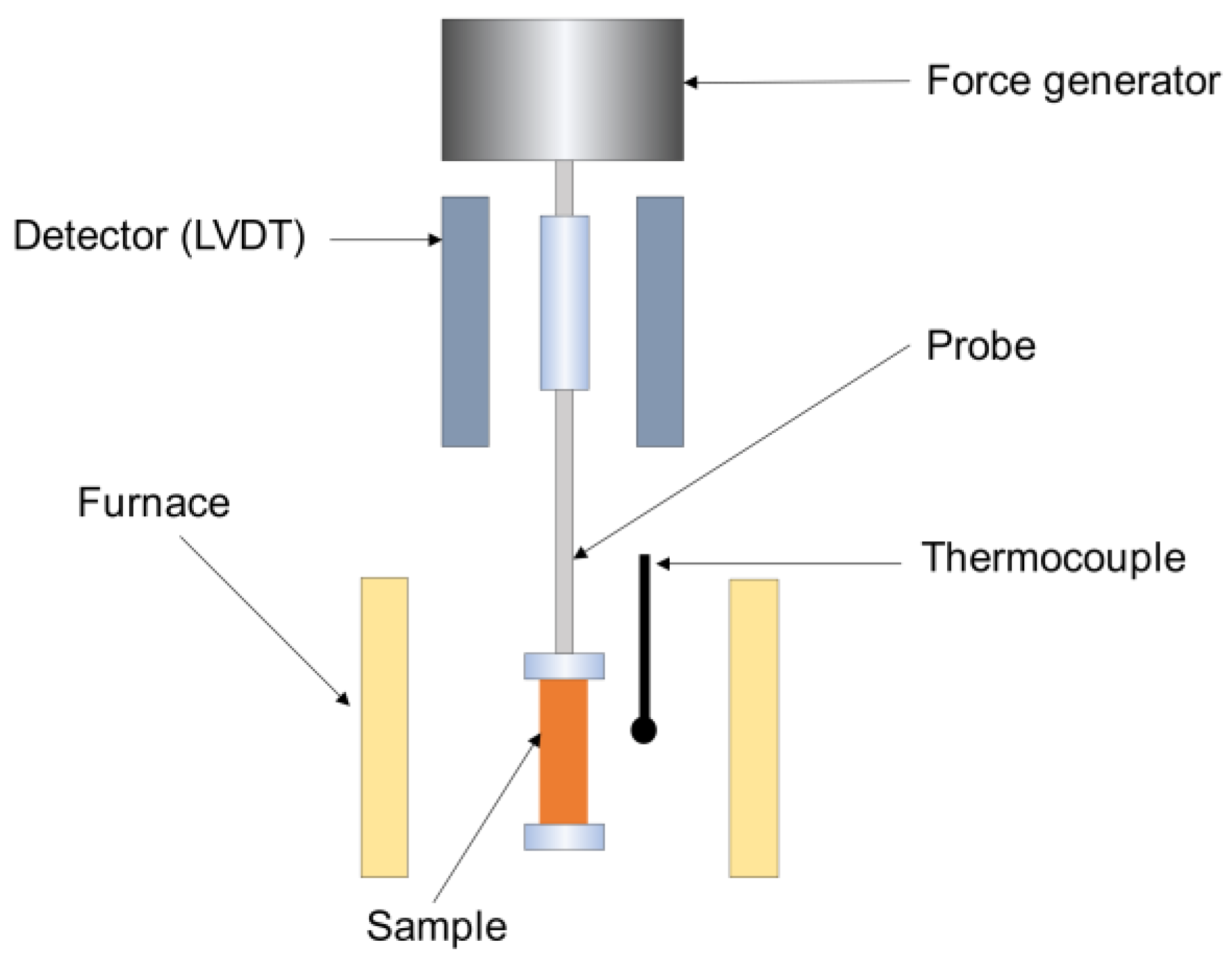
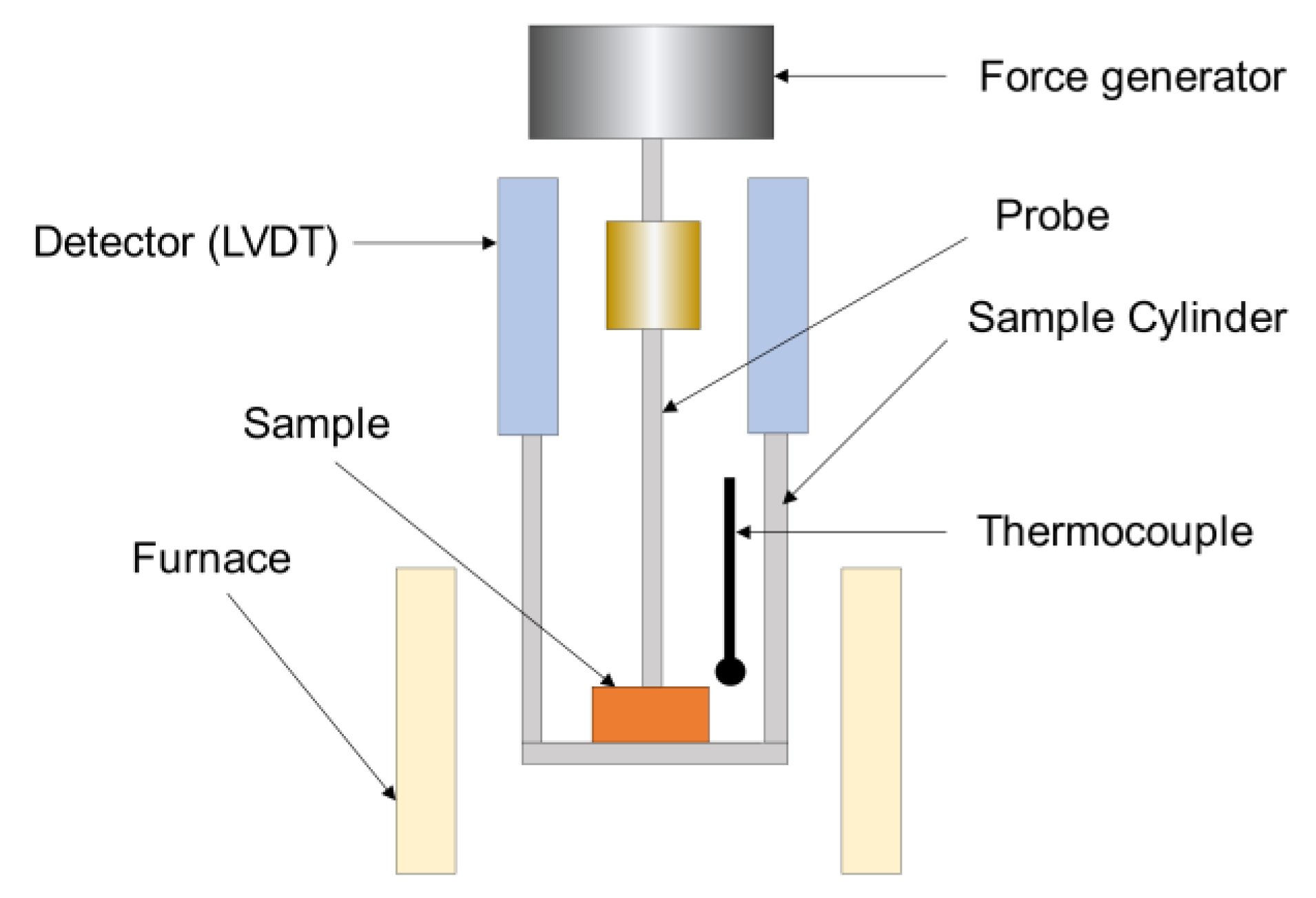
2.2.1. Dynamic Mechanical Analysis (DMA)
2.2.2. Thermomechanical Analysis (TMA)
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.5. Broadband Dielectric Spectroscopy (BbDS)
3. Results and Discussion
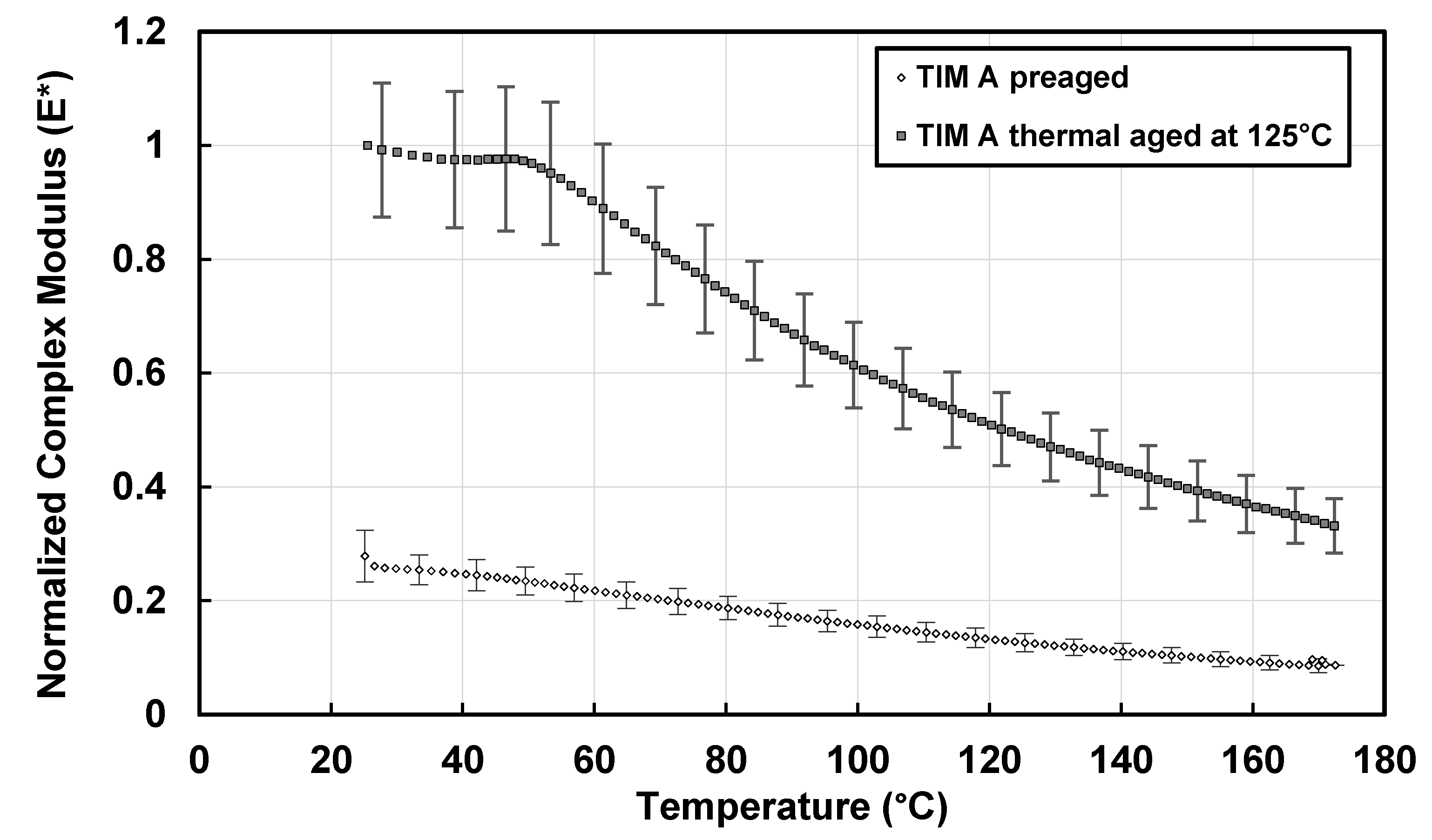
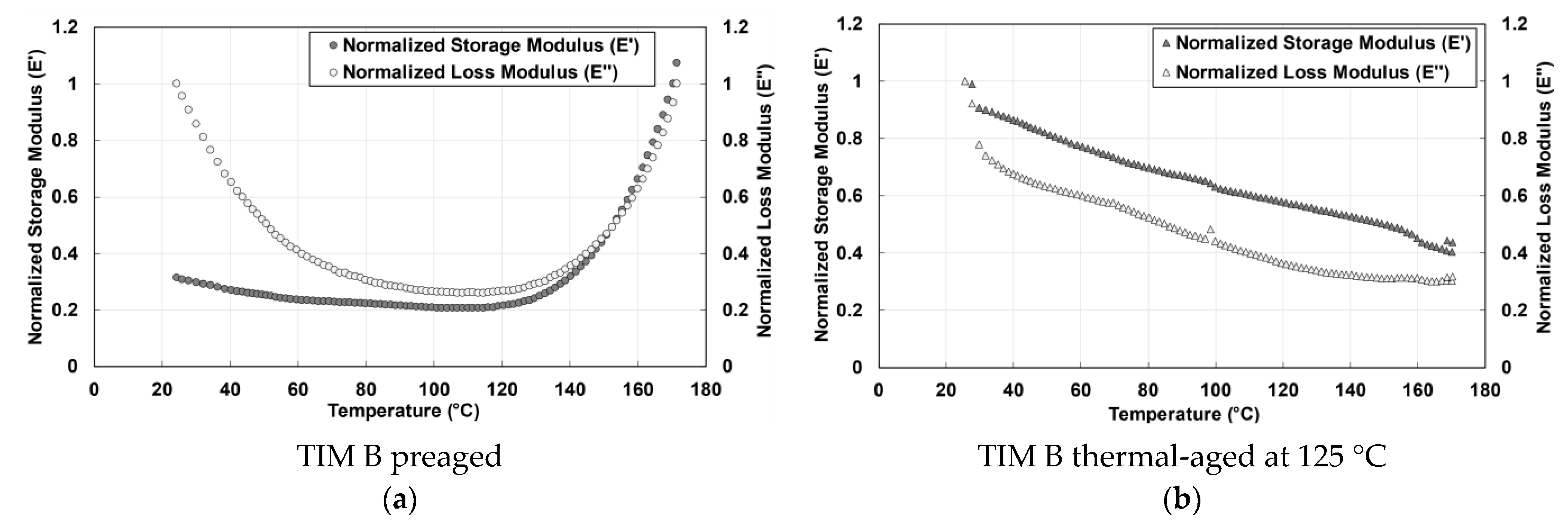
3.1. Dynamic Modulus
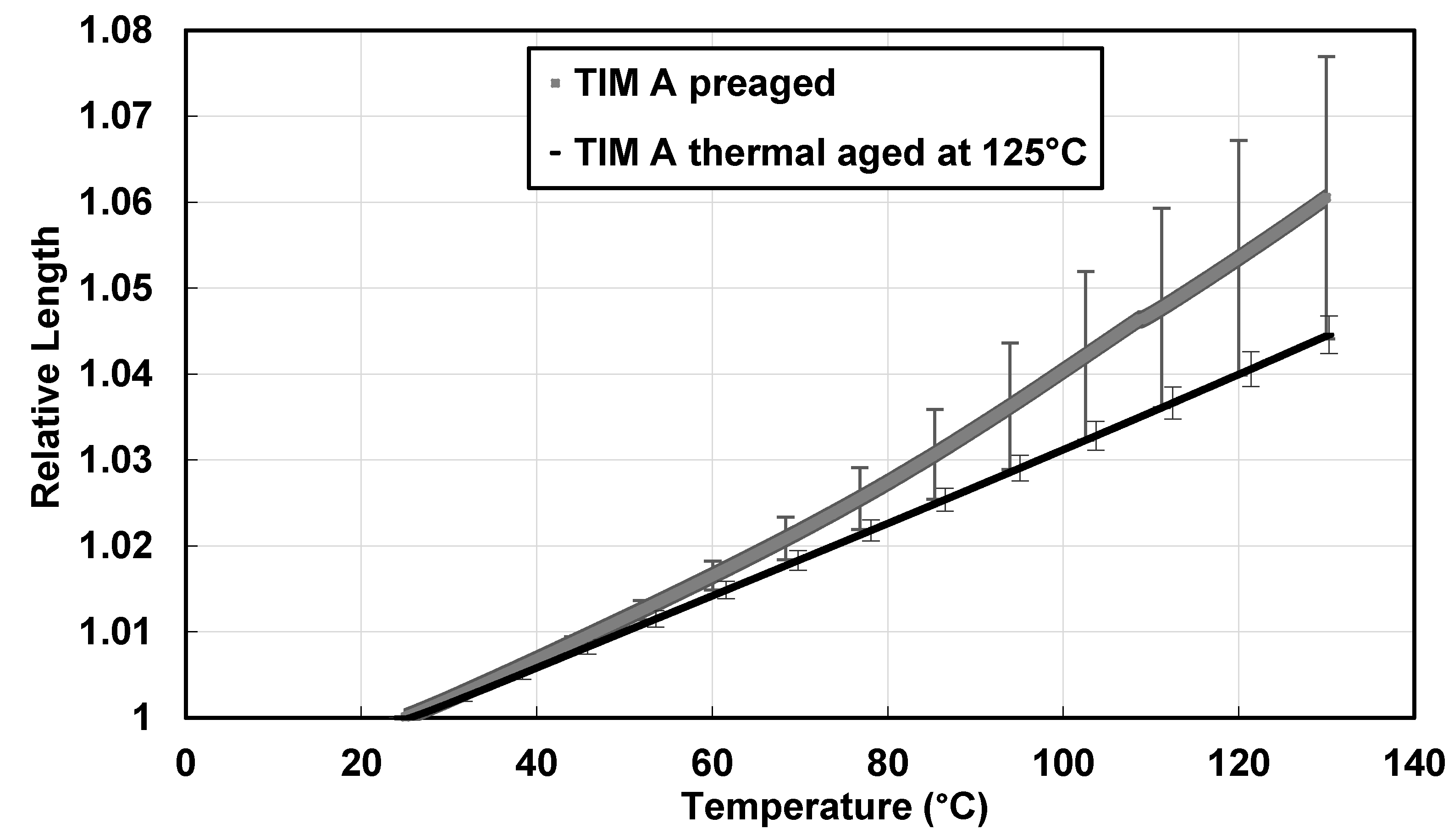
3.2. Thermal Expansion
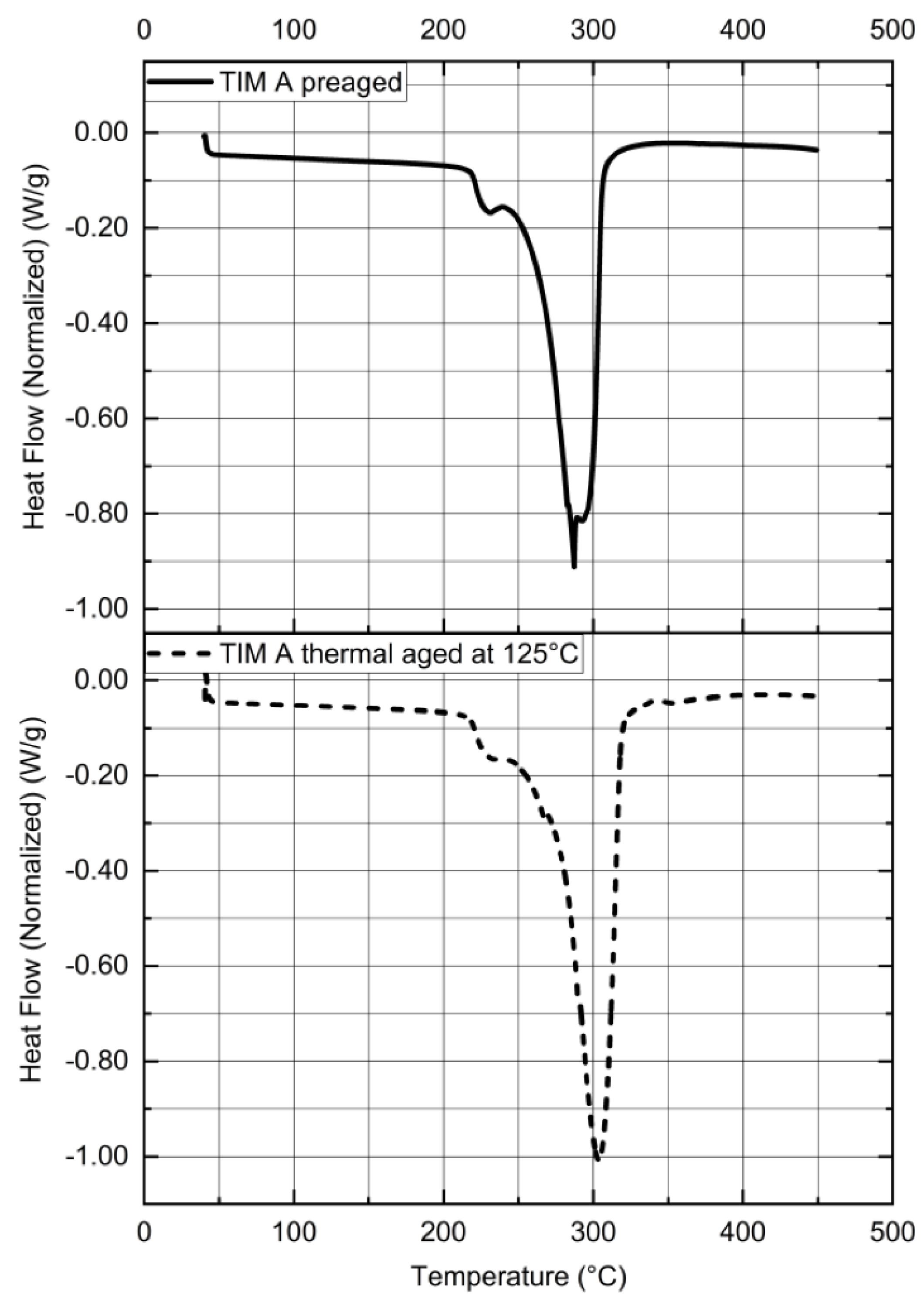
3.3. Thermal Properties
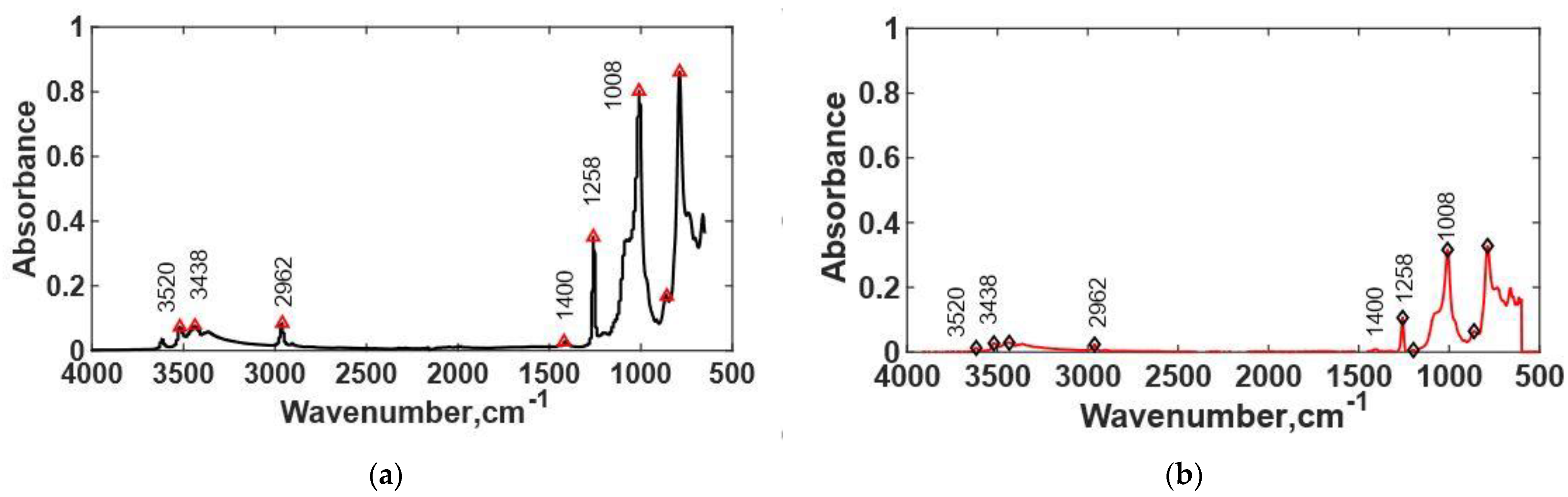
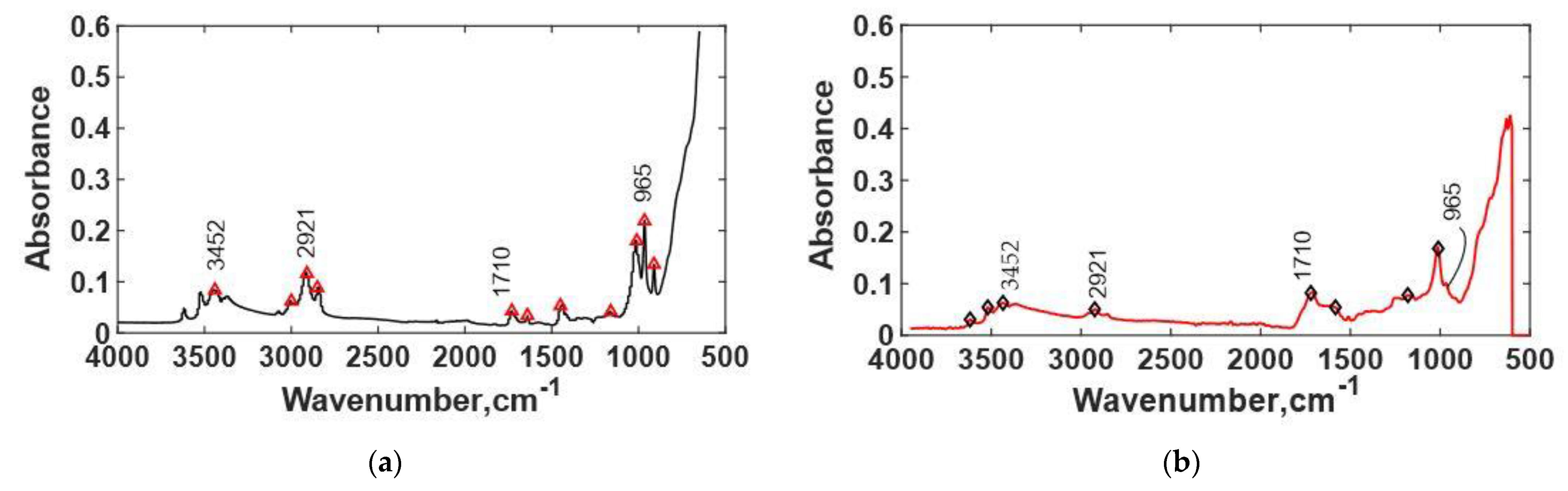
3.4. FTIR Spectra
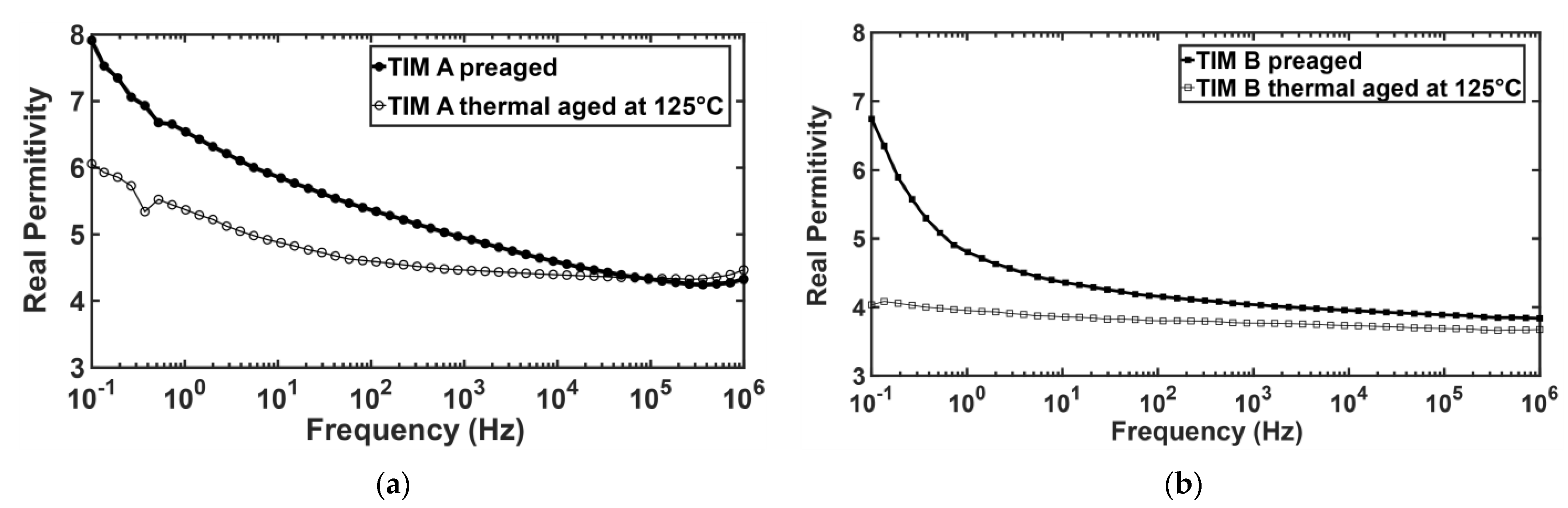
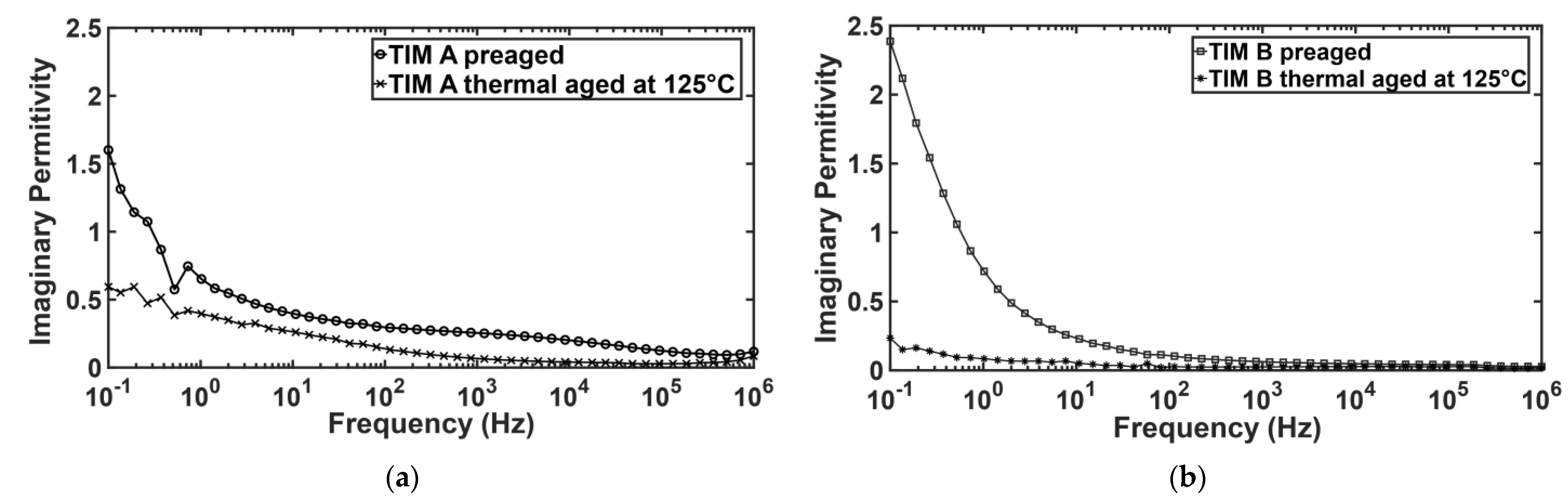
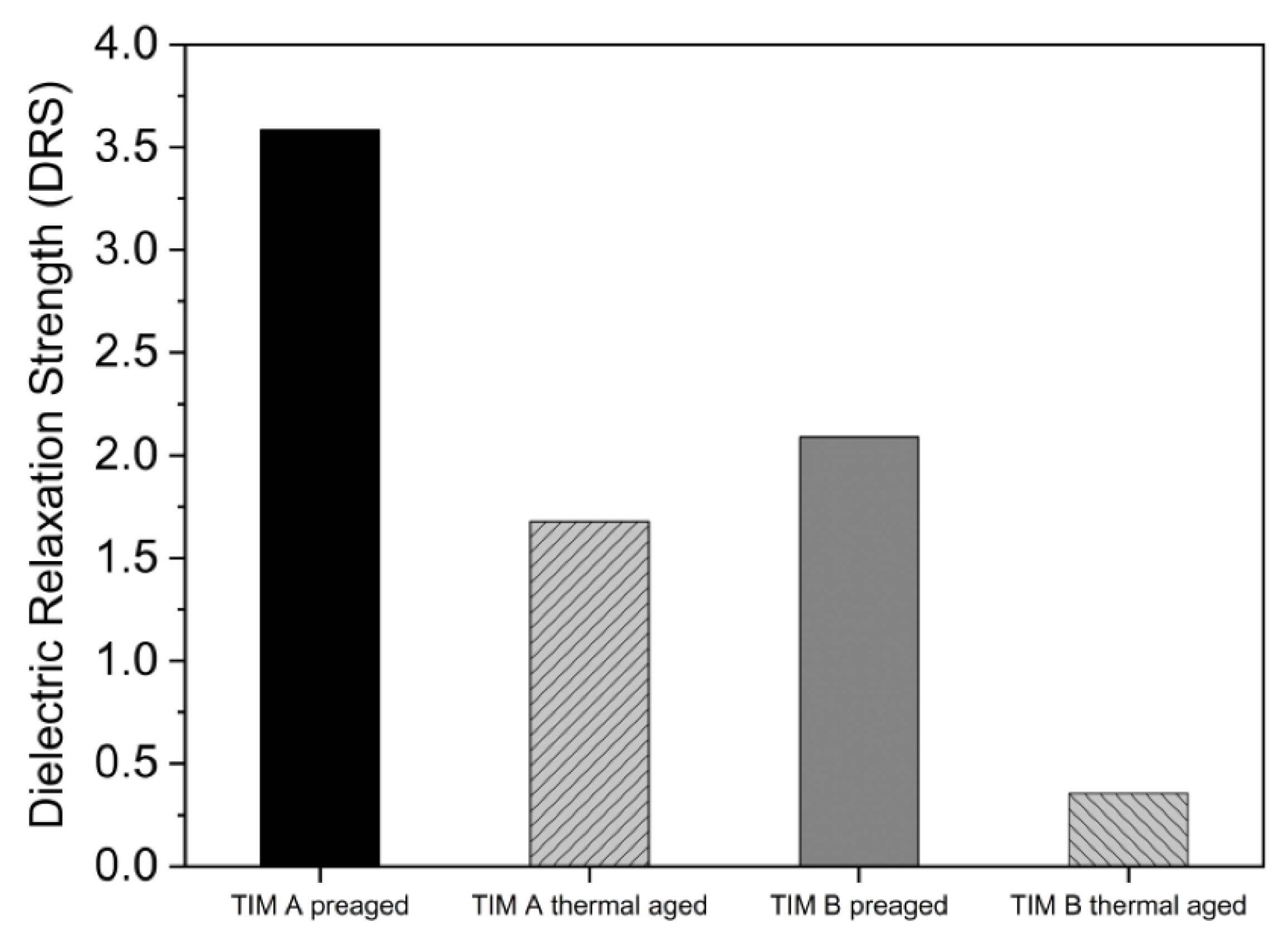
3.5. Dielectric Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khuu, V.; Osterman, M.; Bar-Cohen, A.; Pecht, M. Effects of temperature cycling and elevated temperature/humidity on the thermal performance of thermal interface materials. IEEE Trans. Device Mater. Reliab. 2009, 9, 379–391. [Google Scholar] [CrossRef]
- Due, J.; Robinson, A.J. Reliability of thermal interface materials: A review. Appl. Therm. Eng. 2013, 50, 455–463. [Google Scholar] [CrossRef]
- Sinh, L.H.; Hong, J.-M.; Son, B.T.; Trung, N.N.; Bae, J.-Y. Thermal, Dielectric, and Rheological Properties of Aluminum Nitride/Liquid Crystalline Copoly(ester amide) Composite for the Application of Thermal Interface Materials. Polym. Compos. 2012, 33, 2140–2146. [Google Scholar] [CrossRef]
- Subramanian, V.; Sanchez, J.; Bautista, J.; He, Y.; Wang, J.; Das, A.; Schuldes, J.G.R.; Yazzie, K.; Dhavaleswarapu, H.K.; Malatkar, P. Mechanical Characterization of Thermal Interface Materials and Its Challenges. J. Electron. Packag. Trans. ASME 2019, 141. [Google Scholar] [CrossRef]
- Xu, Y.; Chung, D.D.L.; Mroz, C. Thermally conducting aluminum nitride polymer-matrix composites. Compos. Part A Appl. Sci. Manuf. 2001, 32, 1749–1757. [Google Scholar] [CrossRef]
- Liu, Y. (Ed.) Silicone Dispersions; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-1555-3. [Google Scholar]
- Kitagawa Industries America Inc. Silicone-Free Thermal Pads. Available online: https://www.kgs-ind.com/products/thermal-pads/silicone-free/ (accessed on 12 February 2021).
- Goel, N.; Anoop, T.K.; Bhattacharya, A.; Cervantes, J.A.; Mongia, R.K.; Machiroutu, S.V.; Lin, H.L.; Huang, Y.C.; Fan, K.C.; Denq, B.L.; et al. Technical review of characterization methods for Thermal Interface Materials (TIM). In Proceedings of the 2008 11th Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems, Orlando, FL, USA, 28–31 May 2008; pp. 248–258. [Google Scholar] [CrossRef]
- Fabris, D.; Rosshirt, M.; Cardenas, C.; Wilhite, P.; Yamada, T.; Yang, C.Y. Application of Carbon Nanotubes to Thermal Interface Materials. J. Electron. Packag. Trans. ASME 2011, 133, 1–6. [Google Scholar] [CrossRef]
- Roy, C.K.; Bhavnani, S.; Hamilton, M.C.; Johnson, R.W.; Knight, R.W.; Harris, D.K. Accelerated aging and thermal cycling of low melting temperature alloys as wet thermal interface materials. Microelectron. Reliab. 2015, 55, 2698–2704. [Google Scholar] [CrossRef]
- Misrak, A.; Chauhan, T.; Rajmane, P.; Bhandari, R.; Agonafer, D. Impact of Aging on Mechanical Properties of Thermally Conductive Gap Fillers. J. Electron. Packag. 2020, 142, 1–9. [Google Scholar] [CrossRef]
- Bharatham, L.; Fong, W.S.; Torresola, J.; Koang, C.C. Qualification of Phase Change Thermal Interface Material for Wave Solder Heat Sink on FCBGA Package. In Proceedings of the 2005 7th Electronic Packaging Technology Conference, Singapore, 7–9 December 2005; IEEE: Singapore, 2005; Volume 2, pp. 537–542. [Google Scholar]
- Pramoda, K.P.; Liu, T. Effect of moisture on the dynamic mechanical relaxation of polyamide-6/clay nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 1823–1830. [Google Scholar] [CrossRef]
- Hitachi High-Tech Science Corporation Operational Manual, 0503-513-001E Ver.2.0, TA7000 Series, Dynamic Mechanical Analyzer, DMA7100 2013. Available online: https://www.hitachi-hightech.com/global/product_detail/?pn=ana-dma7100 (accessed on 24 March 2021).
- Meyers, M.A.; Chawla, K.K. Mechanical Behavior of Materials; Cambridge University Press: Cambridge, UK, 2008; ISBN 9780521866750. [Google Scholar]
- Hitachi High-Tech Corporation Principle of Dynamic Mechanical Analysis (DMA). Available online: https://www.hitachi-hightech.com/global/products/science/tech/ana/thermal/descriptions/dma.html (accessed on 24 March 2021).
- Hitachi High-Tech Corporation Principle of Thermomechanical Analysis (TMA). Available online: https://www.hitachi-hightech.com/global/products/science/tech/ana/thermal/descriptions/tma.html (accessed on 30 March 2021).
- Stark, W.; Bohmeyer, W. Non-destructive evaluation (NDE) of composites: Using ultrasound to monitor the curing of composites. In Non-Destructive Evaluation (NDE) of Polymer Matrix Composites; Woodhead Publishing: Cambridge, UK, 2013; pp. 136–181. [Google Scholar]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential scanning calorimetry techniques: Applications in biology and nanoscience. J. Biomol. Tech. 2010, 21, 167–193. [Google Scholar] [PubMed]
- Cassel, R.B. How Tzero™ Technology Improves DSC Performance Part III: The Measurement of Specific Heat Capacity; TA Instruments: New Castle, DE, USA, 2001; Available online: http://www.tainstruments.com/pdf/literature/TA279.pdf (accessed on 4 March 2021).
- TA Instruments. Thermal Analysis & Rheology: Thermal Analysis Review- Modulated DSC Theory. 2012. Available online: https://www.eng.uc.edu/~beaucag/Classes/Characterization/ModulatedDSC_TAinst.pdf (accessed on 4 March 2021).
- ASTM E1269-11. Standard Test Method for Determining Specific Heat Capacity by Differential Scanning Calorimetry; ASTM International: Conshohocken, PA, USA, 2011. [Google Scholar]
- Holde, V.; Kensal Edward, W.; Johnson, C.; Shing Ho, P. Principles of Physical Biochemistry; Prentice Hall: Hoboken, NJ, USA, 2006. [Google Scholar]
- Bacsik, Z.; Mink, J.; Keresztury, G. FTIR spectroscopy of the atmosphere. I. Principles and methods. Appl. Spectrosc. Rev. 2004, 39, 295–363. [Google Scholar] [CrossRef]
- Thomas, S.; Thomas, R.; Zachariah, A.K.; Mishra, R.K. Spectroscopic Methods for Nanomaterials Characterization; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780323461405. [Google Scholar]
- Vadlamudi, V.; Shaik, R.; Raihan, R.; Reifsnider, K.; Iarve, E. Identification of current material state in composites using a dielectric state variable. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105494. [Google Scholar] [CrossRef]
- Polymer Science Learning Center How Polymers Work. Available online: https://pslc.ws/macrog/tg.htm (accessed on 2 March 2021).
- Flory, P.J. Statistical mechanics of swelling of network structures. J. Chem. Phys. 1950, 18, 108–111. [Google Scholar] [CrossRef]
- White, J.R. Polymer ageing: Physics, chemistry or engineering? Time to reflect. Comptes Rendus Chim. 2006, 9, 1396–1408. [Google Scholar] [CrossRef]
- Boparai, K.S.; Singh, R. Thermoplastic Composites for Fused Deposition Modeling Filament: Challenges and Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780128035818. [Google Scholar]
- Duh, Y.S.; Ho, T.C.; Chen, J.R.; Kao, C.S. Study on exothermic oxidation of acrylonitrile-butadiene-styrene (ABS) resin powder with application to ABS processing safety. Polymers 2010, 2, 174–187. [Google Scholar] [CrossRef]
- Aguele, F.O.; Idiaghe, J.A.; Apugo-Nwosu, T.U. A Study of Quality Improvement of Natural Rubber Products by Drying Methods. J. Mater. Sci. Chem. Eng. 2015, 3, 7–12. [Google Scholar] [CrossRef][Green Version]
- Polymer Properties Database Thermal-Oxidative Degradation of Rubber. Available online: http://polymerdatabase.com/polymer chemistry/Thermal Degradation Elastomers.html (accessed on 9 March 2021).
- Polymer Properties Database Elastomers. Available online: https://polymerdatabase.com/Elastomers/Elastomers.html (accessed on 9 March 2021).
- Charles, W.R.; Stanton, J.F.; Feller, T. Low Temperature Behavior and Acceptance Criteria for Elastomeric Bridge Bearings; Transportation Research Board: Washington, DC, USA, 1989. [Google Scholar]
- Boon, A.J. Hock Cleavage—The Cause of Main-chain Scission in Natural Rubber Autoxidation? J. Not. Rubb. Res. 1988, 3, 90–106. [Google Scholar]
- Lucovsky, G.; Mantini, M.J. Low-temperature growth of silicon dioxide films: A study of chemical bonding by ellipsometry and infrared spectroscopy. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 1987, 5, 530. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Silaghi, M.A. (Ed.) Dielectric Material; InTech: London, UK, 2012; ISBN 978-953-51-0764-4. [Google Scholar]
- Sanaeishoar, H.; Sabbaghan, M.; Mohave, F. Synthesis and characterization of micro-mesoporous MCM-41 using various ionic liquids as co-templates. Microporous Mesoporous Mater. 2015, 217, 219–224. [Google Scholar] [CrossRef]
- Michaud, C.F. ‘Chubb’ Ion Exchange: The Role of Cross-Linking and Aging. Available online: http://wcponline.com/2017/05/15/ion-exchange-role-cross-linking-aging/ (accessed on 17 March 2021).








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, A.S.M.R.; Rabby, M.M.; Kabir, M.; Das, P.P.; Bhandari, R.; Raihan, R.; Agonafer, D. A Comparative Study of Thermal Aging Effect on the Properties of Silicone-Based and Silicone-Free Thermal Gap Filler Materials. Materials 2021, 14, 3565. https://doi.org/10.3390/ma14133565
Chowdhury ASMR, Rabby MM, Kabir M, Das PP, Bhandari R, Raihan R, Agonafer D. A Comparative Study of Thermal Aging Effect on the Properties of Silicone-Based and Silicone-Free Thermal Gap Filler Materials. Materials. 2021; 14(13):3565. https://doi.org/10.3390/ma14133565
Chicago/Turabian StyleChowdhury, A S M Raufur, Monjur Morshed Rabby, Mehzabeen Kabir, Partha Pratim Das, Rabin Bhandari, Rassel Raihan, and Dereje Agonafer. 2021. "A Comparative Study of Thermal Aging Effect on the Properties of Silicone-Based and Silicone-Free Thermal Gap Filler Materials" Materials 14, no. 13: 3565. https://doi.org/10.3390/ma14133565
APA StyleChowdhury, A. S. M. R., Rabby, M. M., Kabir, M., Das, P. P., Bhandari, R., Raihan, R., & Agonafer, D. (2021). A Comparative Study of Thermal Aging Effect on the Properties of Silicone-Based and Silicone-Free Thermal Gap Filler Materials. Materials, 14(13), 3565. https://doi.org/10.3390/ma14133565





