Urease Inhibitory Kinetic Studies of Various Extracts and Pure Compounds from Cinnamomum Genus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Plant Extracts and Compounds on Urease Inhibition
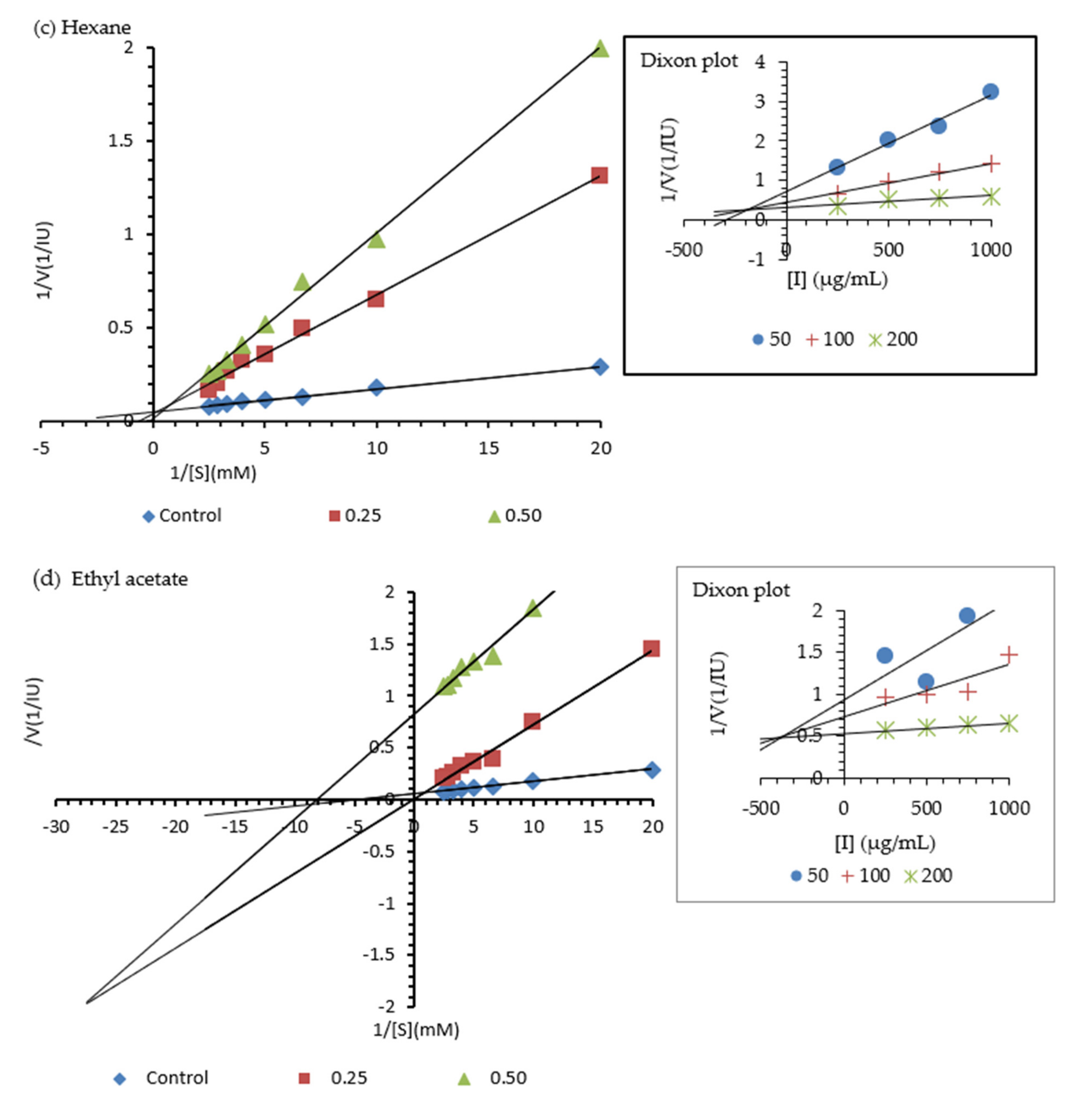
2.2. Urease Inhibition Curve Analysis
2.3. GC–MS Analysis
3. Material and Methods
3.1. Collection of Plant Material
3.2. Chemicals
3.3. Extraction of Plant Material
3.4. Measurement of Urease Inhibitory Activity
3.5. Statistical Analysis
3.6. Determination of Kinetics Parameters
3.7. GC–MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Krajewska, B. Ureases I. Functional, catalytic and kinetic properties: A review. J. Mol. Catal. B Enzym. 2009, 59, 9–21. [Google Scholar] [CrossRef]
- Sirko, A.; Brodzik, R. Plant ureases: Roles and Regulation. Acta Biochim. Pol. 2000, 47, 1189–1195. [Google Scholar] [CrossRef] [Green Version]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Boer, J.L.; Mulrooney, S.B.; Hausinger, R.P. Nickel-dependent metalloenzymes. Arch. Biochem. Biophys. 2014, 15, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Maroney, M.J.; Ciurli, S. Non-redox nickel enzymes. Chem. Rev. 2014, 114, 4206–4228. [Google Scholar] [CrossRef] [Green Version]
- Patra, A.K.; Aschenbach, J.R. Ureases in the gastrointestinal tracts of ruminant and monogastric animals and their implication in Urea-N /ammonia metabolism: A review. J. Adv. Res. 2018, 13, 39–50. [Google Scholar] [CrossRef]
- Eswaran, M.B.; Surendran, S.; Vijayakumar, M.; Ojha, S.K.; Rawat, A.K.S.; Rao, C.V. Gastroprotective activity of Cinnamomum tamala leaves on experimental gastric ulcers in rats. J. Ethnopharmacol. 2010, 128, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Rafatullah, S.; Alqasoumi, S.; Al-Dosary, M.; Al-Yahya, M.; Al-Mofleh, I. Gastroprotective effect of a popular spice cinnamon “Cinnamomum zeylanicum” in rats. Eur. J. Pharmacol. 2011, 668, e42. [Google Scholar] [CrossRef]
- Tanaka, S.; Yoon, Y.H.; Fukui, H.; Tabata, M.; Akira, T.; Okano, K.; Iwai, M.; Iga, Y.; Yokoyama, K. Antiulcerogenic compounds isolated from Chinese cinnamon. Planta Med. 1989, 55, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhu, J.X.; Mo, S.F.; Pan, Y.; Kong, L.D. Effects of Cassia oil on serum and hepatic uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. J. Ethnopharmacol. 2016, 103, 357–365. [Google Scholar] [CrossRef]
- Yang, F.; Long, E.; Wen, J.; Cao, L.; Zhu, C.; Hu, H.; Ruan, Y.; Okanurak, K.; Hu, H.; Wei, X.; et al. Linalool, derived from Cinnamomum camphora (L.) Presl leaf extracts, possesses molluscicidal activity against Oncomelania hupensis and inhibits infection of Schistosoma japonicum. Parasit Vectors. 2014, 407, 3305–3307. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.H.; Racicot, K.; Pilkenton, S.J.; Apostolidis, E. Evaluation of the in vitro anti-hyperglycemic effect of Cinnamomum cassia derived phenolic phytochemicals, via carbohydrate hydrolyzing enzyme inhibition. Plant Foods Hum. Nutr. 2014, 69, 155–160. [Google Scholar] [CrossRef]
- Yang, S.M.; Tsai, K.D.; Wong, H.Y.; Liu, Y.H.; Chen, T.W.; Cherng, J.; Hsu, K.C.; Ang, Y.U.; Chang, J.M. Molecular mechanism of Cinnamomum verum component cuminaldehyde inhibits cell growth and induces cell death in human lung squamous cell carcinoma NCI-H520 cells in vitro and in vivo. J. Cancer 2016, 7, 251–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasoul, M.A.A.; Marei, G.I.K.; Abdelgaleil, S.A.M. Evaluation of antibacterial properties and biochemical effects of monoterpenes on plant pathogenic bacteria. Afr. J. Microbiol. Res. 2012, 6, 3667–3672. [Google Scholar]
- Vallianou, I.; Peroulis, N.; Pantazis, P.; Hatzopoulou-Caldaras, M. Camphene, a Plant Derived Monoterpene, Reduces Plasma Cholesterol and Triglycerides in Hyperlipidemic Rats Independently of HMG-CoA Reductase Activity. PLoS ONE 2011, 6, e20516. [Google Scholar] [CrossRef] [Green Version]
- Thakre, A.D.; Mulange, S.V.; Kodgire, S.S.; Zore, G.B.; Karuppayil, S.M. Effects of Cinnamaldehyde, Ocimene, Camphene, Curcumin and Farnesene on Candida albicans. Adv. Microbiol. 2016, 6, 627–643. [Google Scholar] [CrossRef] [Green Version]
- Romeo, G.R.; Lee, J.; Mulla, C.M.; Noh, Y.; Holden, C.; Lee, B.C. Influence of cinnamon on glycemic control in individuals with prediabetes: A randomized controlled trial. J. Endocr. Soc. 2020, 4, bvaa094. [Google Scholar] [CrossRef]
- Yanakiev, S. Effects of Cinnamon (Cinnamomum spp.) in Dentistry: A Review. Molecules 2020, 25, 4184. [Google Scholar] [CrossRef]
- Tsai, K.D.; Cherng, J.; Liu, Y.H.; Chen, T.W.; Wong, H.Y.; Yang, S.M.; Chou, K.S.; Cherng, J.M. Cinnamomum verum component 2-methoxycinnamaldehyde: A novel antiproliferative drug inducing cell death through targeting both topoisomerase I and II in human colorectal adenocarcinoma COLO 205 cells. Food Nutr. Res. 2016, 60, 31607. [Google Scholar] [CrossRef]
- Balijepalli, M.K.; Buru, A.S.; Sakirolla, R.; Pichika, M.R. Cinnamomum genus: A review on its biological activities. Int. J. Pharm. Pharm. Sci. 2017, 9, 1–11. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.A.S.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complement. Altern. Med. 2013, 13, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Geng, Z.; Zhang, W.; Liang, J.; Wang, C.; Deng, Z.; Du, S. The Chemical Composition of Essential Oils from Cinnamomum camphora and their Insecticidal Activity against the Stored Product Pests. Int. J. Mol. Sci. 2016, 17, 1836. [Google Scholar] [CrossRef]
- Lodhi, M.A.; Hussain, J.; Abbasi, M.A.; Jassbi, A.R.; Choudhary, M.I.; Ahmad, V.U. A New Bacillus pasteurii Urease Inhibitor from Euphorbia decipiens. J. Enzym. Inhib. Med. Chem. 2006, 21, 531–535. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.; Chaudhary, B.A.; Ashraf, M.; Uzair, M.; Janbaz, K.H. Vernonione, A New Urease Inhibitory Carvotacetone Derivative from Vernonia cinerascens. J. Chem. Soc. Pak. 2012, 34, 639–642. [Google Scholar]
- Ramsay, K.S.T.; Wafo, P.; Ali, Z.; Khan, A.; Oluyemisi, O.O.; Marasini, B.P.; Khan, I.A.; Bonaventure, N.T.; Choudhary, M.I.; Rahman, A.U. Chemical constituents of Stereospermum acuminatissimum and their urease and α-chymotrypsin inhibitions. Fitoterapia 2012, 83, 204–208. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]



| Samples * | % Urease Inhibition ** | IC50 (µg/mL) |
|---|---|---|
| C. camphora (Aq) | 35.00 ± 0.01 | 1340 |
| C. camphora (Met) | 70.07 ± 0.03 | 980 |
| C. camphora (H.F.) | 43.52 ± 0.57 | 1210 |
| C. camphora (C.F.) | 17.04 ± 1.38 | N.A. |
| C. camphora (E.F.) | 54.30 ± 1.09 | 1120 |
| C. verum (Aq) | 15.31 ± 0.47 | N.A. |
| C. verum (Met) | 17.92 ± 0.98 | N.A. |
| Camphene | 65.09 ± 0.08 | 0.147 (1.08 µM) |
| Cuminaldehyde | 51.34 ± 0.56 | 0.214 (1.45 µM) |
| Sample | Mode of Inhibition | Ki µg/mL |
|---|---|---|
| Aqueous (E) | Mixed | 260 |
| Methanol (E) | Non-Competitive | 375 |
| Hexane (F) | Competitive | 175 |
| Ethyl acetate (F) Camphene Cuminaldehyde | Mixed Competitive Mixed | 370 195 (1.431 mM) 480 (3.238 mM) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.; Sikri, N.; Chahal, S.; Sharma, J.; Sharma, B.; Yadav, P.; Bhardwaj, M.; Vashishth, D.; Kadyan, P.; Kataria, S.K.; et al. Urease Inhibitory Kinetic Studies of Various Extracts and Pure Compounds from Cinnamomum Genus. Molecules 2021, 26, 3803. https://doi.org/10.3390/molecules26133803
Kumar M, Sikri N, Chahal S, Sharma J, Sharma B, Yadav P, Bhardwaj M, Vashishth D, Kadyan P, Kataria SK, et al. Urease Inhibitory Kinetic Studies of Various Extracts and Pure Compounds from Cinnamomum Genus. Molecules. 2021; 26(13):3803. https://doi.org/10.3390/molecules26133803
Chicago/Turabian StyleKumar, Manoj, Neha Sikri, Sulekha Chahal, Jitender Sharma, Bhavna Sharma, Poonam Yadav, Monika Bhardwaj, Divya Vashishth, Pooja Kadyan, Sudhir Kumar Kataria, and et al. 2021. "Urease Inhibitory Kinetic Studies of Various Extracts and Pure Compounds from Cinnamomum Genus" Molecules 26, no. 13: 3803. https://doi.org/10.3390/molecules26133803






