Safety and Efficacy of SGLT2 Inhibitors: A Multiple-Treatment Meta-Analysis of Clinical Decision Indicators
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
2.4.1. Efficacy of the Main Outcomes and Risk of Adverse Events
2.4.2. Clinical Decision Analysis
2.4.3. Network Meta-Analysis
- Relative rankings of treatments. Once we comparatively estimated the effectiveness of the different SGLT2i types, the next step was to rank the treatments to identify superiority. The probability that each SGLT2i type was the most effective was presented graphically using rankograms [23]. Additionally, the surface under the cumulative ranking (SUCRA) was estimated for each intervention. SUCRA involves the assignment of a numerical value between 0 and 1 to simplify the classification of each intervention in the rankogram. The best intervention would obtain a value for SUCRA close to 1, and the worst intervention would be a value close to 0 [24].
- A comparative network meta-analysis of the SGLT2i effect versus placebo is displayed through league tables [25].
- Sensitivity analysis and small study effect. Sensitivity analyses were conducted to assess the robustness of the summary estimates and to detect whether any particular study represented a large proportion of the heterogeneity. To examine the presence of bias due to the small study effect, a network funnel plot was used to visually scrutinize the criterion of symmetry [26]. All analyses were conducted in Stata 15.0 (Stata, College Station, TX, USA).
3. Results
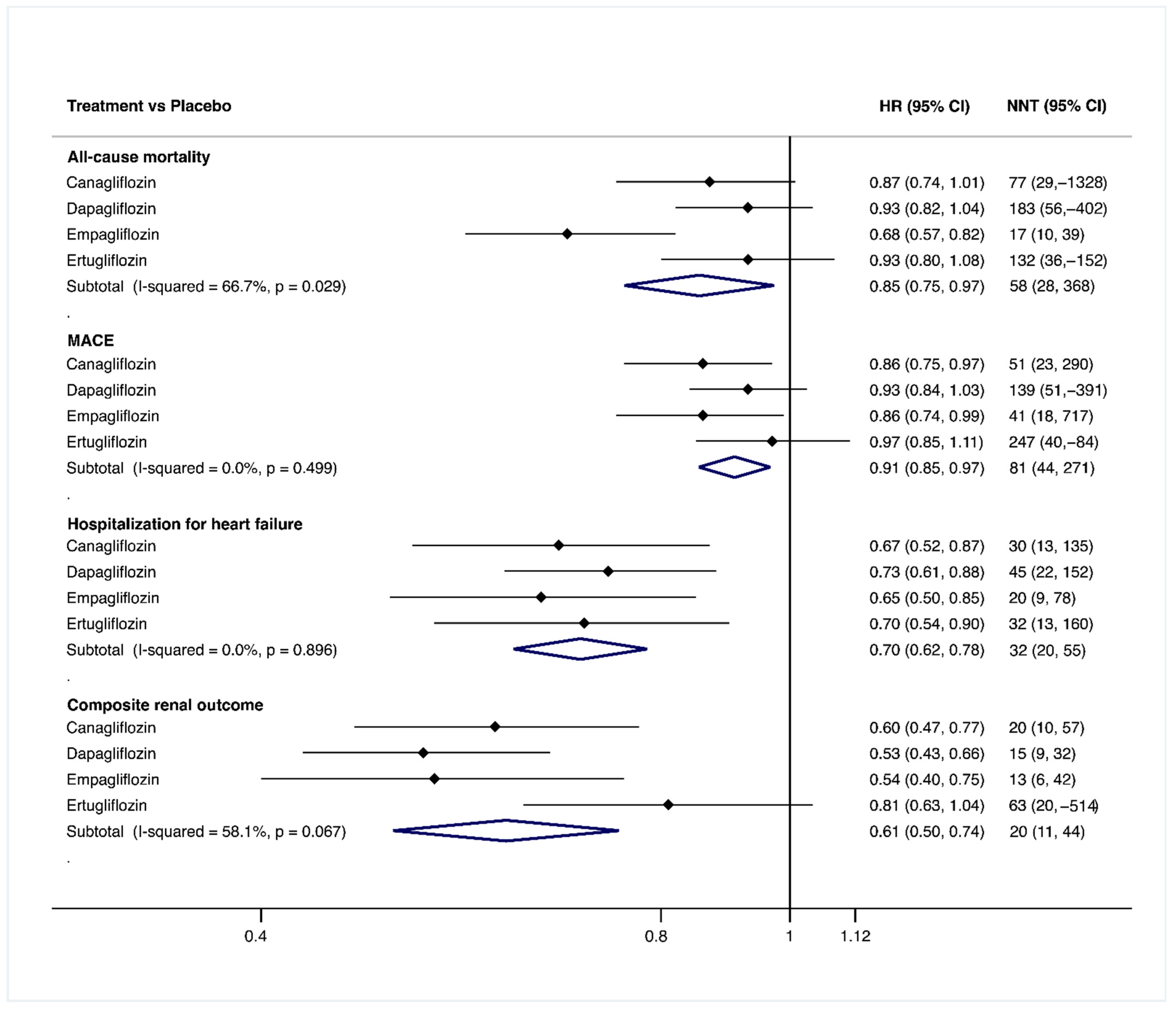
3.1. Efficacy of the Main Outcomes
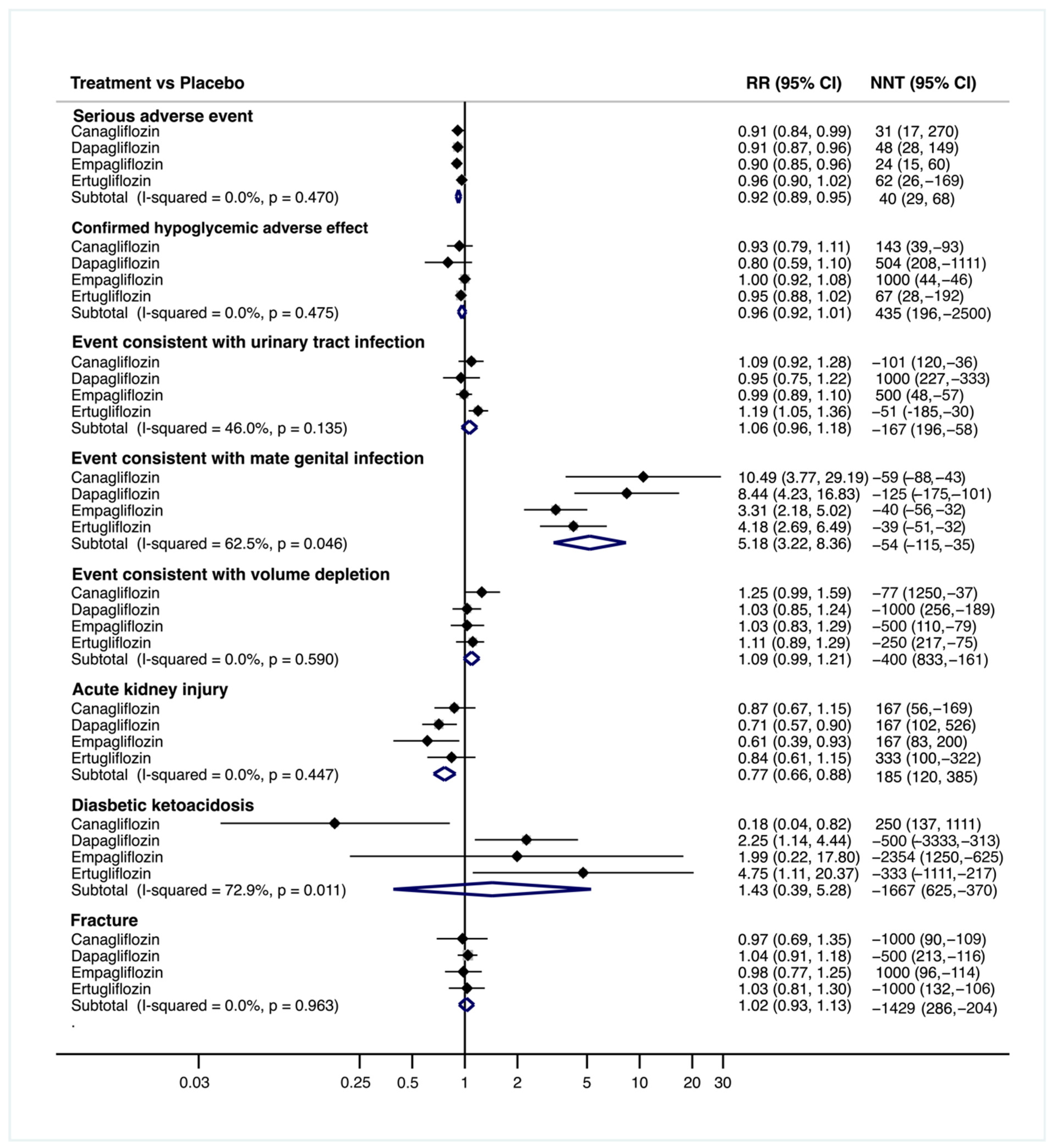
3.2. Risk of Adverse Events
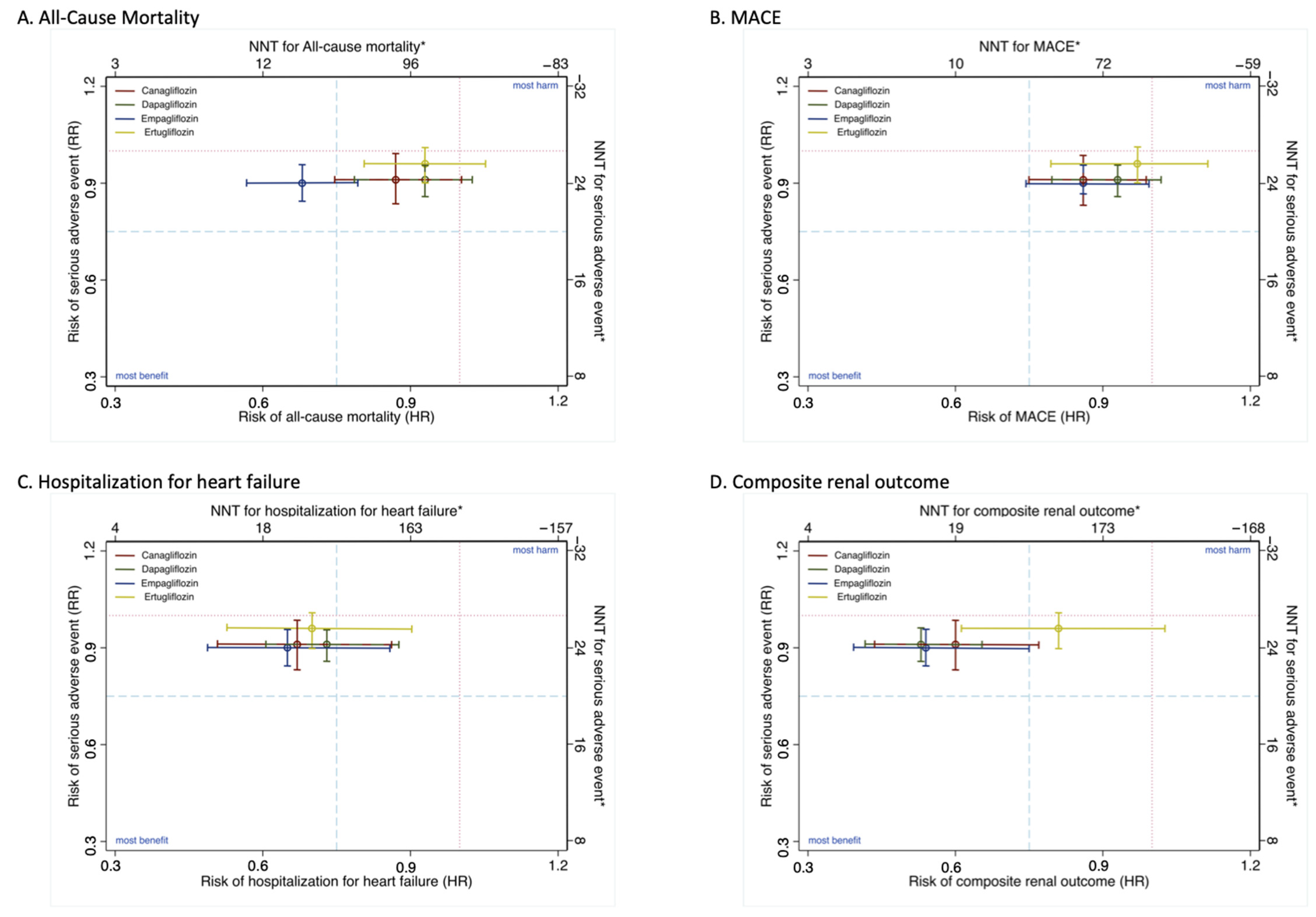
3.3. Clinical Decision Analysis
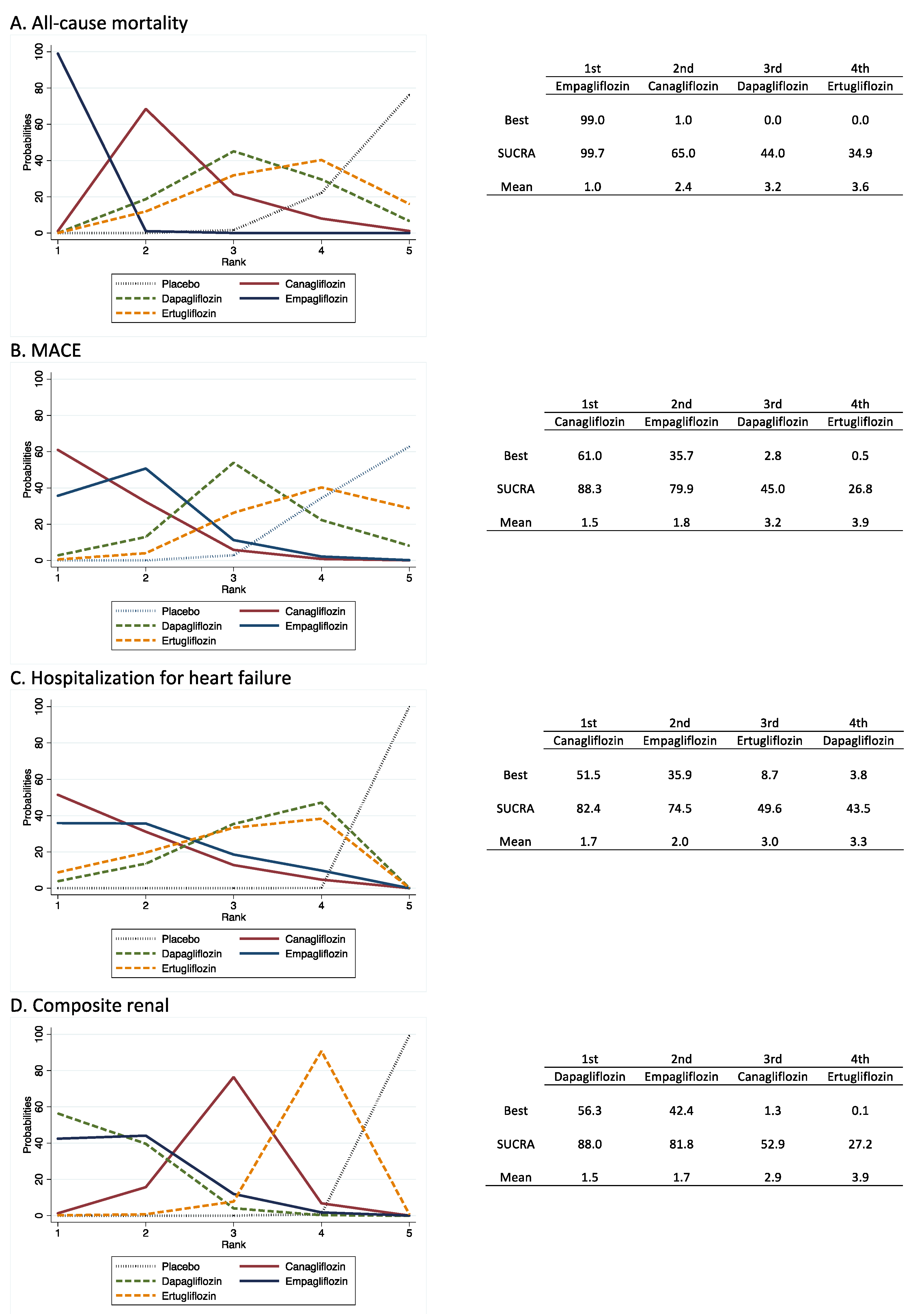
3.4. Treatment Ranking
3.5. Small Study Effects and Publication Bias
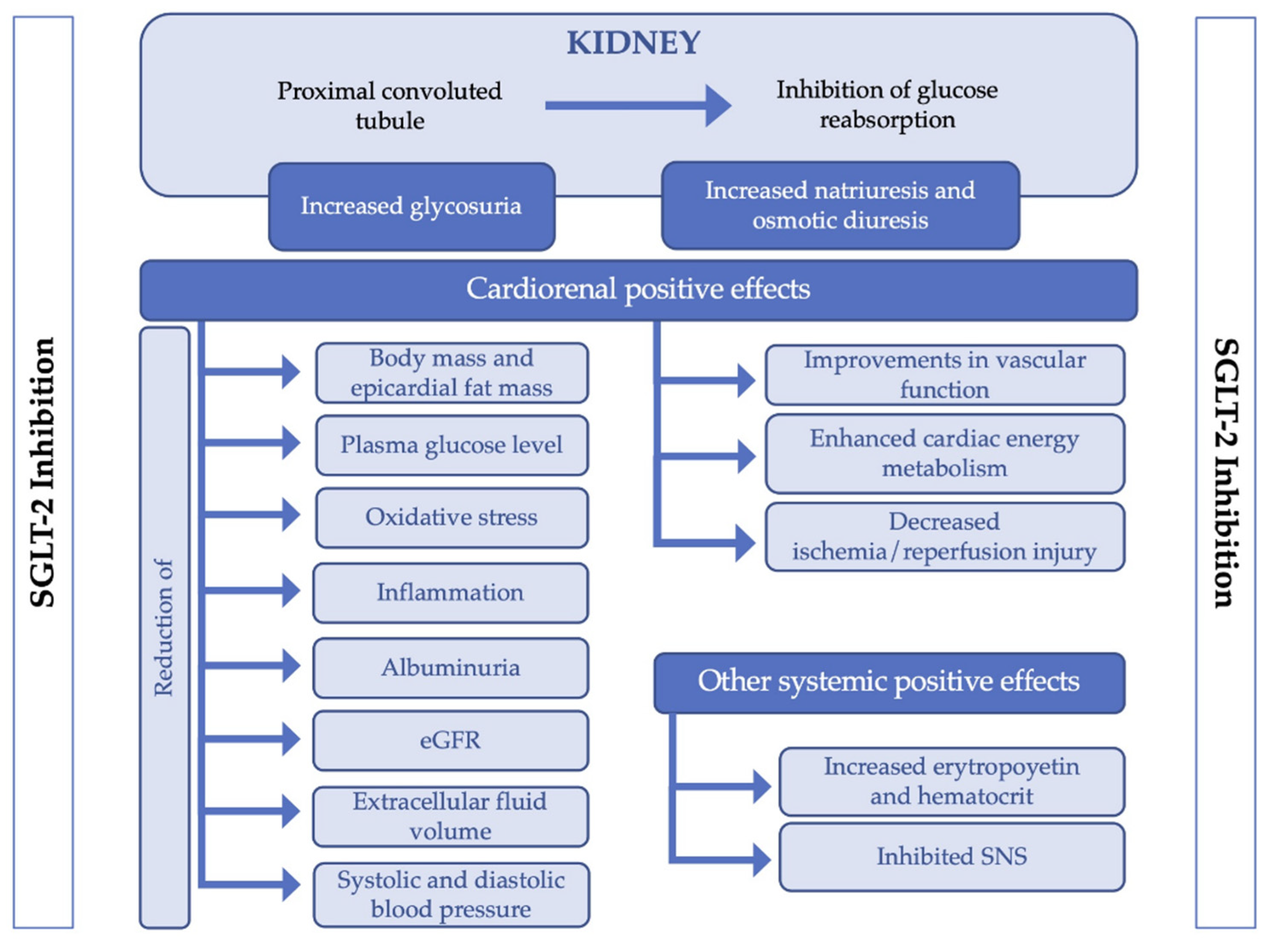
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chao, E.C.; Henry, R.R. SGLT2 inhibition—A novel strategy for diabetes treatment. Nat. Rev. Drug Discov. 2010, 9, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Wang, Z.; Han, R.; Zhang, Z.; Li, Y.; Qin, X.; Zhao, M.; Xiang, R.; Yang, J. Incretin mimetics and sodium-glucose co-transporter 2 inhibitors as monotherapy or add-on to metformin for treatment of type 2 diabetes: A systematic review and network meta-analysis. Acta Diabetol. 2020. [Google Scholar] [CrossRef]
- Shyangdan, D.S.; Uthman, O.A.; Waugh, N. SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. BMJ Open 2016, 6. [Google Scholar] [CrossRef]
- Zaccardi, F.; Webb, D.; Htike, Z.; Youssef, D.; Khunto, K.; Davies, M.J. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: Systematic review and network meta-analysis. Diabetes Obes. Metab. 2016, 18, 783–794. [Google Scholar] [CrossRef]
- Ferreira, G.S.; Veening-Griffioen, D.H.; Boon, W.P.C.; Hooijmans, C.R.; Moors, E.H.M.; Schellekens, H.; van Meer, P.J.K. Comparison of drug efficacy in two animal models of type 2 diabetes: A systematic review and meta-analysis. Eur. J. Pharmacol. 2020, 879, 173153. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A. Risks Associated with SGLT2 Inhibitors: An Overview. Curr. Drug Saf. 2018, 13, 84–91. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Alshnbari, A.S.; Millar, S.A.; O’Sullivan, S.E.; Idris, I. Effect of Sodium-Glucose Cotransporter-2 Inhibitors on Endothelial Function: A Systematic Review of Preclinical Studies. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2020, 11, 1947–1963. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a position statement of the american diabetes association and the european association for the study of diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef]
- Saver, J.L.; Lewis, R.J. Number Needed to Treat: Conveying the Likelihood of a Therapeutic Effect. JAMA 2019, 321, 798–799. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A.A.; Bender, R.; Glasziou, P.; Straus, S.E.; Tricco, A.C. The number needed to treat in pairwise and network meta-analysis and its graphical representation. J. Clin. Epidemiol. 2019, 111, 11–22. [Google Scholar] [CrossRef]
- Hutton, B.; Catala-Lopez, F.; Moher, D. The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA. Med. Clin. 2016, 147, 262–266. [Google Scholar] [CrossRef]
- Higgings, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Higgins, J.; Sterne, J.; Savovic, J.; Page, M.; Hróbjartsson, A.; Boutron, I.; Reeves, B.S.E. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst. Rev. 2016, 10, 29–31. [Google Scholar]
- Puhan, M.A.; Schünemann, H.J.; Murad, M.H.; Li, T.; Brignardello-Petersen, R.; Singh, J.A.; Kessels, A.G.; Guyatt, G.H. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014, 349, g5630. [Google Scholar] [CrossRef]
- Food and Drug Administration What Is a Serious Adverse Event? Available online: https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event (accessed on 12 November 2020).
- Laupacis, A.; Sackett, D.L.; Roberts, R.S. An Assessment of Clinically Useful Measures of the Consequences of Treatment. N. Engl. J. Med. 1988, 318, 1728–1733. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G. Confidence intervals for the number needed to treat. BMJ 1998, 317, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Andersen, P.K. Calculating the number needed to treat for trials where the outcome is time to an event. Br. Med. J. 1999, 319, 1492–1495. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Chaimani, A.; Higgins, J.P.T.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical Tools for Network Meta-Analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P.A. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- White, I. Network meta-analysis. Stata J. 2015, 15, 951–985. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M.; Smith, G.D. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. Br. Med. J. 2001, 323, 101–105. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; Von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B.; et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; De Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Cannon, C.P.; McGuire, D.K.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Charbonnel, B.; Shih, W.J.; Gallo, S.; Masiukiewicz, U.; et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV). Am. Heart J. 2018, 206, 11–23. [Google Scholar] [CrossRef]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.I.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 Inhibitors with Cardiovascular and Kidney Outcomes in Patients with Type 2 Diabetes: A Meta-analysis. JAMA Cardiol. 2020, 75235, 1–11. [Google Scholar] [CrossRef]
- Kluger, A.Y.; Tecson, K.M.; Lee, A.Y.; Lerma, E.V.; Rangaswami, J.; Lepor, N.E.; Cobble, M.E.; McCullough, P.A. Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc. Diabetol. 2019, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Clegg, L.E.; Heerspink, H.J.L.; Penland, R.C.; Tang, W.; Boulton, D.W.; Bachina, S.; Fox, R.D.; Fenici, P.; Thuresson, M.; Mentz, R.J.; et al. Reduction of Cardiovascular Risk and Improved Estimated Glomerular Filtration Rate by SGLT2 Inhibitors, Including Dapagliflozin, Is Consistent Across the Class: An Analysis of the Placebo Arm of EXSCEL. Diabetes Care 2019, 42, 318–326. [Google Scholar] [CrossRef]
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Butler, A.E.; Atkin, S.L.; Katsiki, N.; Sahebkar, A. Sodium–glucose cotransporter 2 inhibitors and inflammation in chronic kidney disease: Possible molecular pathways. J. Cell. Physiol. 2018, 234, 223–230. [Google Scholar] [CrossRef]
- Margonato, D.; Galati, G.; Mazzetti, S.; Cannistraci, R.; Perseghin, G.; Margonato, A.; Mortara, A. Renal protection: A leading mechanism for cardiovascular benefit in patients treated with SGLT2 inhibitors. Heart Fail. Rev. 2021, 26, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.Y.; Foote, C.; Blomster, J.; Toyama, T.; Perkovic, V.; Sundström, J.; Neal, B. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016, 4, 411–419. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, X.; Ilyas, I.; Zheng, X.; Luo, S.; Little, P.J.; Kamato, D.; Sahebkar, A.; Wu, W.; Weng, J.; et al. Impact of sodium glucose cotransporter 2 (SGLT2) inhibitors on atherosclerosis: From pharmacology to pre-clinical and clinical therapeutics. Theranostics 2021, 11, 4502–4515. [Google Scholar] [CrossRef]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef]
- Li, S.; Vandvik, P.O.; Lytvyn, L.; Guyatt, G.H.; Palmer, S.C.; Rodriguez-Gutierrez, R.; Foroutan, F.; Agoritsas, T.; Siemieniuk, R.A.C.; Walsh, M.; et al. SGLT-2 inhibitors or GLP-1 receptor agonists for adults with type 2 diabetes: A clinical practice guideline. BMJ 2021, 373, n1091. [Google Scholar] [CrossRef] [PubMed]
- Zaccardi, F.; Khunti, K.; Marx, N.; Davies, M.J. First-line treatment for type 2 diabetes : Is it too early to abandon metformin ? Lancet 2020, 396, 1705–1707. [Google Scholar] [CrossRef]
- Rahman, W.; Solinsky, P.J.; Munir, K.M.; Lamos, E.M. Pharmacoeconomic evaluation of sodium-glucose transporter-2 (SGLT2) inhibitors for the treatment of type 2 diabetes. Expert Opin. Pharmacother. 2019, 20, 151–161. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Vizcaíno, V.; Díez-Fernández, A.; Álvarez-Bueno, C.; Martínez-Alfonso, J.; Cavero-Redondo, I. Safety and Efficacy of SGLT2 Inhibitors: A Multiple-Treatment Meta-Analysis of Clinical Decision Indicators. J. Clin. Med. 2021, 10, 2713. https://doi.org/10.3390/jcm10122713
Martínez-Vizcaíno V, Díez-Fernández A, Álvarez-Bueno C, Martínez-Alfonso J, Cavero-Redondo I. Safety and Efficacy of SGLT2 Inhibitors: A Multiple-Treatment Meta-Analysis of Clinical Decision Indicators. Journal of Clinical Medicine. 2021; 10(12):2713. https://doi.org/10.3390/jcm10122713
Chicago/Turabian StyleMartínez-Vizcaíno, Vicente, Ana Díez-Fernández, Celia Álvarez-Bueno, Julia Martínez-Alfonso, and Iván Cavero-Redondo. 2021. "Safety and Efficacy of SGLT2 Inhibitors: A Multiple-Treatment Meta-Analysis of Clinical Decision Indicators" Journal of Clinical Medicine 10, no. 12: 2713. https://doi.org/10.3390/jcm10122713
APA StyleMartínez-Vizcaíno, V., Díez-Fernández, A., Álvarez-Bueno, C., Martínez-Alfonso, J., & Cavero-Redondo, I. (2021). Safety and Efficacy of SGLT2 Inhibitors: A Multiple-Treatment Meta-Analysis of Clinical Decision Indicators. Journal of Clinical Medicine, 10(12), 2713. https://doi.org/10.3390/jcm10122713








