Genetic Variability of Mineral Content in Different Grain Structures of Bean Cultivars from Mesoamerican and Andean Gene Pools
Abstract
:1. Introduction
2. Results
2.1. ANOVA: Mineral and Protein Contents in Grains
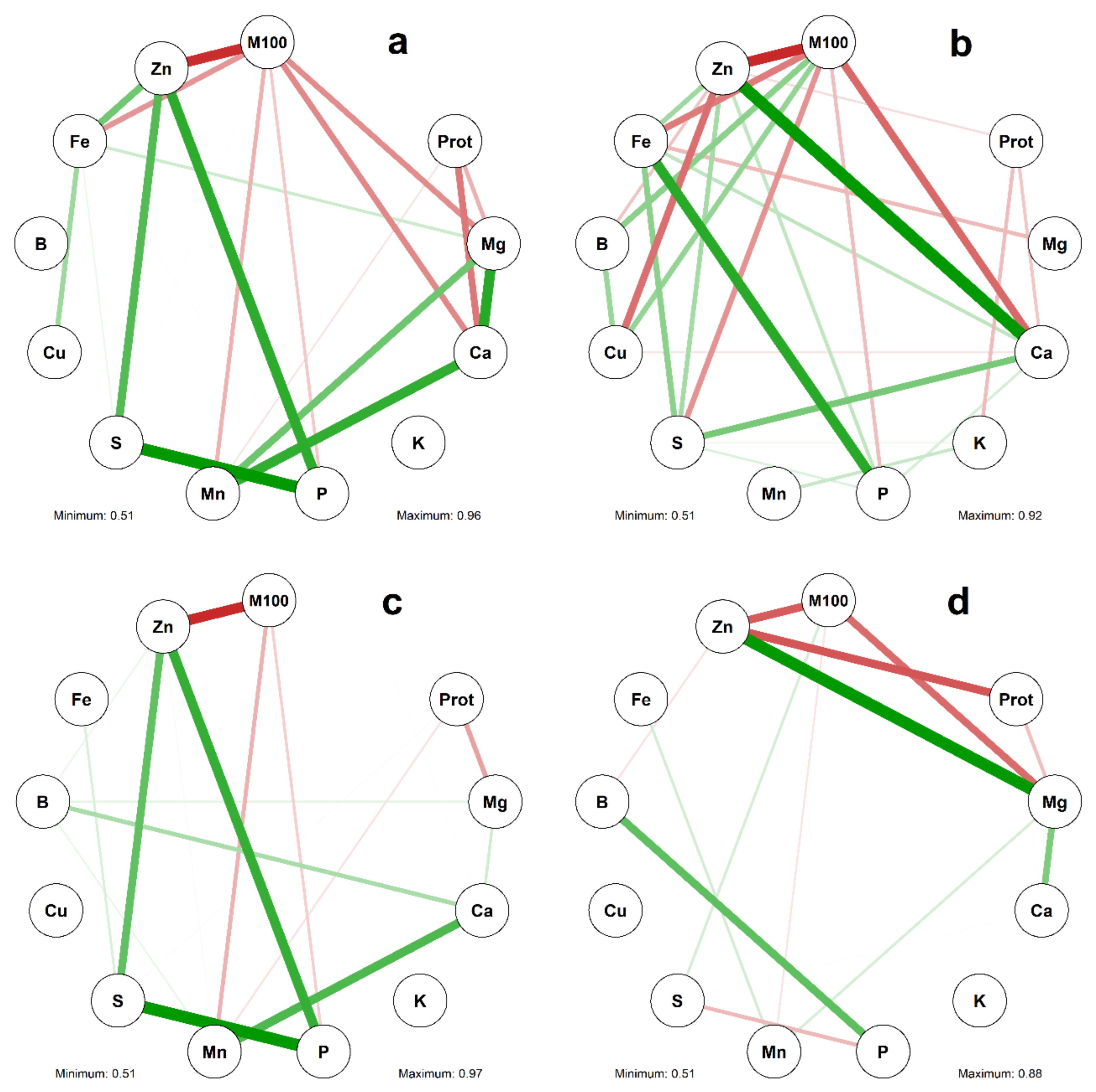
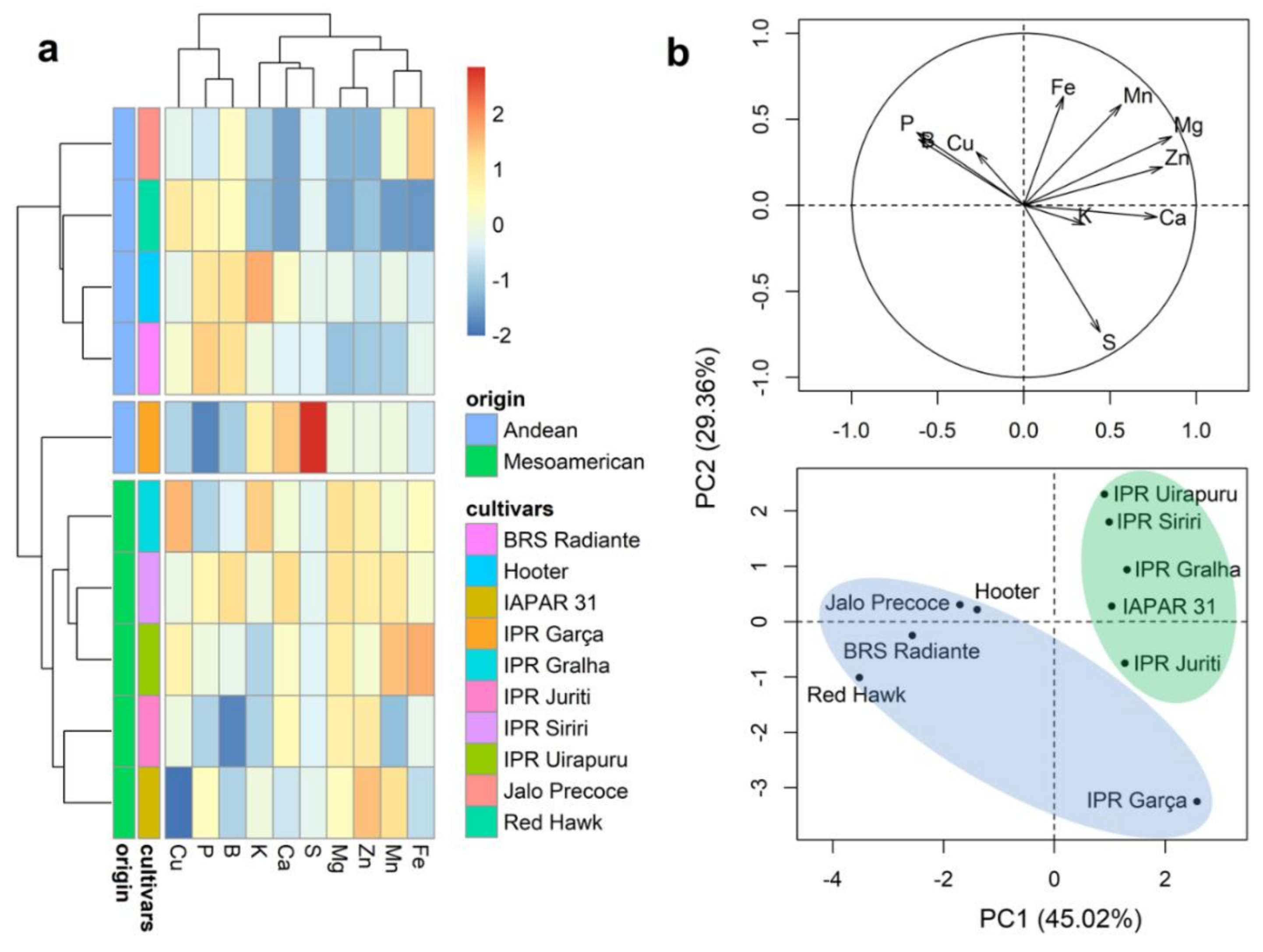
2.2. Correlation between Characters
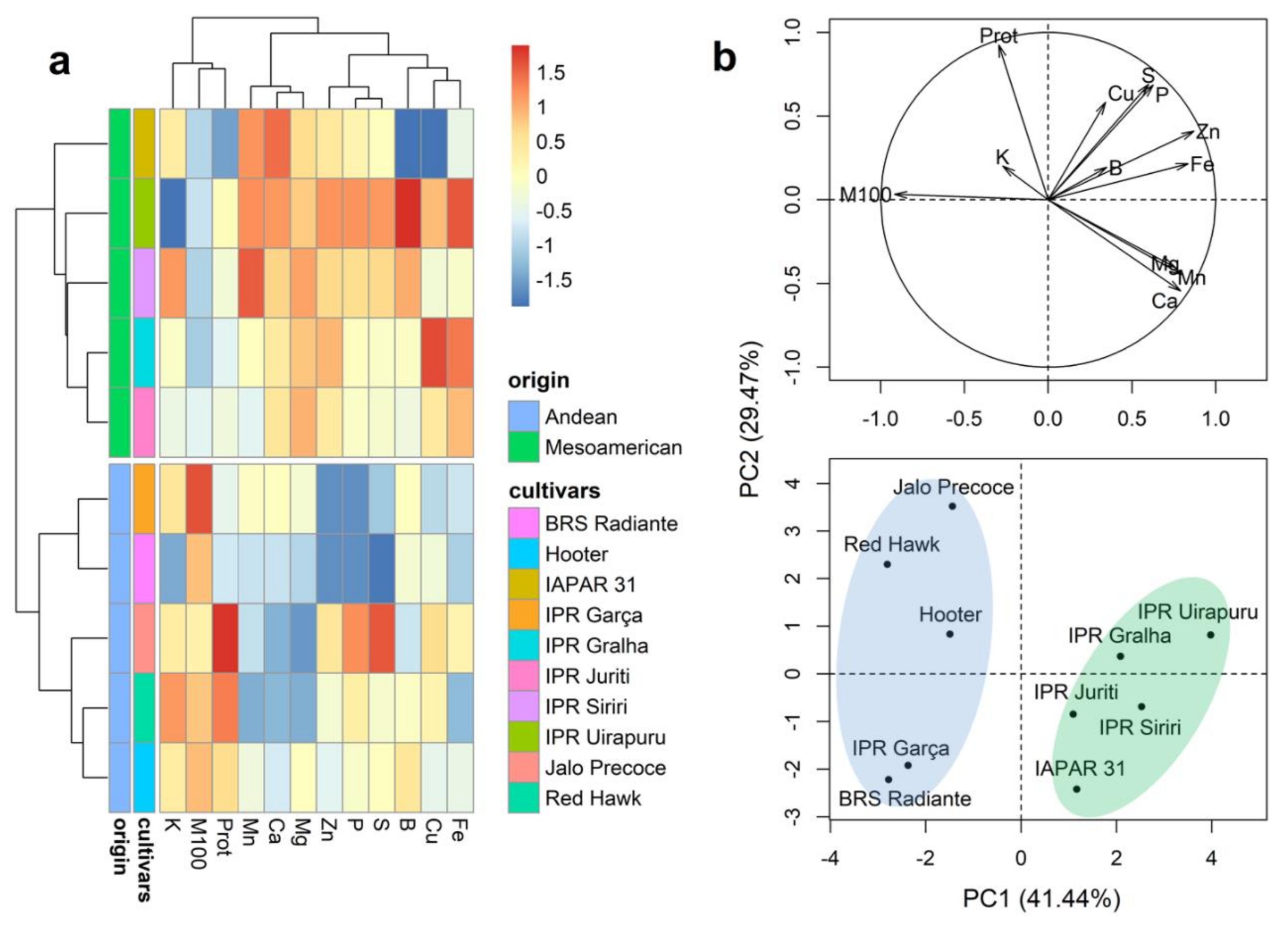
2.3. Mineral Content of Whole Grains
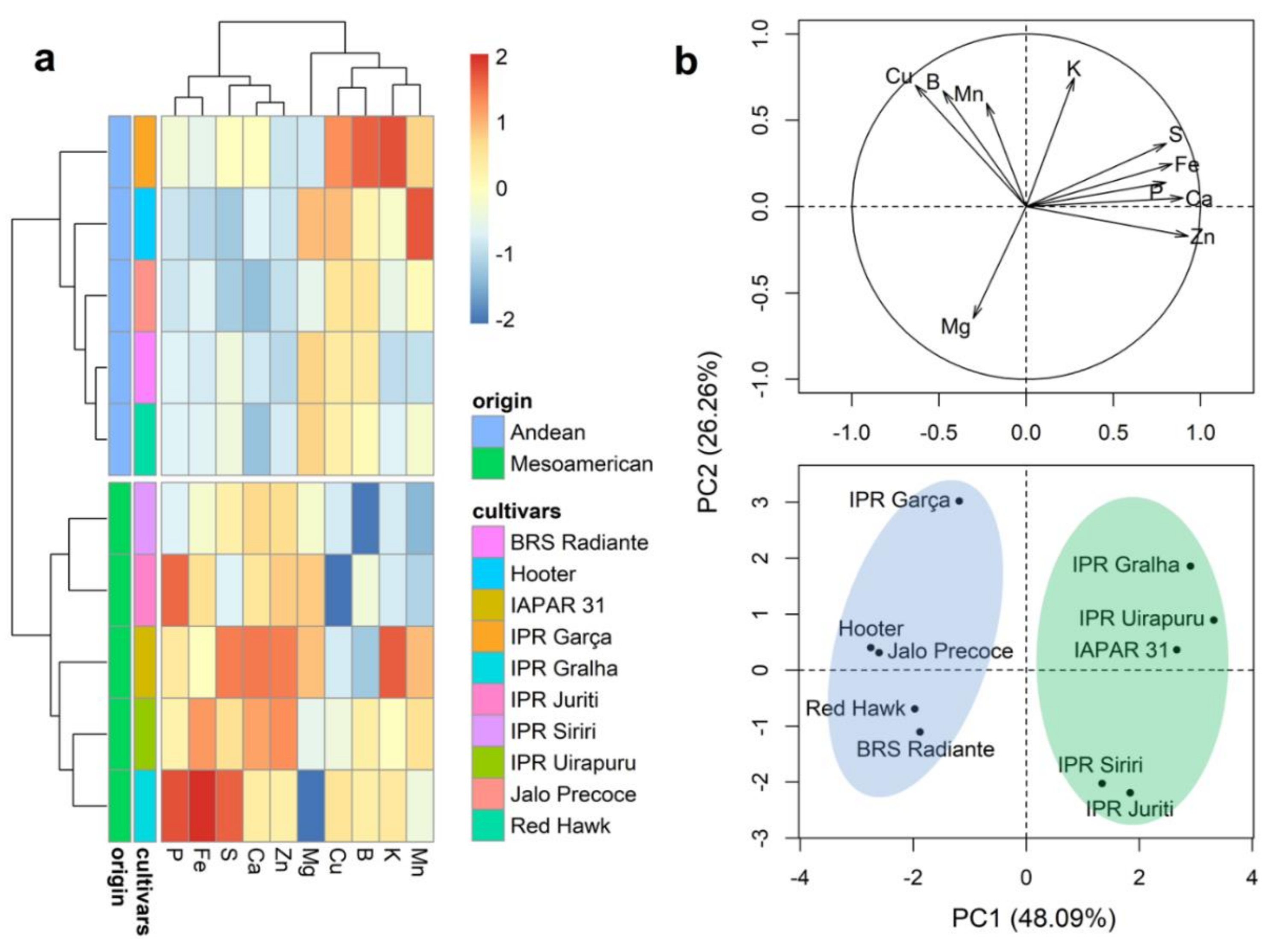
2.4. Mineral Content of the Seed Coat
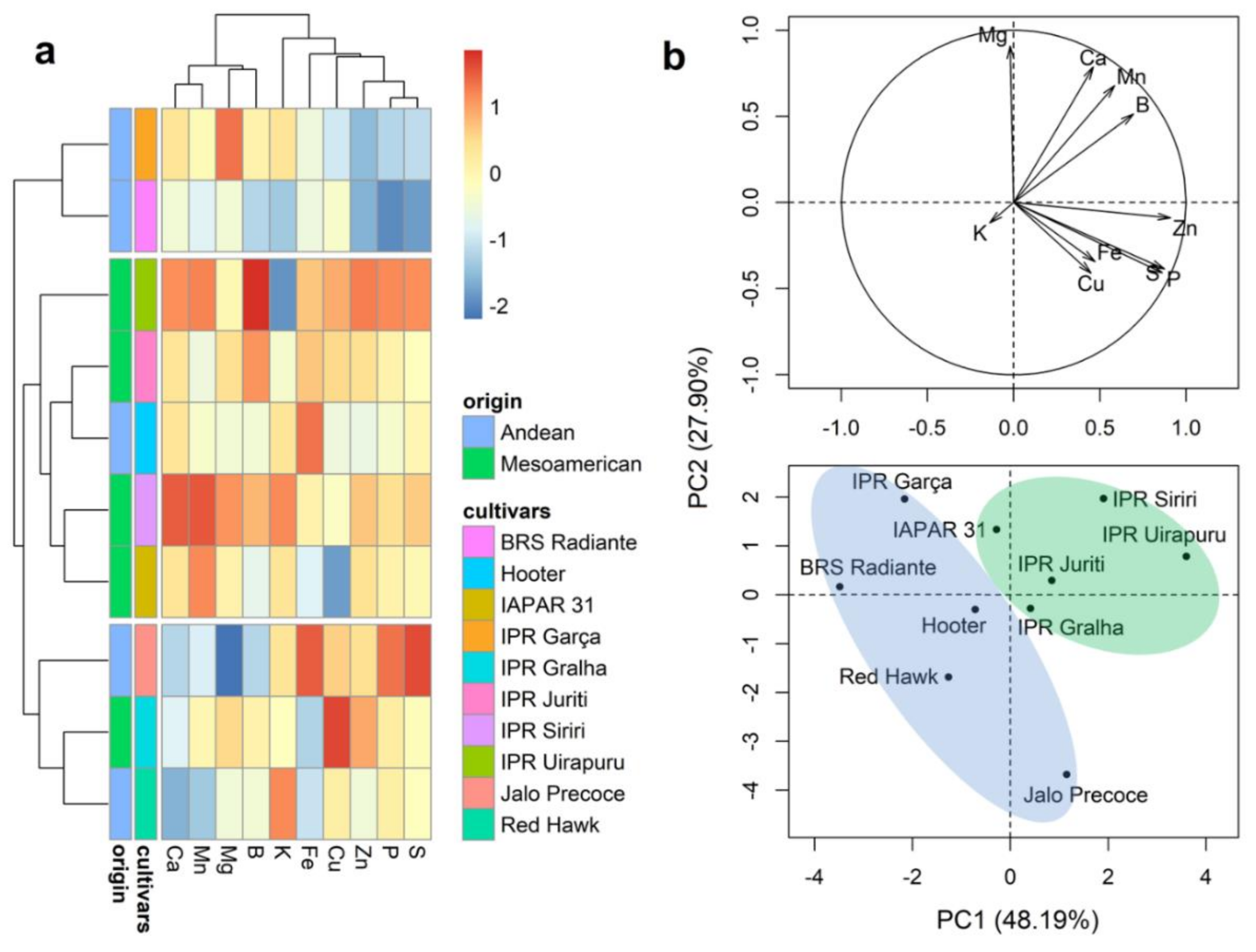
2.5. Mineral Content of Cotyledons
2.6. Mineral Content in the Embryonic Axis
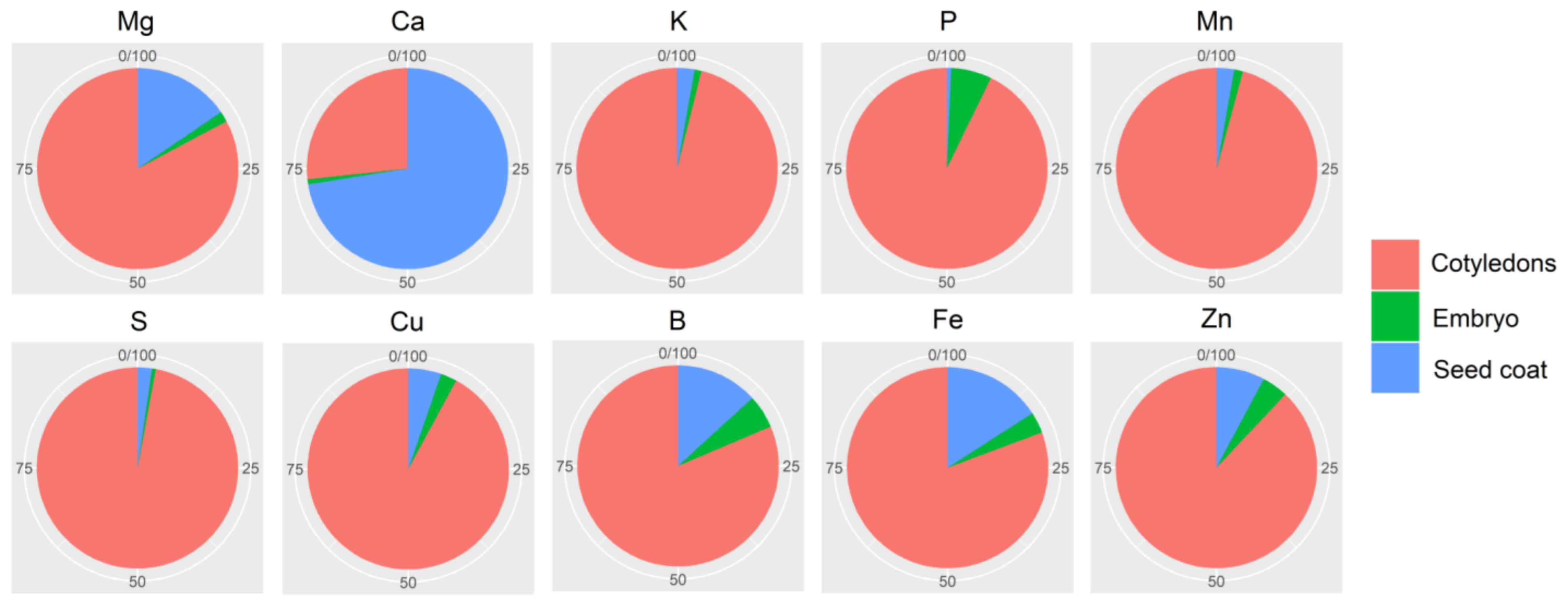
2.7. Mineral Partitioning in Grains
3. Discussion
3.1. Genetic Variability of Mineral Contents
3.2. The Gene Pool and Correlation between Characters
3.3. Macronutrients: Ca, P, Mg, and K
3.4. Micronutrients: Mn, S, Cu, B, Fe, and Zn
4. Material and Methods
4.1. Plant Material and Experimental Design
4.2. Analysis of Mineral Composition and Protein Content
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luna-Vital, D.A.; Mojica, L.; de Mejía, E.G.; Mendoza, S.; Loarca-Piña, G. Biological potential of protein hydroly-sates and peptides from common bean (Phaseolus vulgaris L.): A review. Food Res. Int. 2015, 76, 39–50. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-Rich Dry Common Beans (Phaseolus vulgaris L.) and Their Health Benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef] [Green Version]
- Los, F.G.B.; Zielinski, A.A.F.; Wojeicchowski, J.P.; Nogueira, A.; Demiate, I.M. Beans (Phaseolus vulgaris L.): Whole seeds with complex chemical composition. Curr. Opin. Food Sci. 2018, 19, 63–71. [Google Scholar] [CrossRef]
- Delfini, J.; Moda-Cirino, V.; Neto, J.D.S.; Buratto, J.S.; Ruas, P.M.; Gonçalves, L.S.A. Diversity of nutritional content in seeds of Brazilian common bean germplasm. PLoS ONE 2020, 15, e0239263. [Google Scholar] [CrossRef] [PubMed]
- Castro-Guerrero, N.A.; Isidra-Arellano, M.C.; Mendoza-Cozatl, D.G.; Valdés-López, O. Common Bean: A Legume Model on the Rise for Unraveling Responses and Adaptations to Iron, Zinc, and Phosphate Deficiencies. Front. Plant Sci. 2016, 7, 600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celmeli, T.; Sari, H.; Canci, H.; Sari, D.; Adak, A.; Eker, T.; Toker, C. The Nutritional Content of Common Bean (Phaseolus vulgaris L.) Landraces in Comparison to Modern Varieties. Agronomy 2018, 8, 166. [Google Scholar] [CrossRef] [Green Version]
- Beebe, S.; Gonzalez, A.V.; Rengifo, J. Research on Trace Minerals in the Common Bean. Food Nutr. Bull. 2000, 21, 387–391. [Google Scholar] [CrossRef]
- Akond, A.G.M.; Crawford, H.; Berthold, J.; Talukder, Z.I.; Hossain, K. Minerals (Zn, Fe, Ca and Mg) and Antinutrient (Phytic Acid) Constituents in Common Bean. Am. J. Food Technol. 2011, 6, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chávez-Mendoza, C.; Hernández-Figueroa, K.I.; Sánchez, E. Antioxidant Capacity and Phytonutrient Content in the Seed Coat and Cotyledon of Common Beans (Phaseolus vulgaris L.) from Various Regions in Mexico. Antioxidants 2018, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Blair, M.W. Mineral Biofortification Strategies for Food Staples: The Example of Common Bean. J. Agric. Food Chem. 2013, 61, 8287–8294. [Google Scholar] [CrossRef]
- Figueiredo, M.A.; Boldrin, P.F.; Hart, J.J.; de Andrade, M.J.; Guilherme, L.R.; Glahn, R.P.; Li, L. Zinc and sele-nium accumulation and their effect on iron bioavailability in common bean seeds. Plant Physiol. Biochem. 2017, 111, 193–202. [Google Scholar] [CrossRef]
- Sathe, S.K.; Venkatachalam, M. Bean. In Encyclopedia of Grain Science; Wrigley, C., Corke, H., Walker, C.E., Eds.; Elsevier: St. Louis, MO, USA, 2004; Volume 1, pp. 76–86. [Google Scholar]
- Ribeiro, N.D.; Maziero, S.M.; Prigol, M.; Nogueira, C.W.; Rosa, D.P.; Possobom, M.T.D.F. Mineral concentra-tions in the embryo and seed coat of common bean cultivars. J. Food Compos. Anal. 2012, 26, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Beyer, P. Golden Rice and ‘Golden’crops for human nutrition. New Biotechnol. 2010, 27, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.; Gepts, P. Structure of genetic diversity in the two major gene pools of common bean (Phaseolus vulgaris L., Fabaceae). Theor. Appl. Genet. 2009, 118, 979–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hacisalihoglu, G.; Ozturk, L.; Cakmak, I.; Welch, R.M.; Kochian, L. Genotypic variation in common bean in re-sponse to zinc deficiency in calcareous soil. Plant Soil 2004, 259, 71–83. [Google Scholar] [CrossRef]
- Petry, N.; Boy, E.; Wirth, J.P.; Hurrell, R.F. Review: The Potential of the Common Bean (Phaseolus vulgaris) as a Vehicle for Iron Biofortification. Nutrients 2015, 7, 1144–1173. [Google Scholar] [CrossRef] [Green Version]
- Blair, M.W.; Wu, X.; Bhandari, D.; Astudillo, C. Genetic dissection of ICP-detected nutrient accumulation in the whole seed of common bean (Phaseolus vulgaris L.). Front. Plant Sci. 2016, 7, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jost, E.; Ribeiro, N.D.; Maziero, S.M.; Cerutti, T.; Rosa, D.P. Gene effects of calcium content in bean grains. Ciência Rural 2009, 39, 31–37. [Google Scholar] [CrossRef] [Green Version]
- McClean, P.E.; Lee, R.K.; Otto, C.; Gepts, P.; Bassett, M.J. Molecular and phenotypic mapping of genes controlling seed coat pattern and color in common bean (Phaseolus vulgaris L.). J. Hered. 2002, 93, 148–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariza-Nieto, M.; Blair, M.W.; Welch, R.M.; Glahn, R.P. Screening of Iron Bioavailability Patterns in Eight Bean (Phaseolus vulgaris L.) Genotypes Using the Caco-2 Cell in Vitro Model. J. Agric. Food Chem. 2007, 55, 7950–7956. [Google Scholar] [CrossRef] [PubMed]
- Cominelli, E.; Pilu, R.; Sparvoli, F. Phytic Acid and Mineral Biofortification Strategies: From Plant Science to Breeding and Biotechnological Approaches. Plants 2020, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Hummel, M.; Talsma, E.F.; Taleon, V.; Londoño, L.; Brychkova, G.; Gallego, S.; Raatz, B.; Spillane, C. Iron, Zinc and Phytic Acid Retention of Biofortified, Low Phytic Acid, and Conventional Bean Varieties When Preparing Common Household Recipes. Nutrients 2020, 12, 658. [Google Scholar] [CrossRef] [Green Version]
- Fileppi, M.; Galasso, I.; Tagliabue, G.; Daminati, M.G.; Campion, B.; Doria, E.; Sparvoli, F. Characterisation of structural genes involved in phytic acid biosynthesis in common bean (Phaseolus vulgaris L.). Mol. Breed. 2010, 25, 453–470. [Google Scholar] [CrossRef]
- Caballero, B. Global patterns of child health: The role of nutrition. Ann. Nutr. Metab. 2002, 46, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Goldbach, H.E.; Wimmer, M.A. Boron in plants and animals: Is there a role beyond cell-wall structure? J. Plant Nutr. Soil Sci. 2007, 170, 39–48. [Google Scholar] [CrossRef]
- Métayer, S.; Seiliez, I.; Collin, A.; Duchêne, S.; Mercier, Y.; Geraert, P.A.; Tesseraud, S. Mechanisms through which sulfur amino acids control protein metabolism and oxidative status. J. Nutr. Biochem. 2008, 19, 207–215. [Google Scholar] [CrossRef]
- La Frano, M.R.; de Moura, F.F.; Boy, E.; Lönnerdal, B.; Burri, B.J. Bioavailability of iron, zinc, and provitamin A carotenoids in biofortified staple crops. Nutr. Rev. 2014, 72, 289–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukankusi, C.; Cowling, W.A.; Siddique, K.H.; Li, L.; Kinghorn, B.; Rubyogo, J.C. Diversity Breeding Program on Common Bean (Phaseolus vulgaris L.) Targeting Rapid Cooking and Iron and Zinc Biofortification. Proceedings 2020, 36, 6194. [Google Scholar] [CrossRef] [Green Version]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Balk, J.; Connorton, J.M.; Wan, Y.; Lovegrove, A.; Moore, K.L.; Uauy, C.; Sharp, P.A.; Shewry, P.R. Improving wheat as a source of iron and zinc for global nutrition. Nutr. Bull. 2019, 44, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.W.; Medina, J.I.; Astudillo, C.; Rengifo, J.; Beebe, S.E.; Machado, G.; Graham, R. QTL for seed iron and zinc concentration and content in a Mesoamerican common bean (Phaseolus vulgaris L.) population. Theor. Appl. Genet. 2010, 121, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, S.S.; Ribeiro, N.D.; Jost, E.; Reiniger LR, S.; Rosa, D.P.; Cerutti, T.; Possobom, M.T.D.F. Potential for increasing the zinc content in common bean using genetic improvement. Euphytica 2010, 175, 207–213. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated World Map of the Köppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, F.; Gepts, P.; Lopez, M. Developmental stages in the bean plant. In Bean: Research and Production; Lopez, M., Fernandez, F., Schoonhoven, A., Eds.; CIAT: Cali, Colombia, 1985; pp. 61–78. [Google Scholar]
- Miyazawa, M.; Pavan, M.; Muraoka, T.; Carmo, C.A.F.S.; Mello, W.J. Análise química de tecido vegetal. In Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Silva, F.C., Ed.; EMBRAPA: Brasília, Brazil, 1999; pp. 171–223. [Google Scholar]
- Scott, A.J.; Knott, M. A Cluster Analysis Method for Grouping Means in the Analysis of Variance. Biometrics 1974, 30, 507. [Google Scholar] [CrossRef] [Green Version]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Mojena, R. Hierarchical grouping methods and stopping rules: An evaluation. Comput. J. 1977, 20, 359–363. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes: An R Package for ANOVA and Experimental Designs. Appl. Math. 2014, 5, 2952–2958. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A.; Mundt, F. Package ‘factoextra’. Extr. Vis. Results Multivar. Data Anal. 2017, 76, 337–354. [Google Scholar]
- Kolde, R.; Kolde, M.R. Package ’pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- Epskamp, S.; Cramer, A.O.; Waldorp, L.J.; Schmittmann, V.D.; Borsboom, D. qgraph: Network visualizations of relationships in psychometric data. J. Stat. Softw. 2012, 48, 1–18. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Average ± Standard Deviation | F Test-ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Whole Grain | Seed Coat | Cotyledons | Embryonic Axis | Cultivar (C) | Tissue (T) | C × T | |
| Mass of 100 grains (g) | 38.220 ± 10.920 | – | – | – | ** | – | – |
| Protein content (%) | 17.810 ± 1.480 | – | – | – | ** | – | – |
| Mg (g kg−1) | 1.827 ± 0.119 | 2.396 ± 0.116 | 1.783 ± 0.139 | 1.208 ± 0.112 | ** | ** | * |
| Ca (g kg−1) | 0.656 ± 0.098 | 5.625 ± 1.051 | 0.191 ± 0.021 | 0.406 ± 0.035 | ** | ** | ** |
| K (g kg−1) | 17.731 ± 0.723 | 5.100 ± 1.300 | 15.759 ± 3.134 | 12.576 ± 0.508 | ** | ** | ** |
| P (g kg−1) | 2.817 ± 0.188 | 0.226 ± 0.043 | 2.871 ± 0.137 | 4.262 ± 0.556 | ** | ** | ns |
| S (g kg−1) | 2.679 ± 0.236 | 0.749 ± 0.121 | 2.885 ± 0.143 | 1.288 ± 0.262 | ns | ** | ns |
| Mn (mg kg−1) | 13.712 ± 2.341 | 4.303 ± 0.511 | 14.563 ± 2.052 | 16.020 ± 0.611 | ** | ** | ** |
| Cu (mg kg−1) | 9.269 ± 0.981 | 5.690 ± 0.612 | 9.443 ± 0.823 | 19.151 ± 1.363 | ** | ** | * |
| B (mg kg−1) | 7.238 ± 0.422 | 11.110 ± 0.646 | 6.473 ± 0.313 | 31.213 ± 3.592 | ** | ** | * |
| Fe (mg kg−1) | 42.331 ± 2.871 | 81.134 ± 24.196 | 37.492 ± 2.630 | 112.811 ± 8.292 | ** | ** | ** |
| Zn (mg kg−1) | 22.960 ± 4.277 | 20.375 ± 8.613 | 22.351 ± 2.363 | 73.945 ± 6.627 | ** | ** | ** |
| Cultivar | Color of Seed Coat | Origin | Institution 1 |
|---|---|---|---|
| IPR Siriri | Cream with brown stripes | Mesoamerican | IDR-Paraná |
| IPR Juriti | Cream with brown stripes | Mesoamerican | IDR-Paraná |
| IPR Uirapuru | Black | Mesoamerican | IDR-Paraná |
| IPR Gralha | Black | Mesoamerican | IDR-Paraná |
| IAPAR 31 | Cream with brown spots | Mesoamerican | IDR-Paraná |
| IPR Garça | White | Andean | IDR-Paraná |
| Hooter | Cream with red stripes | Andean | Monsoy Ltd.a. |
| BRS Radiant | Cream with red stripes | Andean | Embrapa |
| Jalo Precoce | Yellow | Andean | Embrapa |
| Red Hawk | Red | Andean | MSU |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeffa, D.M.; Nogueira, A.F.; Buratto, J.S.; Oliveira, R.B.R.d.; Neto, J.d.S.; Moda-Cirino, V. Genetic Variability of Mineral Content in Different Grain Structures of Bean Cultivars from Mesoamerican and Andean Gene Pools. Plants 2021, 10, 1246. https://doi.org/10.3390/plants10061246
Zeffa DM, Nogueira AF, Buratto JS, Oliveira RBRd, Neto JdS, Moda-Cirino V. Genetic Variability of Mineral Content in Different Grain Structures of Bean Cultivars from Mesoamerican and Andean Gene Pools. Plants. 2021; 10(6):1246. https://doi.org/10.3390/plants10061246
Chicago/Turabian StyleZeffa, Douglas Mariani, Alison Fernando Nogueira, Juliana Sawada Buratto, Raquel Barboza Reis de Oliveira, José dos Santos Neto, and Vânia Moda-Cirino. 2021. "Genetic Variability of Mineral Content in Different Grain Structures of Bean Cultivars from Mesoamerican and Andean Gene Pools" Plants 10, no. 6: 1246. https://doi.org/10.3390/plants10061246






