Identification of Abnormal Proteins in Plasma from Gout Patients by LC-MS/MS
Abstract
1. Introduction
2. Materials and Methods
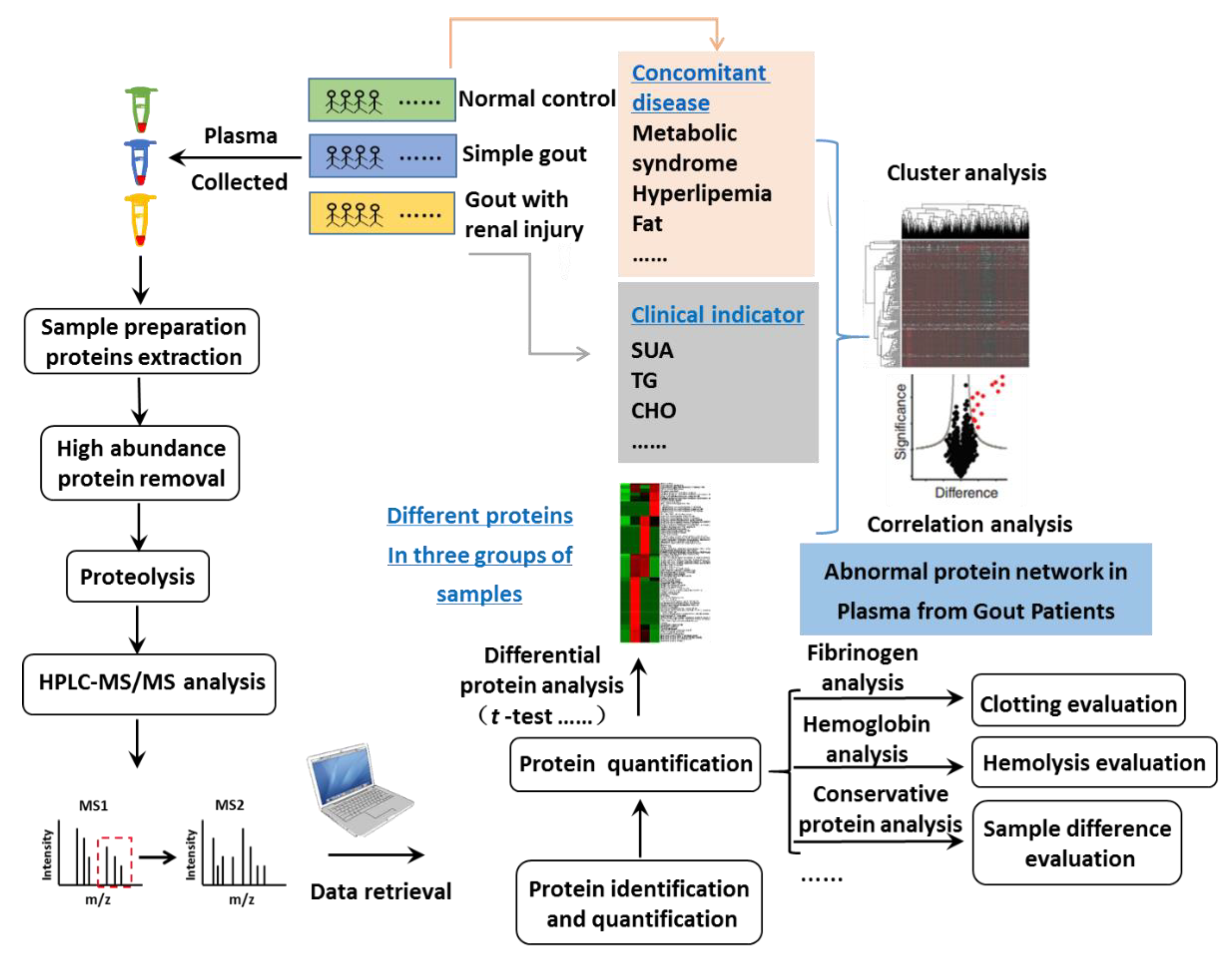
2.1. Study Design
2.2. Methods
2.2.1. The Plasma Sample Collection and Highly Abundant Protein Depletion
2.2.2. Plasma Protein Sample Preparation
2.2.3. LC-MS/MS Analysis
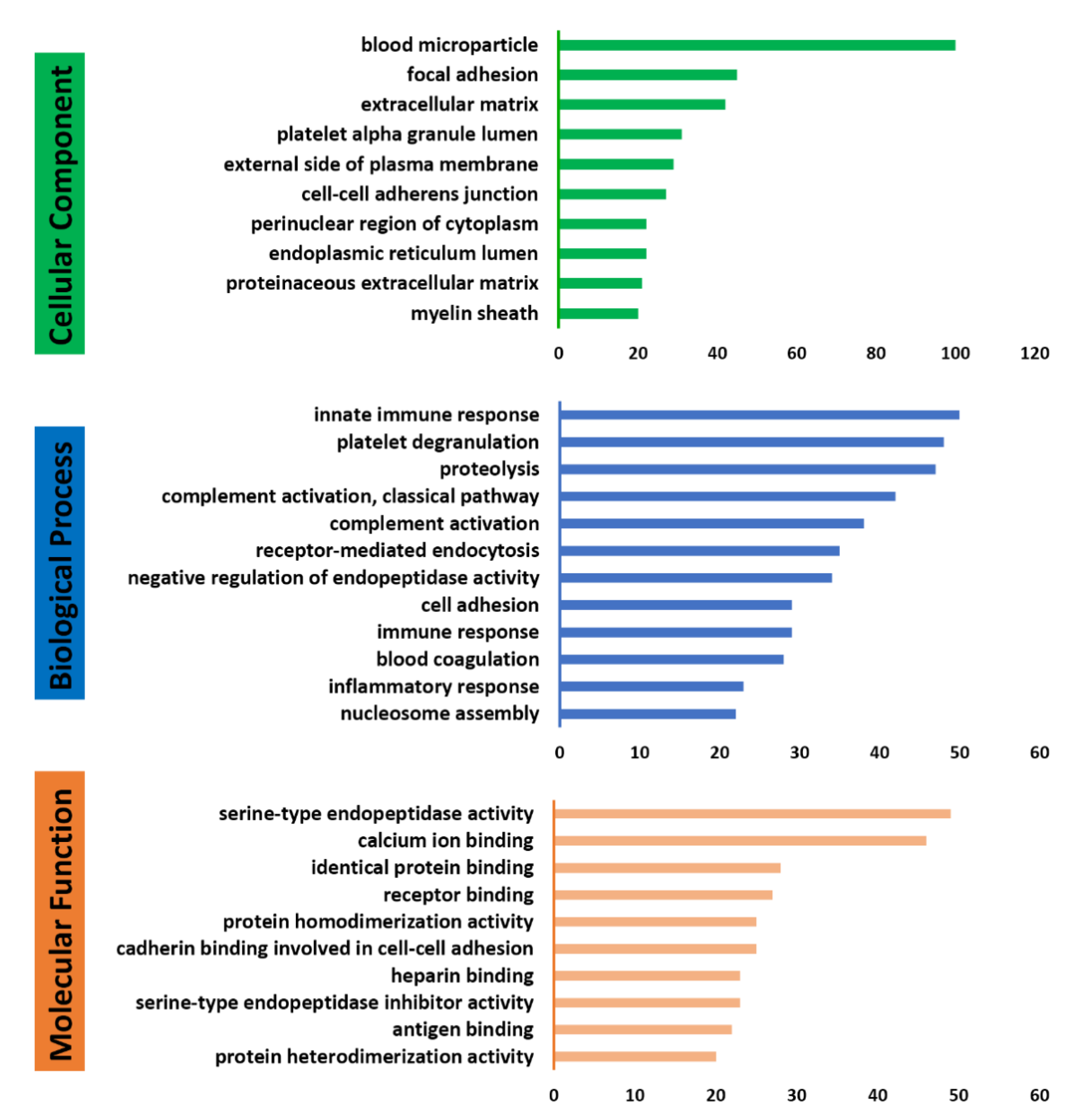
2.2.4. Database Searching and Bioinformatics Analysis
2.2.5. Validation of Identified Proteins by ELISA
2.2.6. Statistical Analysis
3. Results
3.1. Profile of Plasma Proteomes in Gout Study
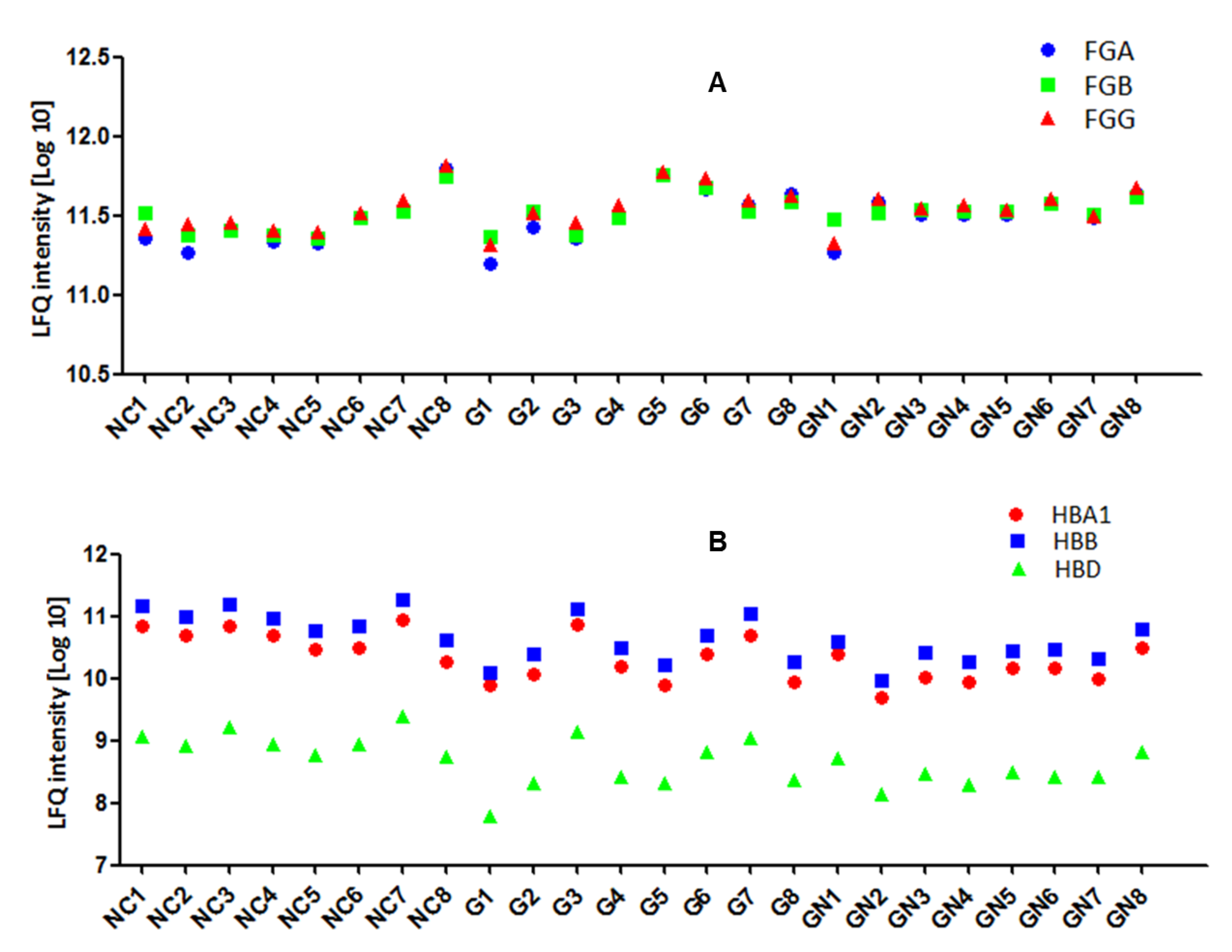
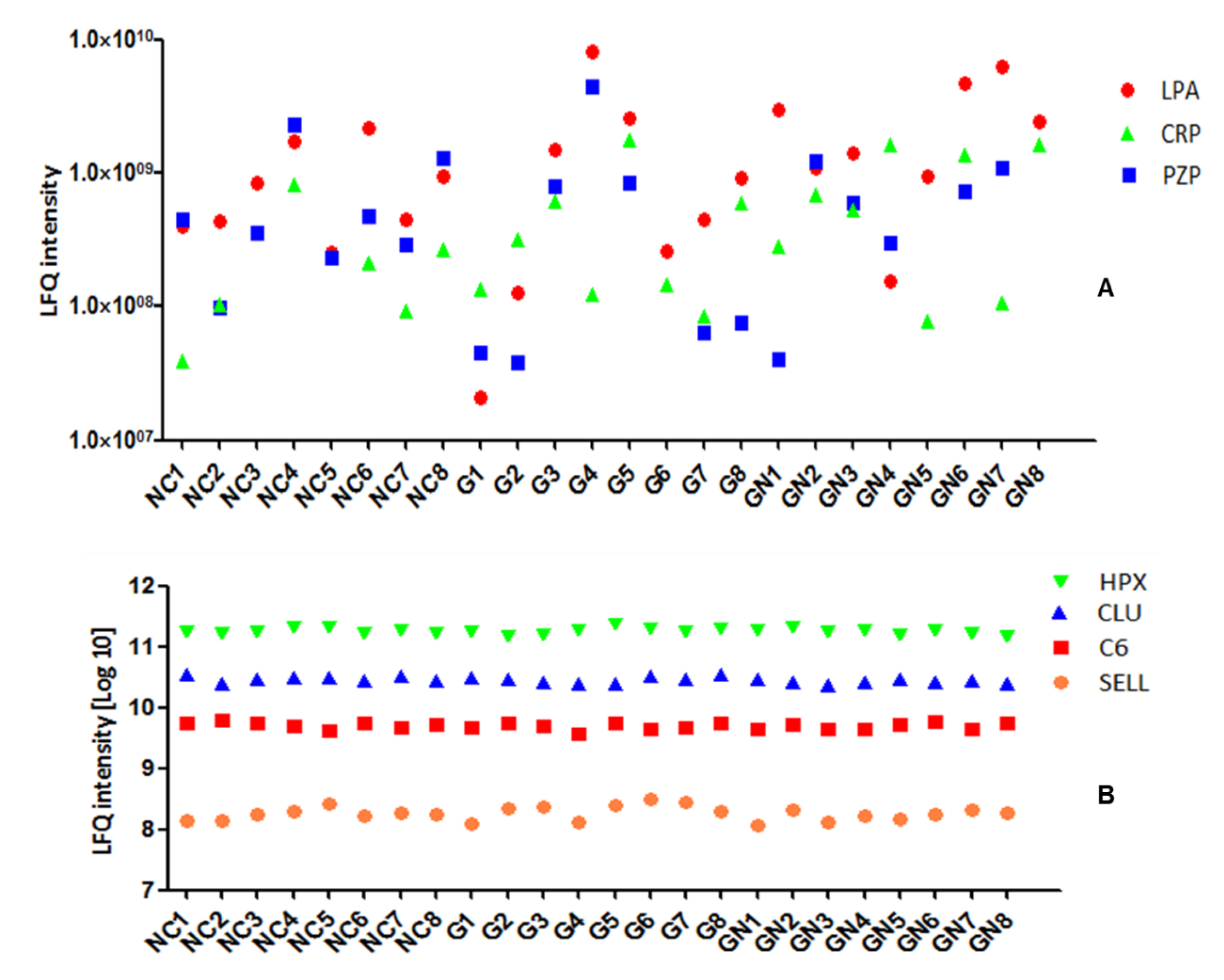
3.2. Plasma Protein Levels Are Individual-Specific
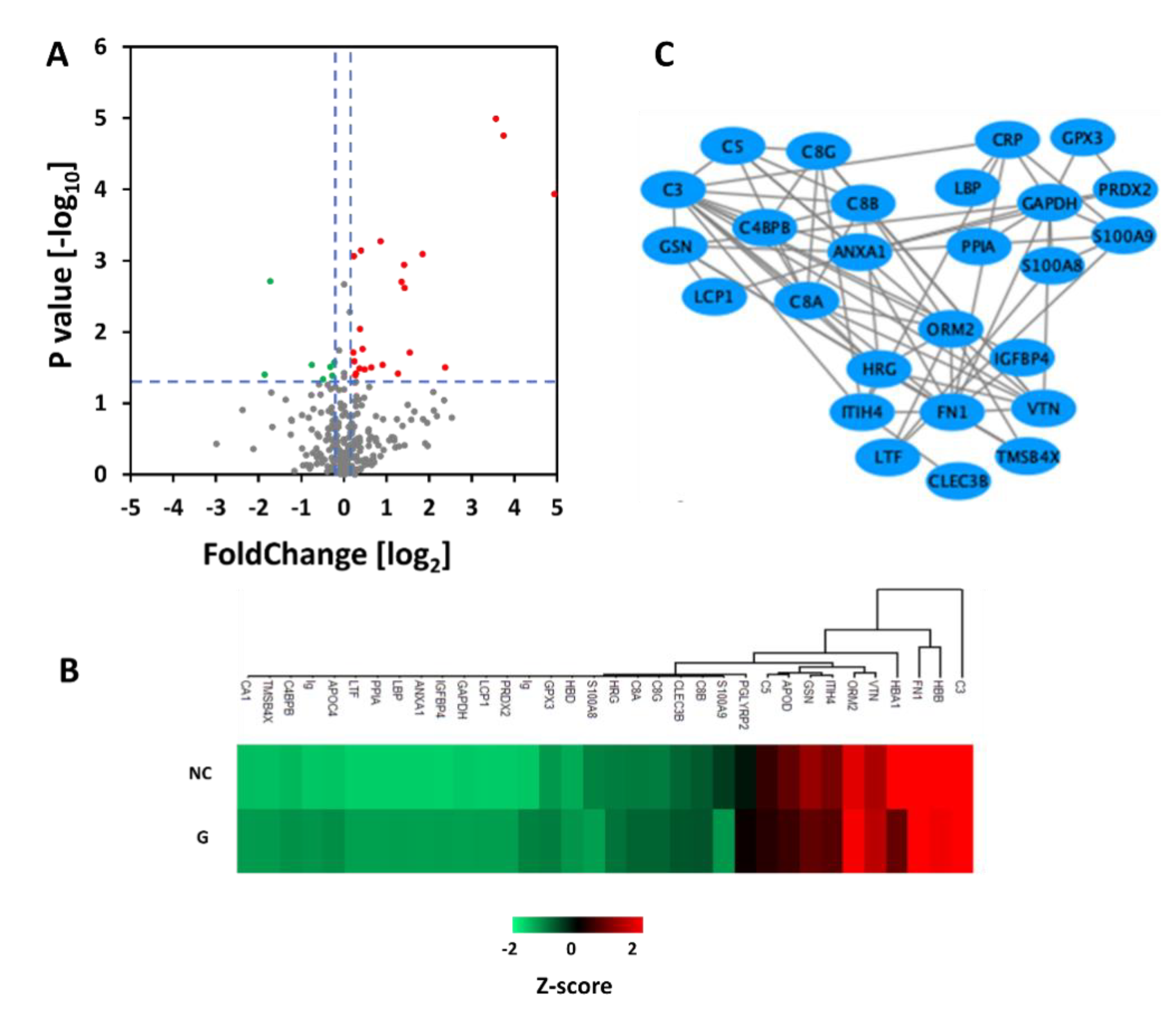
3.3. Comparison of Proteins in Plasma between Normal Control Group and Gout Group
3.4. Comparison of Proteins in Plasma between Gout and Gout with Renal Injury
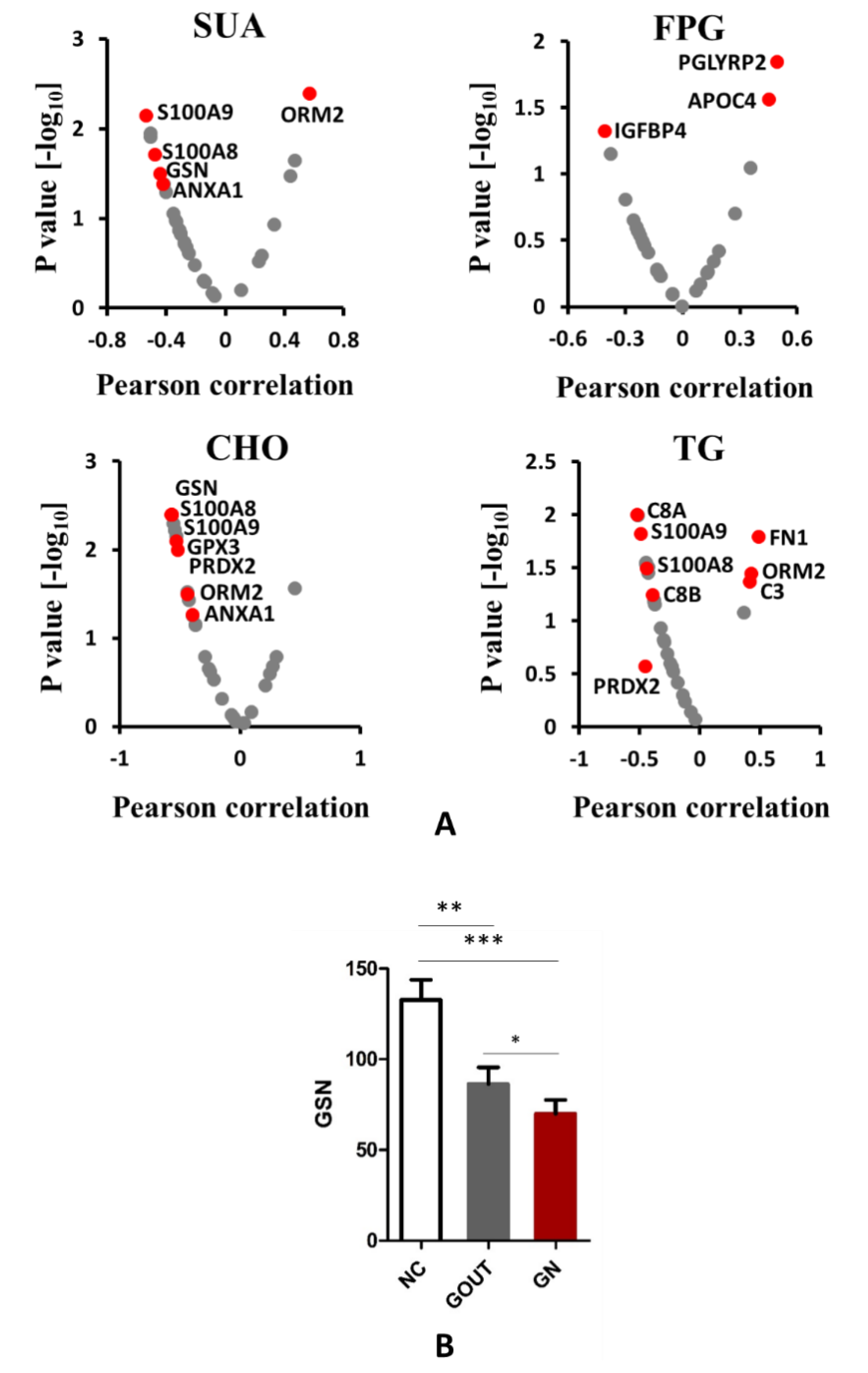
3.5. The Relationship between Plasma Protein and Clinical Biochemical Indexes
3.6. Validation of GSN in Individual Subjects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- C’abau, G.; Crisan, T.O.; Kluck, V.; Popp, R.A.; Joosten, L.A.B. Urate-induced immune programming: Consequences for gouty arthritis and hyperuricemia. Immunol. Rev. 2020, 294, 92–105. [Google Scholar] [CrossRef]
- Kuriyama, S. Dotinurad: A novel selective urate reabsorption inhibitor as a future therapeutic option for hyperuricemia. Clin Exp. Nephrol. 2020, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chang, Y.; Yun, K.E.; Jung, H.S.; Lee, S.J.; Shin, H.; Ryu, S. Development of Nephrolithiasis in Asymptomatic Hyperuricemia: A Cohort Study. Am. J. Kidney Dis. 2017, 70, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Benn, C.L.; Dua, P.; Gurrell, R.; Loudon, P.; Pike, A.; Storer, R.I.; Vangjeli, C. Physiology of Hyperuricemia and Urate-Lowering Treatments. Front. Med. Lausanne 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Mena-Sanchez, G.; Babio, N.; Becerra-Tomas, N.; Martinez-Gonzalez, M.A.; Diaz-Lopez, A.; Corella, D.; Zomeno, M.D.; Romaguera, D.; Vioque, J.; Alonso-Gomez, A.M.; et al. Association between dairy product consumption and hyperuricemia in an elderly population with metabolic syndrome. Nutr. Metab. Cardiovas 2020, 30, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Punzi, L.; Scanu, A.; Galozzi, P.; Luisetto, R.; Spinella, P.; Scire, C.A.; Oliviero, F. One year in review 2020: Gout. Clin. Exp. Rheumatol. 2020, 38, 807–821. [Google Scholar] [PubMed]
- Liu, H.; Zhang, X.M.; Wang, Y.L.; Liu, B.C. Prevalence of hyperuricemia among Chinese adults: A national cross-sectional survey using multistage, stratified sampling. J. Nephrol 2014, 27, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Gaffo, A. Gout epidemiology and comorbidities. Semin. Arthritis Rheu 2020, 50, S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Geyer, P.E.; Albrechtsen, N.J.W.; Tyanova, S.; Grassl, N.; Iepsen, E.W.; Lundgren, J.; Madsbad, S.; Holst, J.J.; Torekov, S.S.; Mann, M. Proteomics reveals the effects of sustained weight loss on the human plasma proteome. Mol. Syst. Biol. 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Geyer, P.E.; Holdt, L.M.; Teupser, D.; Mann, M. Revisiting biomarker discovery by plasma proteomics. Mol. Syst. Biol. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.E.; Tighiouart, H.; Elsayed, E.F.; Griffith, J.L.; Salem, D.N.; Levey, A.S. Uric acid and incident kidney disease in the community. J. Am. Soc. Nephrol. 2008, 19, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Geyer, P.E.; Kulak, N.A.; Pichler, G.; Holdt, L.M.; Teupser, D.; Mann, M. Plasma Proteome Profiling to Assess Human Health and Disease. Cell Syst. 2016, 2, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Aguilar, M.M.; Aparicio-Bautista, D.I.; Ramirez-Salazar, E.G.; Reyes-Grajeda, J.P.; De la Cruz-Montoya, A.H.; Antuna-Puente, B.; Hidalgo-Bravo, A.; Rivera-Paredez, B.; Ramirez-Palacios, P.; Quiterio, M.; et al. Serum Proteomic Analysis Reveals Vitamin D-Binding Protein (VDBP) as a Potential Biomarker for Low Bone Mineral Density in Mexican Postmenopausal Women. Nutrients 2019, 11, 2853. [Google Scholar] [CrossRef] [PubMed]
- Asare-Werehene, M.; Nakka, K.; Reunov, A.; Chiu, C.T.; Lee, W.T.; Abedini, M.R.; Wang, P.W.; Shieh, D.B.; Dilworth, F.J.; Carmona, E.; et al. The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance. Oncogene 2020, 39, 1600–1616. [Google Scholar] [CrossRef] [PubMed]
- Esawy, M.M.; Makram, W.K.; Albalat, W.; Shabana, M.A. Plasma gelsolin levels in patients with psoriatic arthritis: A possible novel marker. Clin. Rheumatol. 2020, 39, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Vaid, B.; Chopra, B.S.; Raut, S.; Sagar, A.; Badmalia, M.D.; Ashish; Khatri, N. Antioxidant and Wound Healing Property of Gelsolin in 3T3-L1 Cells. Oxid. Med. Cell. Longev. 2020, 2020, 4045365. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Chopra, B.S.; Vaid, B.; Sagar, A.; Raut, S.; Badmalia, M.D.; Ashish; Khatri, N. Protective effects of gelsolin in acute pulmonary thromboembolism and thrombosis in the carotid artery of mice. PLoS ONE 2019, 14, e0215717. [Google Scholar] [CrossRef] [PubMed]
- Asare-Werehene, M.; Communal, L.; Carmona, E.; Le, T.; Provencher, D.; Mes-Masson, A.M.; Tsang, B.K. Pre-operative Circulating Plasma Gelsolin Predicts Residual Disease and Detects Early Stage Ovarian Cancer. Sci. Rep. 2019, 9, 13924. [Google Scholar] [CrossRef] [PubMed]
- Feldt, J.; Schicht, M.; Garreis, F.; Welss, J.; Schneider, U.W.; Paulsen, F. Structure, regulation and related diseases of the actin-binding protein gelsolin. Expert Rev. Mol. Med. 2019, 20, e7. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.P.; Bedugnis, A.; Levinson, S.; DiNubile, M.; Stossel, T.; Lu, Q.; Kobzik, L. Delayed Administration of Recombinant Plasma Gelsolin Improves Survival in a Murine Model of Penicillin-Susceptible and Penicillin-Resistant Pneumococcal Pneumonia. J. Infect. Dis. 2019, 220, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
| Clinical Data | Normal Control | Gout | t | p |
|---|---|---|---|---|
| Numbers | 8 | 16 | - | - |
| Age | 57.63 ± 1.19 | 55.88 ± 6.83 | 0.995 | 0.334 |
| BMI (kg/m2) | 24.74 ± 2.50 | 28.08 ± 4.86 | −1.815 | 0.083 |
| SBP (mmHg) | 121.88 ± 14.05 | 135.31 ± 21.01 | −1.627 | 0.118 |
| DBP (mmHg) | 71.13 ± 14.59 | 87.31 ± 14.02 * | −2.631 | 0.015 |
| SUA (umol/L) | 328.50 ± 47.91 | 497.98 ± 126.85 ** | −4.714 | 0.000 |
| Scr (umol/L) | 88.00 ± 5.83 | 97.94 ± 22.45 | −1.663 | 0.113 |
| BUN (mmol/L) | 6.33 ± 1.27 | 5.88 ± 1.48 | 0.728 | 0.474 |
| eGFR (ml/min) | 100.73 ± 7.79 | 95.65 ± 23.49 | 0.784 | 0.442 |
| TG (mmol/L) | 1.02 ± 0.38 | 2.83 ± 1.65 ** | −4.178 | 0.001 |
| CHO (mmol/L) | 4.50 ± 0.65 | 5.55 ± 0.71 ** | −3.510 | 0.002 |
| ALT (IU/L) | 18.38 ± 3.70 | 27.85 ± 7.92 ** | −3.188 | 0.004 |
| FPG (mmol/L) | 5.10 ± 0.30 | 5.29 ± 0.86 | −0.771 | 0.450 |
| Clinical Data | G | GN | t | p |
|---|---|---|---|---|
| Numbers | 8 | 8 | - | - |
| Age | 57.50 ± 6.72 | 54.25 ± 6.98 | 0.948 | 0.359 |
| Course | 14.38 ± 7.80 | 10.00 ± 6.32 | 1.232 | 0.238 |
| BMI (kg/m2) | 25.98 ± 2.53 | 30.19 ± 5.84 | −1.872 | 0.082 |
| SBP (mmHg) | 131.88 ± 27.50 | 138.75 ± 12.75 | −0.641 | 0.536 |
| DBP (mmHg) | 83.13 ± 17.51 | 91.50 ± 8.65 | −1.213 | 0.245 |
| SUA (umol/L) | 491.4 ± 151.1 | 504.56 ± 107.47 | −0.201 | 0.844 |
| Scr (umol/L) | 78.76 ± 8.22 | 117.13 ± 13.09 ** | −7.020 | 0.000 |
| BUN (mmol/L) | 5.07 ± 0.99 | 6.69 ± 1.47 * | −2.58 | 0.022 |
| eGFR(ml/min) | 116.30 ± 11.23 | 74.99 ± 9.01 ** | 8.116 | 0.000 |
| TG (mmol/L) | 3.46 ± 1.93 | 2.20 ± 1.10 | 1.593 | 0.133 |
| CHO (mmol/L) | 5.40 ± 0.57 | 5.71 ± 0.84 | −0.851 | 0.409 |
| ALT (IU/L) | 24.99 ± 4.40 | 30.71 ± 9.81 | −1.506 | 0.154 |
| AST (IU/L) | 24.49 ± 5.97 | 25.34 ± 7.90 | −0.243 | 0.812 |
| FPG (mmol/L) | 5.04 ± 0.75 | 5.53 ± 0.95 | −1.154 | 0.268 |
| HOMA-IR | 2.30 ± 0.49 | 3.84 ± 1.38 * | −2.955 | 0.016 |
| Majority Protein IDs | Protein Names | Gene Names | t | p |
|---|---|---|---|---|
| P00915 | Carbonic anhydrase 1 | CA1 | 2.467 | 0.037 |
| P01024 | Complement C3 | C3 | −2.522 | 0.019 |
| P01031 | Complement C5 | C5 | 2.556 | 0.018 |
| P0DP04 | Ig heavy chain V-III region DOB | −2.201 | 0.039 | |
| P02741 | C-reactive protein | CRP | −2.407 | 0.025 |
| P02751 | Fibronectin | FN1 | −2.111 | 0.046 |
| P02788 | Lactotransferrin | LTF | 2.522 | 0.019 |
| P04004 | Vitronectin | VTN | −2.830 | 0.010 |
| P04083 | Annexin A1 | ANXA1 | 2.403 | 0.047 |
| P04196 | Histidine-rich glycoprotein | HRG | 2.298 | 0.031 |
| P04406 | Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | 5.462 | 0.000 |
| P05090 | Apolipoprotein D | APOD | 2.860 | 0.009 |
| P05109 | Protein S100-A8 | S100A8 | 3.241 | 0.014 |
| P05452 | Tetranectin | CLEC3B | 2.174 | 0.041 |
| P06396 | Gelsolin | GSN | 2.277 | 0.033 |
| P06702 | Protein S100-A9 | S100A9 | 4.003 | 0.005 |
| P07357 | Complement component C8 alpha chain | C8A | 2.080 | 0.049 |
| P07358 | Complement component C8 beta chain | C8B | 3.924 | 0.001 |
| P07360 | Complement component C8 gamma chain | C8G | 2.389 | 0.026 |
| P13796 | Plastin-2 | LCP1 | 4.047 | 0.001 |
| P18428 | Lipopolysaccharide-binding protein | LBP | 2.333 | 0.029 |
| P19652 | Alpha-1-acid glycoprotein 2 | ORM2 | −2.371 | 0.027 |
| P20851 | C4b-binding protein beta chain | C4BPB | 2.263 | 0.034 |
| P22352 | Glutathione peroxidase 3 | GPX3 | 2.568 | 0.018 |
| P22692 | Insulin-like growth factor-binding protein 4 | IGFBP4 | −2.183 | 0.040 |
| P32119 | Peroxiredoxin-2 | PRDX2 | 3.061 | 0.015 |
| P55056 | Apolipoprotein C-IV | APOC4 | −2.972 | 0.007 |
| P62328 | Thymosin beta-4 | TMSB4X | 2.204 | 0.038 |
| P62937 | Peptidyl-prolyl cis-trans isomerase A | PPIA | 2.295 | 0.032 |
| P80748 | Ig lambda chain V-III region LOI | −3.514 | 0.002 | |
| Q14624 | Inter-alpha-trypsin inhibitor heavy chain H4 | ITIH4 | 3.851 | 0.001 |
| Q96PD5 | N-acetylmuramoyl-L-alanine amidase | PGLYRP2 | −2.307 | 0.031 |
| Majority Protein IDs | Protein Names | Gene Names | t | p |
|---|---|---|---|---|
| A0A0B4J1U7 | Ig | IGHV6-1 | 2.735 | 0.023 |
| A0A0C4DH67 | Ig | IGKV1-8 | 2.987 | 0.013 |
| P01833 | Polymeric immunoglobulin receptor | PIGR | 2.465 | 0.039 |
| P02656 | Apolipoprotein C-III | APOC3 | 2.157 | 0.049 |
| P05155 | Plasma protease C1 inhibitor | SERPING1 | −2.400 | 0.031 |
| P05546 | Heparin cofactor 2 | SERPIND1 | 2.612 | 0.021 |
| P0C0L4 | Complement C4-A | C4A | −3.091 | 0.008 |
| P0C0L5 | Complement C4-B | C4B | −2.450 | 0.028 |
| P36955 | Pigment epithelium-derived factor | SERPINF1 | −2.565 | 0.022 |
| Q92954 | Proteoglycan 4 | PRG4 | 2.667 | 0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Dong, H.; Guo, Z.; Zhai, G.; Zhang, K. Identification of Abnormal Proteins in Plasma from Gout Patients by LC-MS/MS. Separations 2021, 8, 85. https://doi.org/10.3390/separations8060085
Shen L, Dong H, Guo Z, Zhai G, Zhang K. Identification of Abnormal Proteins in Plasma from Gout Patients by LC-MS/MS. Separations. 2021; 8(6):85. https://doi.org/10.3390/separations8060085
Chicago/Turabian StyleShen, Lijin, Hanyang Dong, Zhenchang Guo, Guijin Zhai, and Kai Zhang. 2021. "Identification of Abnormal Proteins in Plasma from Gout Patients by LC-MS/MS" Separations 8, no. 6: 85. https://doi.org/10.3390/separations8060085
APA StyleShen, L., Dong, H., Guo, Z., Zhai, G., & Zhang, K. (2021). Identification of Abnormal Proteins in Plasma from Gout Patients by LC-MS/MS. Separations, 8(6), 85. https://doi.org/10.3390/separations8060085






