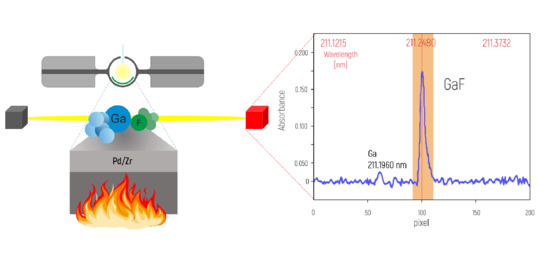
An Improved Methodology for Determination of Fluorine in Biological Samples Using High-Resolution Molecular Absorption Spectrometry via Gallium Fluorine Formation in a Graphite Furnace
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation and Operating Conditions
2.2. Reagents and Solutions
2.3. Sample Preparation
3. Results and Discussion
3.1. Method Development
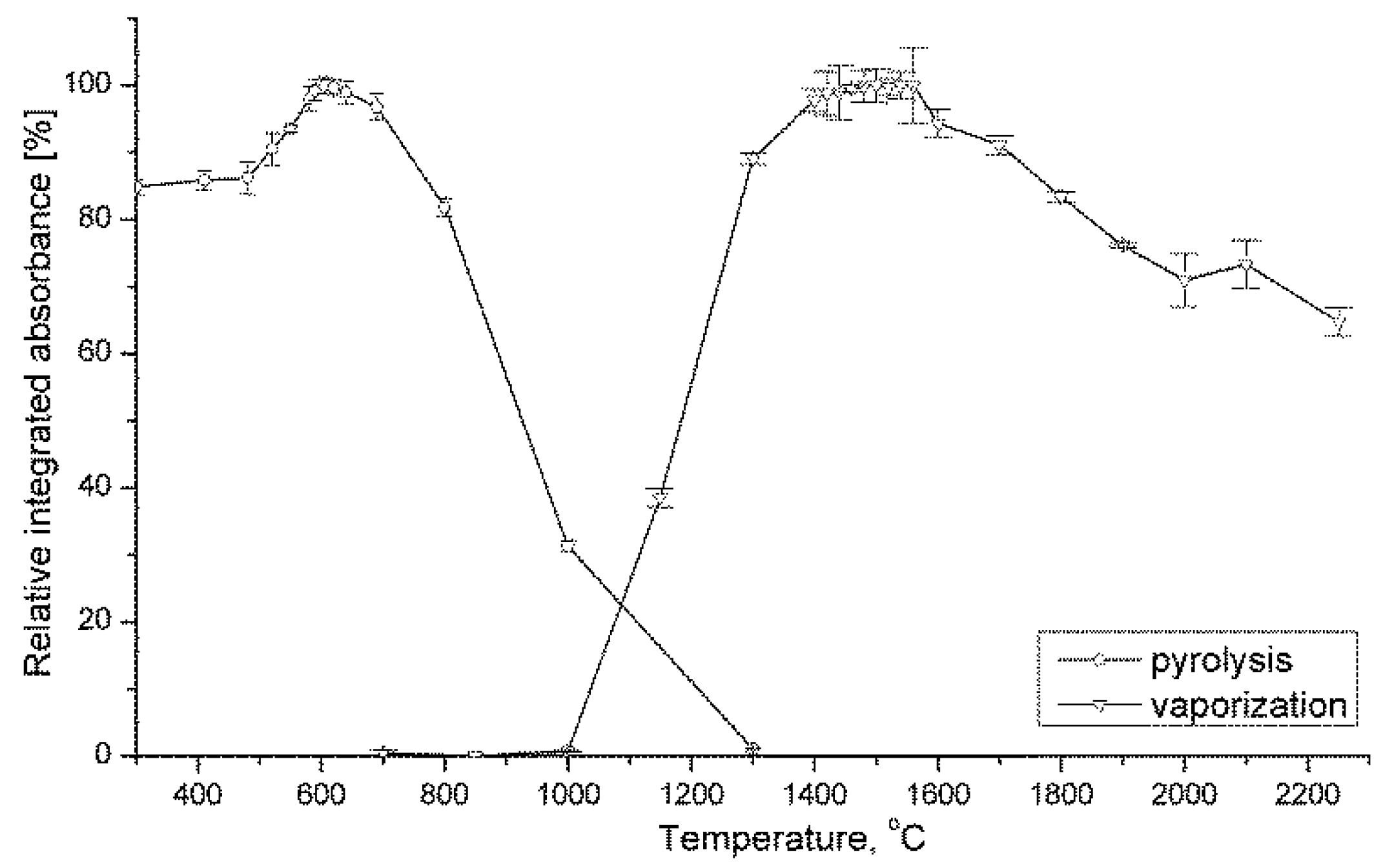
3.1.1. Pyrolysis and Vaporization Conditions
3.1.2. Effect of Pd/Zr Modifier
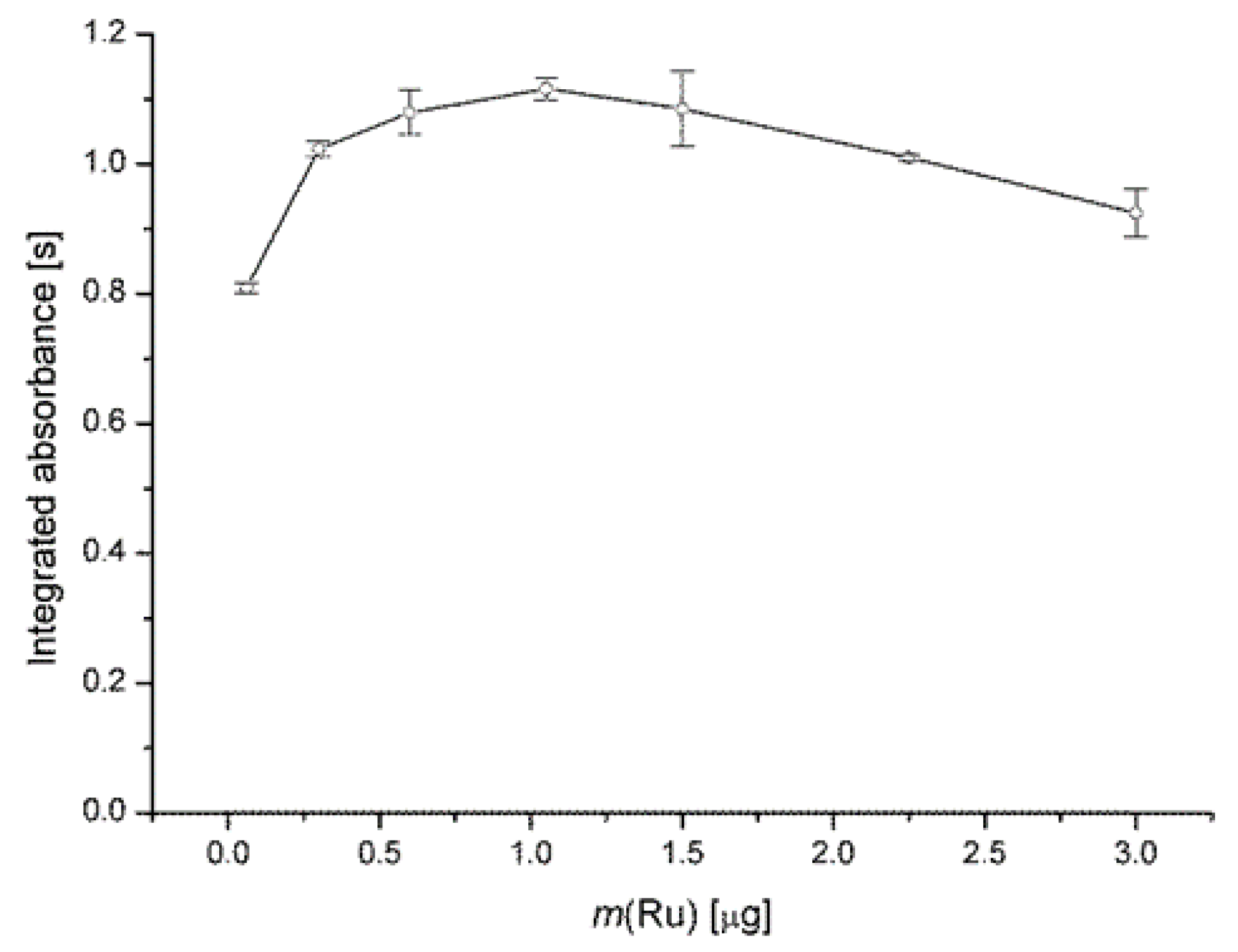
3.1.3. Effect of Ru(III) Modifier
3.1.4. Optimization of the Amount of Gallium
3.2. Method Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brauns, D.H. Quantitative determination of fluorine in organic compounds. J. Res. Natl. Bur. Stand. (1934) 1941, 27, 105. [Google Scholar] [CrossRef]
- Gleisner, H.; Welz, B.; Einax, J.W. Optimization of fluorine determination via the molecular absorption of gallium mono-fluoride in a graphite furnace using a high-resolution continuum source spectrometer. Spectrochim. Acta Part B At. Spectrosc. 2010, 65, 864–869. [Google Scholar] [CrossRef]
- Borjigin, S.; Ashimura, Y.; Yoshioka, T.; Mizoguchi, T. Determination of Fluoride Using Ion-selective Electrodes in the Presence of Aluminum. Anal. Sci. 2009, 25, 1437–1443. [Google Scholar] [CrossRef] [Green Version]
- Hang, Y.-P.; Liu, J.-M. Determination of fluoride by an ion chromatography system using the preconcentration on nanometer-size zirconia. J. Anal. Chem. 2007, 62, 583–587. [Google Scholar] [CrossRef]
- Barghouthi, Z.; Amereih, S. Spectrophotometric Determination of Fluoride in Groundwater Using Resorcin Blue Complexes. Am. J. Anal. Chem. 2012, 03, 651–655. [Google Scholar] [CrossRef] [Green Version]
- Stacewicz, T.; Bulska, E.; Ruszczyńska, A. Cavity Ring-Down Spectroscopy coupled with helium microwave-induced plasma for fluorine detection. Spectrochim. Acta Part B At. Spectrosc. 2010, 65, 306–310. [Google Scholar] [CrossRef]
- Bulska, E.; Jȩdral, W. Application of palladium- and rhodium-plating of the graphite furnace in electrothermal atomic absorption spectrometry. J. Anal. At. Spectrom. 1995, 10, 49–53. [Google Scholar] [CrossRef]
- Bulska, E.; Kandler, W.; Hulanicki, A. Noble metals as permanent modifiers for the determination of mercury by electrothermal atomic absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 1996, 51, 1263–1270. [Google Scholar] [CrossRef]
- Bulska, E. Modifiers in Graphite Furnace Atomic Absorption Spectrometry-Mechanisms and Applications. Encycl. Anal. Chem. 2009, 1–11. [Google Scholar] [CrossRef]
- Wojciechowski, M.; Piaścik, M.; Bulska, E. Noble metal modifiers for antimony determination by graphite furnace atomic absorption spectrometry in biological samples. J. Anal. At. Spectrom. 2001, 16, 99–101. [Google Scholar] [CrossRef]
- Ortner, H.M.; Bulska, E.; Rohr, U.; Schlemmer, G.; Weinbruch, S.; Welz, B. Modifiers and coatings in graphite furnace atomic absorption spectrometry-Mechanisms of action (A tutorial review). Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 1835–1853. [Google Scholar] [CrossRef]
- Welz, B.; Morés, S.; Carasek, E.; Vale, M.G.R.; Okruss, M.; Becker-Ross, H. High-resolution continuum source atomic and molecular absorption spectrometry-A review. Appl. Spectrosc. Rev. 2010, 45, 327–354. [Google Scholar] [CrossRef]
- Resano, M.; Flórez, M.R.; García-Ruiz, E. Progress in the determination of metalloids and non-metals by means of high-resolution continuum source atomic or molecular absorption spectrometry. A critical review. Anal. Bioanal. Chem. 2014, 406, 2239–2259. [Google Scholar] [CrossRef]
- Heitmann, U.; Becker-Ross, H.; Florek, S.; Huang, M.D.; Okruss, M. Determination of non-metals via molecular absorption using high-resolution continuum source absorption spectrometry and graphite furnace atomization. J. Anal. At. Spectrom. 2006, 21, 1314–1320. [Google Scholar] [CrossRef]
- Morés, S.; Monteiro, G.C.; Santos, F.D.S.; Carasek, E.; Welz, B. Determination of fluorine in tea using high-resolution molecular absorption spectrometry with electrothermal vaporization of the calcium mono-fluoride CaF. Talanta 2011, 85, 2681–2685. [Google Scholar] [CrossRef] [Green Version]
- Ozbek, N.; Akman, S. Determination of fluorine in milk samples via calcium-monofluoride by electrothermal molecular absorption spectrometry. Food Chem. 2013, 138, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, N.; Akman, S. Method development for the determination of fluorine in water samples via the molecular absorption of strontium monofluoride formed in an electrothermal atomizer. Spectrochim. Acta Part B At. Spectrosc. 2012, 69, 32–37. [Google Scholar] [CrossRef]
- Ozbek, N.; Akman, S. Molecule Formation Mechanisms of Strontium Mono Fluoride in High-Resolution Continuum Source Electrothermal Atomic Absorption Spectrometry. Anal. Sci. 2013, 29, 741–746. [Google Scholar] [CrossRef] [Green Version]
- Aramendía, M.; Flórez, M.R.; Piette, M.; Vanhaecke, F.; Resano, M. Al determination in whole blood samples as AlF via high-resolution continuum source graphite furnace molecular absorption spectrometry: Potential application to forensic diagnosis of drowning. J. Anal. At. Spectrom. 2011, 26, 1964–1973. [Google Scholar] [CrossRef]
- Bücker, S.; Acker, J. Spectrometric analysis of process etching solutions of the photovoltaic industry-Determination of HNO3, HF, and H2SiF 6 using high-resolution continuum source absorption spectrometry of diatomic molecules and atoms. Talanta 2012, 94, 335–341. [Google Scholar] [CrossRef]
- Boschetti, W.; Dessuy, M.B.; Pizzato, A.H.; Vale, M.G.R. New analytical method for total fluorine determination in soil samples using high-resolution continuum source graphite furnace molecular absorption spectrometry. Microchem. J. 2017, 130, 276–280. [Google Scholar] [CrossRef]
- Machado, P.M.; Morés, S.; Pereira, É.R.; Welz, B.; Carasek, E.; De Andrade, J.B. Fluorine determination in coal using high-resolution graphite furnace molecular absorption spectrometry and direct solid sample analysis. Spectrochim. Acta Part B At. Spectrosc. 2015, 105, 18–24. [Google Scholar] [CrossRef]
- Ozbek, N.; Akman, S. Solid sampling determination of total fluorine in baby food samples by high-resolution continuum source graphite furnace molecular absorption spectrometry. Food Chem. 2016, 211, 180–184. [Google Scholar] [CrossRef]
- Ozbek, N.; Akman, S. Optimization and Application of a Slurry Sampling Method for the Determination of Total Fluorine in Flour Using a High-Resolution Continuum Source Graphite Furnace Molecular Absorption Spectrometer. Food Anal. Methods 2016, 9, 2925–2932. [Google Scholar] [CrossRef]
- Ozbek, N.; Akman, S. Application of Solid Sampling for the Determination of Total Fluorine in Fish and Seafood by High-Resolution Continuum Source Graphite Furnace Molecular Absorption Spectrometry. Anal. Lett. 2018, 51, 2776–2789. [Google Scholar] [CrossRef]
- Borges, A.R.; Duarte, Á.T.; da Potes, M.L.; Silva, M.M.; Vale, M.G.R.; Welz, B. Fluorine in eye shadow: Development of method using high-resolution continuum source graphite furnace molecular absorption spectrometry via calcium mono-fluoride with direct solid sample introduction. Microchem. J. 2016, 124, 410–415. [Google Scholar] [CrossRef]
- Gleisner, H.; Einax, J.W.; Morés, S.; Welz, B.; Carasek, E. A fast and accurate method for the determination of total and soluble fluorine in toothpaste using high-resolution graphite furnace molecular absorption spectrometry and its comparison with established techniques. J. Pharm. Biomed. Anal. 2011, 54, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; McNee, D.; Gleisner, H.; Raab, A.; Kyeremeh, K.; Jaspars, M.; Krupp, E.; Deng, H.; Feldmann, J. Fluorine speciation analysis using reverse phase liquid chromatography coupled off-line to continuum source molecular absorption spectrometry (CS-MAS): Identification and quantification of novel fluorinated organic compounds in environmental and biologica. Anal. Chem. 2012, 84, 6213–6219. [Google Scholar] [CrossRef]
- Qin, Z.; Raab, A.; Krupp, E.; Deng, H.; Feldmann, J. Mining complex bacteria media for all fluorinated compounds made possible by using HPLC coupled parallel to fluorine-specific and molecular specific detection. J. Anal. At. Spectrom. 2013, 28, 877. [Google Scholar] [CrossRef]
- Würtenberger, I.; Gust, R. A highly sensitive method for in vitro testing of fluorinated drug candidates using high-resolution continuum source molecular absorption spectrometry (HR-CS MAS). Anal. Bioanal. Chem. 2014, 406, 3431–3442. [Google Scholar] [CrossRef]
- Metzger, M.; Ley, P.; Sturm, M.; Meermann, B. Screening method for extractable organically bound fluorine (EOF) in river water samples by means of high-resolution–continuum source graphite furnace molecular absorption spectrometry (HR-CS GF MAS). Anal. Bioanal. Chem. 2019, 411, 4647–4660. [Google Scholar] [CrossRef]
- Gehrenkemper, L.; Simon, F.; Roesch, P.; Fischer, E.; von der Au, M.; Pfeifer, J.; Cossmer, A.; Wittwer, P.; Vogel, C.; Simon, F.G.; et al. Determination of organically bound fluorine sum parameters in river water samples—comparison of combustion ion chromatography (CIC) and high resolution-continuum source-graphite furnace molecular absorption spectrometry (HR-CS-GFMAS). Anal. Bioanal. Chem. 2021, 413, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; Huang, M.-D.; Becker-Roß, H.; Florek, S.; Ott, I.; Gust, R. Quantification of the fluorine containing drug 5-fluorouracil in cancer cells by GaF molecular absorption via high-resolution continuum source molecular absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2012, 69, 50–55. [Google Scholar] [CrossRef]
- Gawor, A.; Konopka, A.; Ruszczyńska, A.; Pączek, L.; Gajewski, Z.; Bulska, E. Molecular absorption and mass spectrometry for complementary analytical study of fluorinated drugs in animal organisms. J. Anal. At. Spectrom. 2020, 35, 1840–1847. [Google Scholar] [CrossRef]
- Kowalewska, Z.; Brzezińska, K.; Zieliński, J.; Pilarczyk, J. Method development for determination of organic fluorine in gasoline and its components using high-resolution continuum source flame molecular absorption spectrometry with gallium fluoride as a target molecule. Talanta 2021, 227, 122205. [Google Scholar] [CrossRef]
- Ley, P.; Sturm, M.; Ternes, T.A.; Meermann, B. High-resolution continuum source graphite furnace molecular absorption spectrometry compared with ion chromatography for quantitative determination of dissolved fluoride in river water samples. Anal. Bioanal. Chem. 2017, 409, 6949–6958. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.D.; Becker-Ross, H.; Florek, S.; Heitmann, U.; Okruss, M. The influence of calcium and magnesium on the phosphorus monoxide molecular absorption signal in the determination of phosphorus using a continuum source absorption spectrometer and an air-acetylene flame. J. Anal. At. Spectrom. 2006, 21, 346–349. [Google Scholar] [CrossRef]
- Shirai, T.; Reader, J.; Kramida, A.E.; Sugar, J. Spectral Data for Gallium: Ga I through Ga XXXI. J. Phys. Chem. Ref. Data 2007, 36, 509–615. [Google Scholar] [CrossRef]




| Step | Process | Temperature [°C ] | Ramp [°C/s] | Hold [s] |
|---|---|---|---|---|
| 1 | Drying I | 80 | 7 | 2 |
| 2 | Drying II | 95 | 10 | 20 |
| 3 | Drying III | 120 | 20 | 10 |
| 4 | Drying IV | 350 | 200 | 10 |
| 5 | Pretreatment | 1100 | 300 | 10 |
| 6 | Cooling | 70 | -- | 20 |
| 7 | Sample injection | 70 | - | - |
| 8 | Drying | 105 | 20 | 30 |
| 9 | Pyrolysis | 600 | 550 | 10 |
| 10 | Auto Zero | 600 | - | 5 |
| 11 | Vaporization | 1540 | 1540 | 7 |
| 12 | Clean | 2450 | 500 | 4 |
| Mode of Application | Components | Concentration | Volume [µL] |
|---|---|---|---|
| Thermal deposition | Pd + Zr | 0.15% m/v Pd, 30 mg/L Zr | 5 |
| Thermal deposition | Ga in HNO3 | 20 g/L | 25 |
| Added with the sample | Ru in HCl | 0.2 g/L Ru | 5 |
| Added with the sample | Ga in HNO3 | 2 g/L | 10 |
| Added with the sample | CH3COONa | 1 g/L | 5 |
| Added in step 7 | sample or standard | variable | 20 |
| CRM | Origin | Certified/Added F Content [µg/L] | Found F Content [µg/L] | Recovery Factor [%] |
|---|---|---|---|---|
| CRANBERRY-05 | lake water | 70 ± 26 | 72 ± 2 | 103 |
| ONTARIO-12 | lake water | 114 ± 24 | 106 ± 1 | 93 |
| NIST 1577c | bovine liver | 25 1 | 26.4 ± 0.3 | 106 |
| NIST 1577c | bovine liver | 50 1 | 50.0 ± 1.1 | 100 |
| MODAS-5 | cod tissue | 25 1 | 25.7 ± 0.7 | 103 |
| MODAS-5 | cod tissue | 50 1 | 49.7 ± 1.0 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawor, A.; Tupys, A.; Ruszczyńska, A.; Bulska, E. An Improved Methodology for Determination of Fluorine in Biological Samples Using High-Resolution Molecular Absorption Spectrometry via Gallium Fluorine Formation in a Graphite Furnace. Appl. Sci. 2021, 11, 5493. https://doi.org/10.3390/app11125493
Gawor A, Tupys A, Ruszczyńska A, Bulska E. An Improved Methodology for Determination of Fluorine in Biological Samples Using High-Resolution Molecular Absorption Spectrometry via Gallium Fluorine Formation in a Graphite Furnace. Applied Sciences. 2021; 11(12):5493. https://doi.org/10.3390/app11125493
Chicago/Turabian StyleGawor, Andrzej, Andrii Tupys, Anna Ruszczyńska, and Ewa Bulska. 2021. "An Improved Methodology for Determination of Fluorine in Biological Samples Using High-Resolution Molecular Absorption Spectrometry via Gallium Fluorine Formation in a Graphite Furnace" Applied Sciences 11, no. 12: 5493. https://doi.org/10.3390/app11125493
APA StyleGawor, A., Tupys, A., Ruszczyńska, A., & Bulska, E. (2021). An Improved Methodology for Determination of Fluorine in Biological Samples Using High-Resolution Molecular Absorption Spectrometry via Gallium Fluorine Formation in a Graphite Furnace. Applied Sciences, 11(12), 5493. https://doi.org/10.3390/app11125493