Biodegradable Antimicrobial Films for Food Packaging: Effect of Antimicrobials on Degradation
Abstract
:1. Introduction
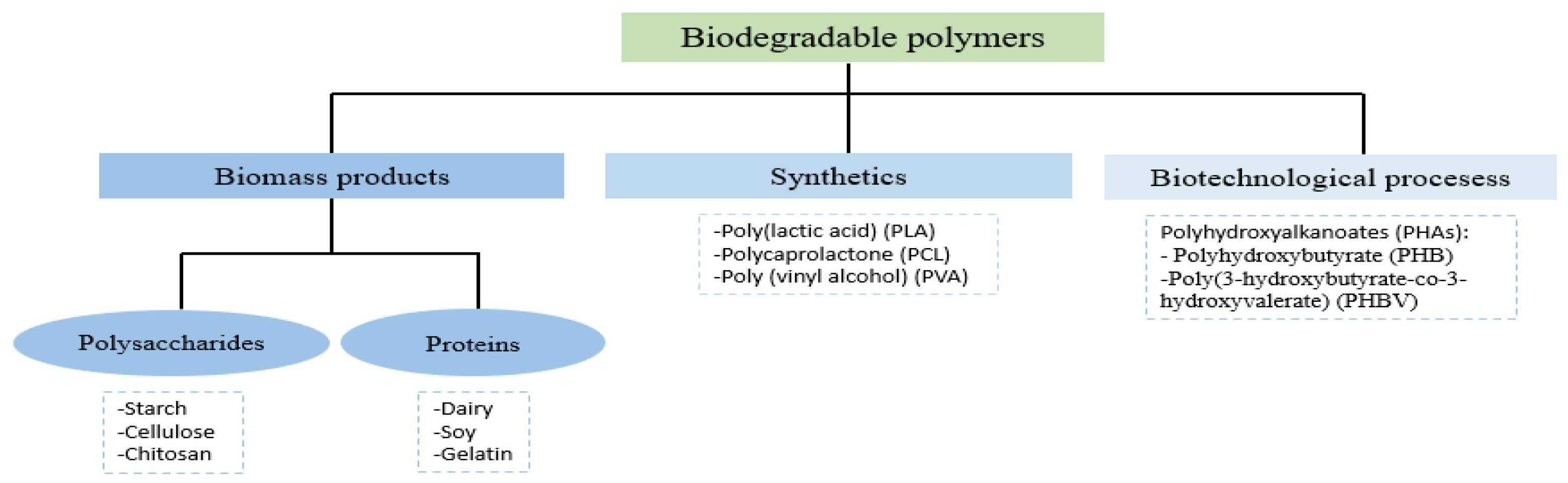
2. Antimicrobial Packaging Materials Based on Biodegradable Polymers
2.1. Polymers from Biomass
2.2. Synthetic Polymers
2.3. Polymers from Microorganisms
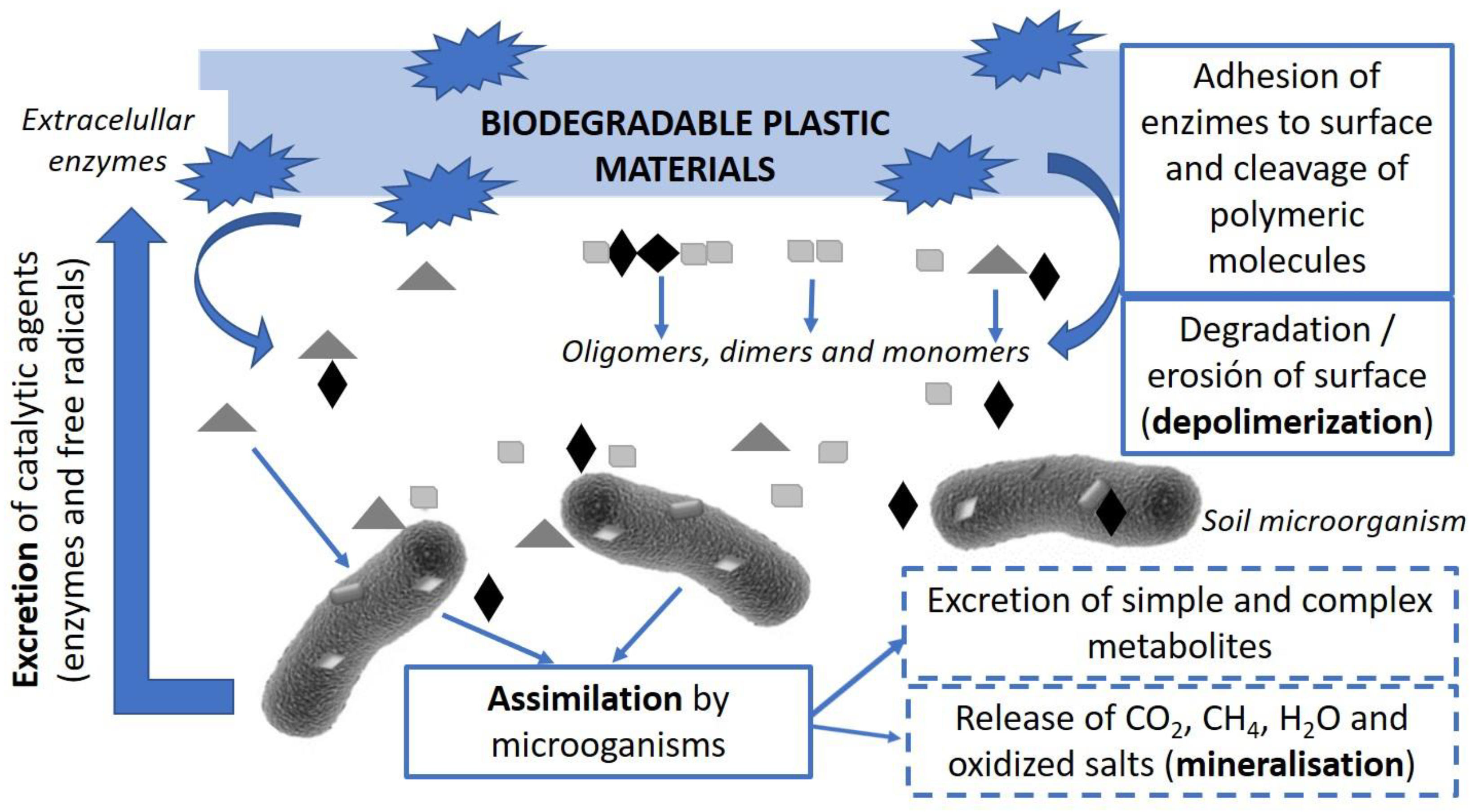
3. Polymer Biodegradation Studies in Different Media
4. Effect of Antimicrobials on the Biodegradation of Polymer Based Active Packaging Materials
5. Final Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Alvarado Mendoza, D.L. Envasado en la Industria de Alimentos y sus Nuevas Tendencias. Bachelor’s Thesis, Universidad Autónoma Agraria, Saltillo, Mexico, 2009. [Google Scholar]
- Asociación Española de Basuras Marinas (AEBAM). ¿Qué Son Las Basuras Marinas? 2020. Available online: https://aebam.org/basuras-marinas/ (accessed on 14 November 2020).
- Arrieta, M.P.; Peltzer, M.A.; Garrigós Selva, M.C.; Jímenez Migallón, A. Envases alimentarios sostenibles. Biopelículas activas obtenidas a partir de proteínas lácteas. Segur. Medio Ambient. 2011, 121, 46–56. [Google Scholar]
- Labeaga, A. Polímeros Biodegradables. Importancia y potenciales Aplicaciones. Master’s Thesis, Universidad Nacional de Educación a Distancia, Madrid, Spain, 2018. [Google Scholar]
- European Bioplastics, Nova Institute. 2019. Available online: www.european-biplastics.org/market (accessed on 8 September 2020).
- Tubón, I. Formulación, Elaboración y Evaluación de Bioenvase para Caramelos a Base de Almidón de Yuca, Sacarosa y Gelatina. Bachelor’s Thesis, Universidad Nacional de Chimborazo, Riobamba, Ecuador, 2013. [Google Scholar]
- Vermeiren, F.L.; Devlieghere, J.D. Effectiveness of some recent antimicrobial packaging concepts. Food Aditives Contam. 2002, 19, 163–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas, C.F. Envases Activos e Inteligentes: Control de la Calidad y Seguridad del Producto; Instituto Tecnólogico Embalaje Logística: Vitoria, Spain, 2012. [Google Scholar]
- Ross, G.; Ross, S.; Tighe, B.J. Bioplastics: New Routes, New Products; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780323358248. [Google Scholar] [CrossRef]
- Valencia-Sullca, C.; Vargas, M.; Atarés, L.; Chiralt, A. Thermoplastic cassava starch-chitosan bilayer films containing essential oils. Food Hydrocoll. 2018, 75, 107–115. [Google Scholar] [CrossRef]
- Valencia-Sullca, C.; Atarés, L.; Vargas, M.; Chiralt, A. Physical and Antimicrobial Properties of Compression-Molded Cassava Starch-Chitosan Films for Meat Preservation. Food Bioprocess Technol. 2018, 11, 1339–1349. [Google Scholar] [CrossRef]
- Moreno, O.; Atarés, L.; Chiralt, A.; Cruz-Romero, M.C.; Kerry, J. Starch-gelatin antimicrobial packaging materials to extend the shelf life of chicken breast fillets. LWT 2018, 97, 483–490. [Google Scholar] [CrossRef]
- Silveira, M.P.; Silva, H.C.; Pimentel, I.C.; Poitevin, C.G.; da Costa Stuart, A.K.; Carpiné, D.; de Matos Jorge, L.M.; Jorge, R.M.M. Development of active cassava starch cellulose nanofiber-based films incorporated with natural antimicrobial tea tree essential oil. J. Appl. Polym. Sci. 2020, 137, 1–11. [Google Scholar] [CrossRef]
- Souza, A.C.; Goto, G.E.O.; Mainardi, J.A.; Coelho, A.C.V.; Tadini, C.C. Cassava starch composite films incorporated with cinnamon essential oil: Antimicrobial activity, microstructure, mechanical and barrier properties. LWT Food Sci. Technol. 2013, 54, 346–352. [Google Scholar] [CrossRef]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Development and characterization of active films based on starch-PVA, containing silver nanoparticles. Food Packag. Shelf Life 2016, 10, 16–24. [Google Scholar] [CrossRef]
- Hasan, M.; Gopakumar, D.A.; Olaiya, N.G.; Zarlaida, F.; Alfian, A.; Aprinasari, C.; Alfatah, T.; Rizal, S.; Khalil, H.P.S.A. Evaluation of the thermomechanical properties and biodegradation of brown rice starch-based chitosan biodegradable composite films. Int. J. Biol. Macromol. 2020, 156, 896–905. [Google Scholar] [CrossRef]
- Syafiq, R.; Sapuan, S.M.; Zuhri, M.R.M. Antimicrobial activity, physical, mechanical and barrier properties of sugar palm based nanocellulose/starch biocomposite films incorporated with cinnamon essential oil. J. Mater. Res. Technol. 2021, 11, 144–157. [Google Scholar] [CrossRef]
- Shapi’i, R.A.; Othman, S.H.; Nordin, N.; Kadir Basha, R.; Nazli Naim, M. Antimicrobial properties of starch films incorporated with chitosan nanoparticles: In vitro and in vivo evaluation. Carbohydr. Polym. 2020, 230, 115602. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and Antimicrobial Properties of Starch-PVA Blend Films as Affected by the Incorporation of Natural Antimicrobial Agents. Foods 2015, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Perdana, M.I.; Ruamcharoen, J.; Panphon, S.; Leelakriangsak, M. Antimicrobial activity and physical properties of starch/chitosan film incorporated with lemongrass essential oil and its application. LWT 2021, 141, 110934. [Google Scholar] [CrossRef]
- Dhumal, C.V.; Ahmed, J.; Bandara, N.; Sarkar, P. Improvement of antimicrobial activity of sago starch/guar gum bi-phasic edible films by incorporating carvacrol and citral. Food Packag. Shelf Life 2019, 21, 100380. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, J.; Dong, H.; Hou, H.; Guo, P. Properties and antimicrobial activities of starch-sodium alginate composite films incorporated with sodium dehydroacetate or rosemary extract. J. Appl. Polym. Sci. 2013, 127, 1951–1958. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.; Chen, P.; Duan, M.; Lin, X.; Zhang, Y. Biodegradation analysis of polyvinyl alcohol during the compost burial course. J. Basic Microbiol. 2019, 59, 368–374. [Google Scholar] [CrossRef]
- De Moraes, J.O.; Hilton, S.T.; Moraru, C.I. The effect of Pulsed Light and starch films with antimicrobials on Listeria innocua and the quality of sliced cheddar cheese during refrigerated storage. Food Control 2020, 112, 107134. [Google Scholar] [CrossRef]
- Moreno, O.; Gil, À.; Atarés, L.; Chiralt, A. Active starch-gelatin films for shelf-life extension of marinated salmon. LWT Food Sci. Technol. 2017, 84, 189–195. [Google Scholar] [CrossRef]
- Ali, A.; Chen, Y.; Liu, H.; Yu, L.; Baloch, Z.; Khalid, S.; Zhu, J.; Chen, L. Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 2019, 129, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Serna, C.L.; Rodríguez de, S.A.; Albán, A.F. Ácido Poliláctico (PLA): Propiedades y Aplicaciones. Ing. Compet. 2011, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wei, D.; Gong, W.; Zheng, A.; Guan, Y. Hydrogen-Bond Assembly of Poly(vinyl alcohol) and Polyhexamethylene Guanidine for Nonleaching and Transparent Antimicrobial Films. ACS Appl. Mater. Interfaces 2018, 10, 37535–37543. [Google Scholar] [CrossRef] [PubMed]
- Hajji, S.; Chaker, A.; Jridi, M.; Maalej, H.; Jellouli, K.; Boufi, S.; Nasri, M. Structural analysis, and antioxidant and antibacterial properties of chitosan-poly (vinyl alcohol) biodegradable films. Environ. Sci. Pollut. Res. 2016, 23, 15310–15320. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.E.; Kamvari-Moghaddam, A. Degradation Rate of Bioresorbable Materials; Synthetic Bioresorbable Polymers; Woodhead Publishing Limited: Sawston, UK, 2008; pp. 43–66. ISBN 9781845693299. [Google Scholar]
- Perveen, R.; Nasar, A. Multiwalled Carbon Nanotube-Based Nanocomposites for Artificial Bone Grafting; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128137574. [Google Scholar]
- Ahmed, J.; Hiremath, N.; Jacob, H. Antimicrobial, Rheological, and Thermal Properties of Plasticized Polylactide Films Incorporated with Essential Oils to Inhibit Staphylococcus aureus and Campylobacter jejuni. J. Food Sci. 2016, 81, E419–E429. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Jiang, L.; Yu, M.; Dong, W.; Chen, M. Green Antibacterial Nanocomposites from Poly(lactide)/Poly(butylene adipate-co-terephthalate)/Nanocrystal Cellulose-Silver Nanohybrids. ACS Sustain. Chem. Eng. 2016, 4, 6417–6426. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Aliotta, L.; Vannozzi, A.; Morganti, P.; Panariello, L.; Danti, S.; Neri, S.; Fernandez-Avila, C.; Fusco, A.; Donnarumma, G.; et al. Properties and skin compatibility of films based on poly(lactic acid) (PLA) bionanocomposites incorporating chitin nanofibrils (CN). J. Funct. Biomater. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodayari, M.; Basti, A.A.; Khanjari, A.; Misaghi, A.; Kamkar, A.; Shotorbani, P.M.; Hamedi, H. Effect of poly(lactic acid) films incorporated with different concentrations of Tanacetum balsamita essential oil, propolis ethanolic extract and cellulose nanocrystals on shelf life extension of vacuum-packed cooked sausages. Food Packag. Shelf Life 2019, 19, 200–209. [Google Scholar] [CrossRef]
- Muller, J.; Casado Quesada, A.; González-Martínez, C.; Chiralt, A. Antimicrobial properties and release of cinnamaldehyde in bilayer films based on polylactic acid (PLA) and starch. Eur. Polym. J. 2017, 96, 316–325. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Vargas, M.; Chiralt, A.; Kenny, J.M. Effects of chitosan on the physicochemical and antimicrobial properties of PLA films. J. Food Eng. 2013, 119, 236–243. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Jakubowska, E.; Tarach, I.; Sedlarik, V.; Pummerova, M. Antibacterial films based on PVA and PVA-chitosan modified with poly(hexamethylene guanidine). Polymers 2019, 11, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghighi, H.; Leugoue, S.K.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Fava, P.; Pulvirenti, A. Development of antimicrobial films based on chitosan-polyvinyl alcohol blend enriched with ethyl lauroyl arginate (LAE) for food packaging applications. Food Hydrocoll. 2020, 100, 105419. [Google Scholar] [CrossRef]
- Suganthi, S.; Vignesh, S.; Kalyana Sundar, J.; Raj, V. Fabrication of PVA polymer films with improved antibacterial activity by fine-tuning via organic acids for food packaging applications. Appl. Water Sci. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Physicochemical and bioactivity of cross-linked chitosan-PVA film for food packaging applications. Int. J. Biol. Macromol. 2009, 45, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, P.; Zhou, F.; Zhao, Y.; Ren, L.; Yuan, X. Antimicrobial eugenol-loaded electrospun membranes of poly(ε-caprolactone)/gelatin incorporated with REDV for vascular graft applications. Colloids Surf. B Biointerfaces 2018, 162, 335–344. [Google Scholar] [CrossRef]
- Salević, A.; Prieto, C.; Cabedo, L.; Nedović, V.; Lagaron, J.M. Physicochemical, antioxidant and antimicrobial properties of electrospun poly(ε-caprolactone) films containing a solid dispersion of sage (Salvia officinalis L.) extract. Nanomaterials 2019, 9, 270. [Google Scholar] [CrossRef] [Green Version]
- Takala, P.N.; Vu, K.D.; Salmieri, S.; Khan, R.A.; Lacroix, M. Antibacterial effect of biodegradable active packaging on the growth of Escherichia coli, Salmonella typhimurium and Listeria monocytogenes in fresh broccoli stored at 4 °C. LWT Food Sci. Technol. 2013, 53, 499–506. [Google Scholar] [CrossRef]
- Lyu, J.S.; Lee, J.S.; Han, J. Development of a biodegradable polycaprolactone film incorporated with an antimicrobial agent via an extrusion process. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Yu, L.; Feng, M.; Meng, L.; Bai, Y.; Ali, A.; Liu, H.; Chen, L. Development and characterization of biodegradable antimicrobial packaging films based on polycaprolactone, starch and pomegranate rind hybrids. Food Packag. Shelf Life 2018, 18, 71–79. [Google Scholar] [CrossRef]
- Uzunlu, S.; Niranjan, K. Laboratory antimicrobial activity of cinnamaldehyde and pomegranate-based polycaprolactone films. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Tampau, A.; González-Martínez, C.; Chiralt, A. Release kinetics and antimicrobial properties of carvacrol encapsulated in electrospun poly-(ε-caprolactone) nanofibres. Application in starch multilayer films. Food Hydrocoll. 2018, 79, 158–169. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Hustede, E.; Liebergesell, M.; Pieper, U.; Timm, A.; Valentin, H. Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol. Lett. 1992, 103, 217–230. [Google Scholar] [CrossRef]
- Erceg, M.; Kovačić, T.; Klarić, I. Thermal degradation of poly(3-hydroxybutyrate) plasticized with acetyl tributyl citrate. Polym. Degrad. Stab. 2005, 90, 313–318. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Serrano-Aroca, Á. Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate): Enhancement strategies for advanced applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef] [Green Version]
- Basnett, P.; Marcello, E.; Lukasiewicz, B.; Nigmatullin, R.; Paxinou, A.; Ahmad, M.H.; Gurumayum, B.; Roy, I. Antimicrobial materials with lime oil and a poly(3-hydroxyalkanoate) produced via valorisation of sugar cane molasses. J. Funct. Biomater. 2020, 11, 24. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Martínez-Abad, A.; Fabra, M.J.; Olivera, C.; Reis, M.; Lagarón, J.M. Stabilization of antimicrobial silver nanoparticles by a polyhydroxyalkanoate obtained from mixed bacterial culture. Int. J. Biol. Macromol. 2014, 71, 103–110. [Google Scholar] [CrossRef]
- Xu, P.; Yang, W.; Niu, D.; Yu, M.; Du, M.; Dong, W.; Chen, M.; Jan Lemstra, P.; Ma, P. Multifunctional and robust polyhydroxyalkanoate nanocomposites with superior gas barrier, heat resistant and inherent antibacterial performances. Chem. Eng. J. 2020, 382, 122864. [Google Scholar] [CrossRef]
- Xavier, J.R.; Babusha, S.T.; George, J.; Ramana, K.V. Material Properties and Antimicrobial Activity of Polyhydroxybutyrate (PHB) Films Incorporated with Vanillin. Appl. Biochem. Biotechnol. 2015, 176, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Correa, J.P.; Molina, V.; Sanchez, M.; Kainz, C.; Eisenberg, P.; Massani, M.B. Improving ham shelf life with a polyhydroxybutyrate/polycaprolactone biodegradable film activated with nisin. Food Packag. Shelf Life 2017, 11, 31–39. [Google Scholar] [CrossRef]
- Narayanan, A.; Ramana, K.V. Synergized antimicrobial activity of eugenol incorporated polyhydroxybutyrate films against food spoilage microorganisms in conjunction with pediocin. Appl. Biochem. Biotechnol. 2013, 170, 1379–1388. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, Q.; Sun, Z.; Li, G.; Ren, X.; Liang, J.; Huang, T.S. Preparation and characterization of electrospun antimicrobial fibrous membranes based on polyhydroxybutyrate (PHB). Fibers Polym. 2015, 16, 1751–1758. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Torres-Giner, S.; Enescu, D.; Cabedo, L.; Cerqueira, M.A.; Pastrana, L.M.; Lagaron, J.M. Electrospun active biopapers of food waste derived poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with short-term and long-term antimicrobial performance. Nanomaterials 2020, 10, 506. [Google Scholar] [CrossRef] [Green Version]
- Requena, R.; Jiménez, A.; Vargas, M.; Chiralt, A. Poly[(3-hydroxybutyrate)-co-(3-hydroxyvalerate)] active bilayer films obtained by compression moulding and applying essential oils at the interface. Polym. Int. 2016, 65, 883–891. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Bernardos, A.; Martínez-Máñez, R.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Electrospun antimicrobial films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) containing eugenol essential oil encapsulated in mesoporous silica nanoparticles. Nanomaterials 2019, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Sabharwal, P.K.; Chattopadhyay, S.; Singh, H. Preparation and characterization of antimicrobial, biodegradable, triclosan-incorporated polyhydroxybutyrate-co-valerate films for packaging applications. J. Appl. Polym. Sci. 2018, 135, 1–10. [Google Scholar] [CrossRef]
- Requena, R.; Vargas, M.; Chiralt, A. Eugenol and carvacrol migration from PHBV films and antibacterial action in different food matrices. Food Chem. 2019, 277, 38–45. [Google Scholar] [CrossRef]
- Requena, R.; Vargas, M.; Chiralt, A. Obtaining antimicrobial bilayer starch and polyester-blend films with carvacrol. Food Hydrocoll. 2018, 83, 118–133. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.E. Polymer biodegradation: Mechanisms and estimation techniques—A review. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef]
- Muniyasamy, S.; Anstey, A.; Reddy, M.M.; Misra, M.; Mohanty, A. Biodegradability and compostability of lignocellulosic based composite materials. J. Renew. Mater. 2013, 1, 253–272. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of bioplastic packaging materials: An overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Volova, T.G.; Boyandin, A.N.; Vasiliev, A.D.; Karpov, V.A.; Prudnikova, S.V.; Mishukova, O.V.; Boyarskikh, U.A.; Filipenko, M.L.; Rudnev, V.P.; Bá Xuân, B.; et al. Biodegradation of polyhydroxyalkanoates (PHAs) in tropical coastal waters and identification of PHA-degrading bacteria. Polym. Degrad. Stab. 2010, 95, 2350–2359. [Google Scholar] [CrossRef]
- Harrison, J.P.; Boardman, C.; O’Callaghan, K.; Delort, A.M.; Song, J. Biodegradability standards for carrier bags and plastic films in aquatic environments: A critical review. R. Soc. Open Sci. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- UNE-EN 13432. Requirements for Packaging Recoverable through Composting and Biodegradation Test Scheme and evaluation Criteria for the Final Acceptance of Packaging. 2001. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0024465 (accessed on 10 January 2021).
- Madejón, E.; Jesús Díaz, M.; López, R.; Cabrera, F. New approaches to establish optimum moisture content for compostable materials. Bioresour. Technol. 2002, 85, 73–78. [Google Scholar] [CrossRef]
- Miyatake, F.; Iwabuchi, K. Effect of compost temperature on oxygen uptake rate, specific growth rate and enzymatic activity of microorganisms in dairy cattle manure. Bioresour. Technol. 2006, 97, 961–965. [Google Scholar] [CrossRef]
- Suler, D.J.; Finstein, M.S. Effect of temperature, aeration, and moisture on CO2 formation in bench scale, continuously thermophilic composting of solid waste. Appl. Environ. Microbiol. 1977, 33, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Monedero, M.A.; Roig, A.; Paredes, C.; Bernal, M.P. Nitrogen transformation during organic waste composting by the Rutgers system and its effects on pH, EC and maturity of the composting mixtures. Bioresour. Technol. 2001, 78, 301–308. [Google Scholar] [CrossRef]
- Bidlingmaier, W.; Müsken, J. Compost Science and Technology; Odor Emissions from Composting Plants; Elsevier: Amsterdam, The Netherlands, 2007; Volume 8, pp. 215–328. ISBN 9780080439600. [Google Scholar]
- Luo, Y.; Lin, Z.; Guo, G. Biodegradation Assessment of Poly (Lactic Acid) Filled with Functionalized Titania Nanoparticles (PLA/TiO2) under Compost Conditions. Nanoscale Res. Lett. 2019, 14, 56. [Google Scholar] [CrossRef] [Green Version]
- Luzi, F.; Fortunati, E.; Puglia, D.; Petrucci, R.; Kenny, J.M.; Torre, L. Study of disintegrability in compost and enzymatic degradation of PLA and PLA nanocomposites reinforced with cellulose nanocrystals extracted from Posidonia Oceanica. Polym. Degrad. Stab. 2015, 121, 105–115. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Gu, J.; Tan, H. Physicochemical evolutions of starch/poly (lactic acid) composite biodegraded in real soil. J. Environ. Manag. 2018, 228, 223–231. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Laforsch, C.; Greiner, A.; Agarwal, S. Fate of So-Called Biodegradable Polymers in Seawater and Freshwater. Glob. Challenges 2017, 1, 1700048. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, M.P.; Aliaga, C.; Fito, C.; Hortal, M. Compostability assessment of nano-reinforced poly(lactic acid) films. Waste Manag. 2016, 48, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Sanahuja, A.; Casado-Coy, N.; Simó-Cabrera, L.; Sanz-Lázaro, C. Monitoring polymer degradation under different conditions in the marine environment. Environ. Pollut. 2020, 259. [Google Scholar] [CrossRef]
- Volova, T.G.; Gladyshev, M.I.; Trusova, M.Y.; Zhila, N.O. Degradation of polyhydroxyalkanoates in eutrophic reservoir. Polym. Degrad. Stab. 2007, 92, 580–586. [Google Scholar] [CrossRef]
- Thellen, C.; Coyne, M.; Froio, D.; Auerbach, M.; Wirsen, C.; Ratto, J.A. A processing, characterization and marine biodegradation study of melt-extruded polyhydroxyalkanoate (PHA) films. J. Polym. Environ. 2008, 16, 1–11. [Google Scholar] [CrossRef]
- Briassoulis, D.; Pikasi, A.; Briassoulis, C.; Mistriotis, A. Disintegration behaviour of bio-based plastics in coastal zone marine environments: A field experiment under natural conditions. Sci. Total Environ. 2019, 688, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, Z.; Mawad, D.; Crosky, A. Soil Biodegradation of Unidirectional Polyhydroxybutyrate-Co-Valerate (PHBV) Biocomposites Toughened With Polybutylene-Adipate-Co-Terephthalate (PBAT) and Epoxidized Natural Rubber (ENR). Front. Mater. 2019, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Muniyasamy, S.; Ofosu, O.; Thulasinathan, B.; Thondi Rajan, A.S.; Ramu, S.M.; Soorangkattan, S.; Muthuramalingam, J.B.; Alagarsamy, A. Thermal-chemical and biodegradation behaviour of alginic acid treated flax fibres/ poly(hydroxybutyrate-co-valerate) PHBV green composites in compost medium. Biocatal. Agric. Biotechnol. 2019, 22, 101394. [Google Scholar] [CrossRef]
- Sashiwa, H.; Fukuda, R.; Okura, T.; Sato, S.; Nakayama, A. Microbial degradation behavior in seawater of polyester blends containing poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx). Mar. Drugs 2018, 16, 34. [Google Scholar] [CrossRef] [Green Version]
- Nevoralová, M.; Koutný, M.; Ujčić, A.; Starý, Z.; Šerá, J.; Vlková, H.; Šlouf, M.; Fortelný, I.; Kruliš, Z. Structure Characterization and Biodegradation Rate of Poly(ε-caprolactone)/Starch Blends. Front. Mater. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Saika, A.; Nomura, K.; Watanabe, T.; Watanabe, T.; Fujimoto, Y.; Enoki, M.; Sato, T.; Kato, C.; Kanehiro, H. Biodegradation of aliphatic polyesters soaked in deep seawaters and isolation of poly(ε-caprolactone)-degrading bacteria. Polym. Degrad. Stab. 2011, 96, 1397–1403. [Google Scholar] [CrossRef]
- Adhikari, D.; Mukai, M.; Kubota, K.; Kai, T.; Kaneko, N.; Araki, K.S.; Kubo, M. Degradation of Bioplastics in Soil and Their Degradation Effects on Environmental Microorganisms. J. Agric. Chem. Environ. 2016, 5, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.X.; Wang, X.L.; Wang, Y.Z. Biodegradation behavior of PHAs with different chemical structures under controlled composting conditions. Polym. Test. 2011, 30, 372–380. [Google Scholar] [CrossRef]
- Tosin, M.; Weber, M.; Siotto, M.; Lott, C.; Innocenti, F.D. Laboratory test methods to determine the degradation of plastics in marine environmental conditions. Front. Microbiol. 2012, 3, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Sen, C.; Das, M. Biodegradability of Starch Based Self-Supporting Antimicrobial Film and Its Effect on Soil Quality. J. Polym. Environ. 2018, 26, 4331–4337. [Google Scholar] [CrossRef]
- Ulloa, P.A.; Vidal, J.; Lopéz de Dicastillo, C.; Rodriguez, F.; Guarda, A.; Cruz, R.M.S.; Galotto, M.J. Development of poly(lactic acid) films with propolis as a source of active compounds: Biodegradability, physical, and functional properties. J. Appl. Polym. Sci. 2019, 136, 1–11. [Google Scholar] [CrossRef]
- Gonçalves, S.P.C.; Strauss, M.; Martinez, D.S.T. The Positive Fate of Biochar Addition to Soil in the Degradation of PHBV-Silver Nanoparticle Composites. Environ. Sci. Technol. 2018, 52, 13845–13853. [Google Scholar] [CrossRef]
- Pavoni, J.M.F.; Luchese, C.L.; Tessaro, I.C. Impact of acid type for chitosan dissolution on the characteristics and biodegradability of cornstarch/chitosan based films. Int. J. Biol. Macromol. 2019, 138, 693–703. [Google Scholar] [CrossRef]
- Cano, A.I.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Biodegradation behavior of starch-PVA films as affected by the incorporation of different antimicrobials. Polym. Degrad. Stab. 2016, 132, 11–20. [Google Scholar] [CrossRef]
- Wang, H.; Wei, D.; Zheng, A.; Xiao, H. Soil burial biodegradation of antimicrobial biodegradable PBAT films. Polym. Degrad. Stab. 2015, 116, 14–22. [Google Scholar] [CrossRef]
- Norcino, L.B.; Mendes, J.F.; Natarelli, C.V.L.; Manrich, A.; Oliveira, J.E.; Mattoso, L.H.C. Pectin films loaded with copaiba oil nanoemulsions for potential use as bio-based active packaging. Food Hydrocoll. 2020, 106, 105862. [Google Scholar] [CrossRef]
- Tampau, A.; González-Martínez, C.; Chiralt, A. Biodegradability and disintegration of multilayer starch films with electrospun PCL fibres encapsulating carvacrol. Polym. Degrad. Stab. 2020, 173. [Google Scholar] [CrossRef]
- Arrieta, M.P.; García, A.D.; López, D.; Fiori, S.; Peponi, L. Antioxidant bilayers based on PHBV and plasticized electrospun PLA-PHB fibers encapsulating catechin. Nanomaterials 2019, 9, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.; Zou, P.; Xiong, H.; Tang, H. Effect of nano-SiO2 on the performance of starch/polyvinyl alcohol blend films. Carbohydr. Polym. 2008, 72, 521–526. [Google Scholar] [CrossRef]
- Capelezzo, A.P.; Mohr, L.C.; Dalcanton, F.; Barreta, C.R.D.M.; Martins, M.A.P.M.; Fiori, M.A.; De Mello, J.M.M. Polimero biodegradavel antimicrobiano através da aditivacao com compostos a base de zinco. Quim. Nova 2018, 41, 367–374. [Google Scholar] [CrossRef]
| Biodegradable Polymer | Antimicrobial | Microbiological Tests | Microorganisms | Results | Reference |
|---|---|---|---|---|---|
| Cassava starch/cellulose nanofibers | Tea tree essential oil | In vitro | E. coli S. aureus C. albicans | S. aureus: inhibition of 73% C. albicans: inhibition of 65% E. coli: no effect | [13] |
| Cassava starch | Cinnamon essential oil | In vitro | P. commune E. amstelodami | Mayor inhibition of E. amstelodami | [14] |
| Pea starch/PVA | Silver nanoparticles | In vitro | L. innocua E. coli A. niger P. expansum | Microbial growth inhibition | [15] |
| Brown rice starch /chitosan | Chitosan | In vitro | E. coli S. aureus | Microbial growth inhibition | [16] |
| Cassava starch/chitosan | Oregano and cinnamon leaf essential oils | Pork meat | Total aerobic and coliform | No growth inhibition | [10] |
| Cassava starch/chitosan | Chitosan | Pork meat | Total aerobic and coliform | Microbial growth inhibition | [11] |
| Sugar palm starch/nanocrystalline celulose | Cinnamon essential oil | In vitro | B. susbtilis S. aureus E. coli | Microbial growth inhibition | [17] |
| Tapioca starch | Chitosan | In vitro/Cherry tomato | B. cereus S. aureus E. coli, S. typhimurium | Microbial growth inhibition | [18] |
| Corn starch/bovine gelatin | N-α-lauroyl-l-arginine ethyl ester monohydrochloride | Chicken breast | Psychotrophic bacteria, lactic acid bacteria, anaerobic, total coliforms, E.coli | Microbial growth inhibition | [12] |
| Pea starch/PVA | Neem and oregano essential oils | In vitro | L. innocua and E. coli | Microbial growth inhibition | [19] |
| Cassava starch/chitosan | Lemongrass essential oil | In vitro | Mesophillic bacteria | Microbial growth inhibition | [20] |
| Sago starch/guar gum | Carvacrol and citral | In vitro | B. cereus E. coli | Microbial growth inhibition | [21] |
| Oxidized and Acetylated Corn Starch / Sodium Alginate | Sodium dehydroacetate and rosemary extract | In vitro | E. coli A. niger | Microbial growth inhibition | [22] |
| Hydroxypropyl-high-amylose starch | Pomegranate peel powder | In vitro | S. aureus Salmonella | Greater action against S. aureus than against Salmonella | [23] |
| Antimicrobial starch | Sodium benzoate and citric acid | Cheddar cheese | L. innocua | Microbial growth inhibition | [24] |
| Corn starch/bovine gelatine | Ethyl lauroyl arginate (LAE) | Marinated salmon | L. innocua | Microbial growth inhibition | [25] |
| Biodegradable Polymer | Antimicrobial | Microbiological Tests | Microorganisms | Results | Reference |
|---|---|---|---|---|---|
| PLA | Essential oils (clove, cinnamon and garlic) | In vitro | C. jejuni S. aureus | Garlic oil: limited antimicrobial activity Clove and cinnamon oils: more effective against C. jejuni than against S. aureus | [32] |
| PLA/PBAT | Cellulose-silver nanocrystals | In vitro | E. coli S. Aureus | Limited antimicrobial activity | [33] |
| PLA/PBS | Chitin nanofrils | In vitro | S. aureus Enterobacter spp. | No microbial inhibition | [34] |
| PLA | Propolis ethanolic extract | In vitro | Gram positivas Gram negativas | No efective | [35] |
| PLA | Propolis ethanolic extract Essential oil of Tanacetum balsamita | In vitro | B. cereus Gram positive Gram negative | Limited antimicrobial activity | [35] |
| PLA | Propolis ethanolic extract Tanacetum balsamita essential oil | Sausages | Lactic acid, aerobic esophilic and psychotrophic bacterias | Extended shelf-life of sausages | [35] |
| PLA/Starch | Cinnamaldehyde | In vitro | E. coli L. innocua | Inhibition of microbial growth | [36] |
| PLA | Chitosan | Pork meat | Total aerobes and coliform | Inhibition of microbial growth | [37] |
| PVA | poly (hexamethylene guanidine) | In vitro | S. aureus E. coli | Inhibition of microbial growth | [38] |
| PVA-Chitosan | Ethyl Lauroyl Arginate (LAE) | In vitro | C. jejuni, S. typhimurium E.coli, L. monocytogenes | Inhibition of microbial growth with 5–10% LAE | [39] |
| PVA | Lactic, tartaric and malic acids | In vitro | S. aureus E. coli | Greater inhibition with lactic acid, followed by malic and tartaric acids | [40] |
| PVA-Chitosan | Chitosan | Minimally processed tomato | S. aureus E. coli B. subtilis | Greater inhibitory effect in E. coli and B. subtilis than in S. aureus | [41] |
| PCL/short chain peptide (REDV) | Eugenol | In vitro | E. coli S. aureus | Limited antimicrobial activity | [42] |
| PCL | Solid extract of sage | In vitro | E. coli S. aureus | Limited antimicrobial activity. More effective against S. aureus | [43] |
| PCL | Organic acids, rosmarinic acid extract and Asian essential oil blend. | Broccoli | E. coli S. typhimurium | Inhibition of microbial growth | [44] |
| PCL | Grapefruit seed extract | In vitro | L. monocytogenes | Inhibition of microbial growth | [45] |
| PCL/starch | Pomegranate rind powder | In vitro | S. aureus | Inhibition of microbial growth at high concentrations | [46] |
| PCL | Cinnamaldehyde | In vitro | E. coli S. aureus | Inhibition of microbial growth | [47] |
| PCL | Methanolic extract of pomegranate | In vitro | E. coli S. aureus | 6–7 days growth delay | [47] |
| PCL | Freeze dried pomegranate arils | In vitro | E. coli S. aureus | 2-day growth delay | [47] |
| PCL | Pomegranate seed flour | In vitro | E. coli S. aureus | 2-day growth delay | [47] |
| PCL/starch | Carvacrol | In vitro | E. coli L. innoua | Inhibition of E.coli | [48] |
| Biodegradable Polymer | Antimicrobial | Microbiological Tests | Microorganisms | Results | Reference |
|---|---|---|---|---|---|
| PHA | Lime oil | In vitro | E. coli S. aureus | Antimicrobial effectiveness against S. aureus | [52] |
| PHBV | Silver nanoparticles | In vitro | S. enterica L. monocytogenes | Effective against S.enterica and not effective against L. monocytogenes | [53] |
| PHA | Alkyl quaternary ammonium salts | In vitro | E. coli S. aureus | Inhibition of microbial growth | [54] |
| PHB | Vanillin | In vitro | E. coli S. typhimurium S. flexneri S. aureus | Minimum concentration to reduce microbial activity: 80 μg/g PHB | [55] |
| PHB/PCL/Organic clays | Nisin | In vitro/ham slices | L. plantarum | Inhibition of microbial growth | [56] |
| PHB | Eugenol and pediocin | In vitro | S.aureus E. coli S. typhimurium B. cereus | Inhibition of microbial growth | [57] |
| PHB/PSPH | Chlorine | In vitro | S.aureus E. coli | Inhibition of microbial growth | [58] |
| PHBV | ZnO nanoparticles and oregano essential oil | In vitro | E. coli S. aureus | Significant microbial growth inhibition | [59] |
| PHBV | ZnO nanoparticles and oregano essential oil acting synergistically | In vitro | E. coli S. aureus | Greater microbial inhibition than that of pure antimicrobials | [59] |
| PHBV | Oregano essential oil | In vitro | E. coli L. innocua | Significant microbial inhibition | [60] |
| PHBV | Carvacrol | In vitro | E. coli L. innocua | Significant microbial inhibition | [60] |
| PHBV/Silica mesoporous support | Eugenol essential oil | In vitro | E. coli S. aureus | Microbial inhibition | [61] |
| PHBV | Triclosan | In vitro | E. coli S. aureus | Effective microbial inhibition | [62] |
| PHBV | Carvacrol/Eugenol | In vitro | E.coli L. innocua | Effective microbial inhibition | [63] |
| Bioplastics | Type of Environment | Conditions | Control Method | Biodegradation Period (days) | Biodegradation (%) Others Data | Reference |
|---|---|---|---|---|---|---|
| PLA | ||||||
| PLA | Compost | 58 °C | Produced CO2 | 80 | 78.9 | [78] |
| PLA/TiO2 nanocomposites | Compost | 58 °C | Produced CO2 | 80 | Between 85 and 97.8 | [78] |
| PLA | Compost | 58 °C, 50% humidity | Weight loss | 14 | >90 | [79] |
| PLA/CNC nanocomposites | Compost | 58 °C, 50% humidity | Weight loss | 14 | >90 | [79] |
| PLA | Soil | - | Weight loss | 70 | 0.15 | [80] |
| PLA/Starch | Soil | - | Weight loss | 70 | 16 | [80] |
| PLA | Sea water and freshwater | 25 °C and fluorescence light (16 h light and 8 h dark) | Weight loss | 365 | Non-significant degradation | [81] |
| PLA | Compost | 58 °C | Produced CO2 | 130 | 90 | [82] |
| PLA | Sea water | Without sediment, in euphotic and aphotic conditions | Weight loss | 365 | PLA > PET | [83] |
| PLGA | Sea water and fresh water | 25 °C and fluorescence light (16 h light and 8 h dark) | Weight loss | 270 | 100 | [81] |
| PHAs | ||||||
| 3-PHB | Sea water | 28.75 ± 1.65 °C 53% salinity pH 7.0–7.5 | Weight loss | 160 (films) 80–160 (pellets) | 58 (films) 38 (pellets) | [70] |
| PHB/PHBV | River water | Eutrophic recreation. (1 m depth) | Weight loss Degradation rate (DR) | 31–42 | 34.6–43.5% DR: 0.011–0.014 d−1 | [84] |
| PHB | River water | Eutrophic recreation (1 m depth) | Weight loss Degradation rate (DR) | 22–45 | 93% DR: 0.008–0.174 d−1 | [84] |
| PHB | Sea water and fresh water | 25 °C and fluorescence light (16 h light and 8 h dark) | Weight loss | 365 | 8.5 | [81] |
| 3-PHB/3-PHV | Sea water | 28.75 ± 1.65 °C 53% salinity pH 7.0–7.5 | Weight loss | 160 (films) 80–160 (pellets) | 54 (films) (13 pellets) | [70] |
| PHBV | Sea water | Laboratory (static), 30 °C. (With sediment, 75 mL) Aquarium (dynamic): 12–22 °C. With and without sediment. | Produced CO2 and Weight loss (WL) | 38–90 | % CO2: 70% WL static: 75–85% WL dynamic: 33–50% | [85] |
| PHB | Sea water | Laboratory (static): 30 °C. (With sediment, 75 mL) Aquarium (dynamic): 12–22 °C. With and without sediment. | Produced CO2 and weight loss (WL) | 18–100 | % CO2: 80–90% WL static: 90% WL dynamic: <90% | [85] |
| PHB | Sea water | Intertidal zone, pelagic (10 m depth), benthic (20 m depth). | Degradation rate (DR) | - | DR: Benthic > Intertidal > Pelagic | [86] |
| PHBV | Soil | - | Weight loss | 112 | 0.5 | [87] |
| PHBV flax | Soil | - | Weight loss | 112 | 6 | [87] |
| PHBV/PBAT/ flax | Soil | - | Weight loss | 112 | 9 | [87] |
| PHBV/ENR flax | Soil | - | Weight loss | 112 | 17 | [87] |
| PHBV | Compost | 58 °C | Produced CO2 | 100 | 63.2 | [88] |
| PHBV/flaxseed fibers | Compost | 58 °C | Produced CO2 | 100 | 85.6 | [88] |
| PHBV/flax/ alginic | Compost | 58 °C | Produced CO2 | 100 | 88.0 | [88] |
| PHBHHx/PBAT | Sea water | - | Weight loss | 28 | 31 (ratio 100/0) 19 (ratio 80/20) 10 (ratio 60/40) 3 (ratio 40/60) 1 (ratio 0/100) | [89] |
| PHBHHx/PBS | Sea water | - | Weight loss | 28 | 51 (ratio 100/0) 41(ratio 80/20) 18 (ratio 60/40) 5 (ratio 40/60) 1 (ratio 0/100) | [89] |
| PHBHHx/PLA | Sea water | - | Weight loss | 28 | 34 (ratio 100/0) 33 (ratio 80/20) 32 (ratio 60/40) 26 (ratio 40/60) 1 (ratio 0/100) | [89] |
| PCL | ||||||
| PCL | Compost | 58 °C | Produced CO2 | 72 | ~100 | [90] |
| PCL/TPS | Compost | 58 °C | Produced CO2 | 72 | ~90 (ratio 50/50) ~95 (ratio 30/70) | [90] |
| PCL | Soil | 30 °C | Weight loss | 90 | 2.5 | [45] |
| PCL | Sea water | Depth: 321 m, 350 m, 612 m. Low temperatures and high hydrostatic pressure. | Resistance to break, (RB) and surface morphology (SM) | 270–360 | RB decrease: 0–20% SM: abundant pores and heterogeneous cracks | [91] |
| PCL | Sea water and fresh water | 25 °C and fluorescence light (16 h light and 8 h dark) | Weight loss | 365 | Non-significant degradability | [81] |
| Others | ||||||
| PBS/Starch | Soil | 25 °C, 60% humidity | Weight loss | 28 | 7 (films) 24 (powdered) | [92] |
| PBS | Soil | 25 °C, 60% humidity | Weight loss | 28 | 1 (films) 16.8 (powdered) | [92] |
| PVA | Compost | - | Iodometric analysis | 8 | 51–79 | [23] |
| PBS | Sea water | Depth: 321 m, 350 m, 612 m. Low temperatures and high hydrostatic pressure. | Resistance to break, RB) and surface morphology (SM) | 360 | RB decrease ≈ 100% SM: rough surface with many stains | [91] |
| PBSe | Sea water | Intertidal zone, pelagic (10 m depth), benthic (20 m depth). | Weight loss and Degradation rate (DR) | - | DT: Benthic > Intertidal > Pelagic | [86] |
| PBSet | Sea water | Intertidal zone, pelagic (10 m depth), benthic (20 m depth). | Weight loss Degradation rate (DR) | - | DT: Benthic > Intertidal > Pelagic | [86] |
| Polymer | Antimicrobial | Type of Environment | Main Feature | Reference |
|---|---|---|---|---|
| Starch/PVA | Sodium propionate | Soil | The antimicrobial did not interfere with biodegradation. 90% degradation in 28 days | [97] |
| PLA | Propolis (crude propolis and its ethanolic extract) | Soil | Propolis promoted biodegradation | [98] |
| PHBV | Silver nanoparticles | Soil | Biochar accelerated biodegradation. Silver nanoparticles significantly reduced biodegradability | [99] |
| Maize starch/chitosan | Chitosan | Compost | In 15 days, the chitosan did not negatively affect the biodegradation | [100] |
| Brown rice starch/chitosan | Chitosan | Compost | Biodegradation was faster with higher proportion of starch | [16] |
| Starch/PVA | Neem oil, oregano essential oil and silver nanoparticles | Compost | The oils improved the biodegradation of films Silver nanoparticles inhibited biodegradation | [101] |
| PBAT/ thermoplastic starch | Polyhexamethylene Guanidine Hydrochloride (PHPG) | Soil | Antimicrobial delayed the biodegradation | [102] |
| Pectin | Copaiba oil | Soil | Delay biodegradation of polymer | [103] |
| Starch/PCL | Carvacrol | Compost | Carvacrol delayed biodegradation | [104] |
| PHBV/PLA-PHB | Catechin | Compost | Catechin delayed disintegration process Lactic acid accelerated it | [105] |
| Starch/PVA | Silicon oxide nanoparticles | Soil | Silicon oxide nanoparticles did not affected biodegradation | [106] |
| Ecoflex® | Zinc oxide nanoparticles and microcapsules with ionic zinc | Soil | Zinc compounds did not affect biodegradation process | [107] |
| PCL | Grapefruit seed extract (GSE) | Soil | Biodegradation was faster as the incorporated amount of GSE increased | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-García, E.; Vargas, M.; González-Martínez, C.; Chiralt, A. Biodegradable Antimicrobial Films for Food Packaging: Effect of Antimicrobials on Degradation. Foods 2021, 10, 1256. https://doi.org/10.3390/foods10061256
Hernández-García E, Vargas M, González-Martínez C, Chiralt A. Biodegradable Antimicrobial Films for Food Packaging: Effect of Antimicrobials on Degradation. Foods. 2021; 10(6):1256. https://doi.org/10.3390/foods10061256
Chicago/Turabian StyleHernández-García, Eva, María Vargas, Chelo González-Martínez, and Amparo Chiralt. 2021. "Biodegradable Antimicrobial Films for Food Packaging: Effect of Antimicrobials on Degradation" Foods 10, no. 6: 1256. https://doi.org/10.3390/foods10061256






