Production, Optimization and Characterization of Polylactic Acid Microparticles Using Electrospray with Porous Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Electrospray Solutions
2.2. Characterization of Physical Properties of Solutions
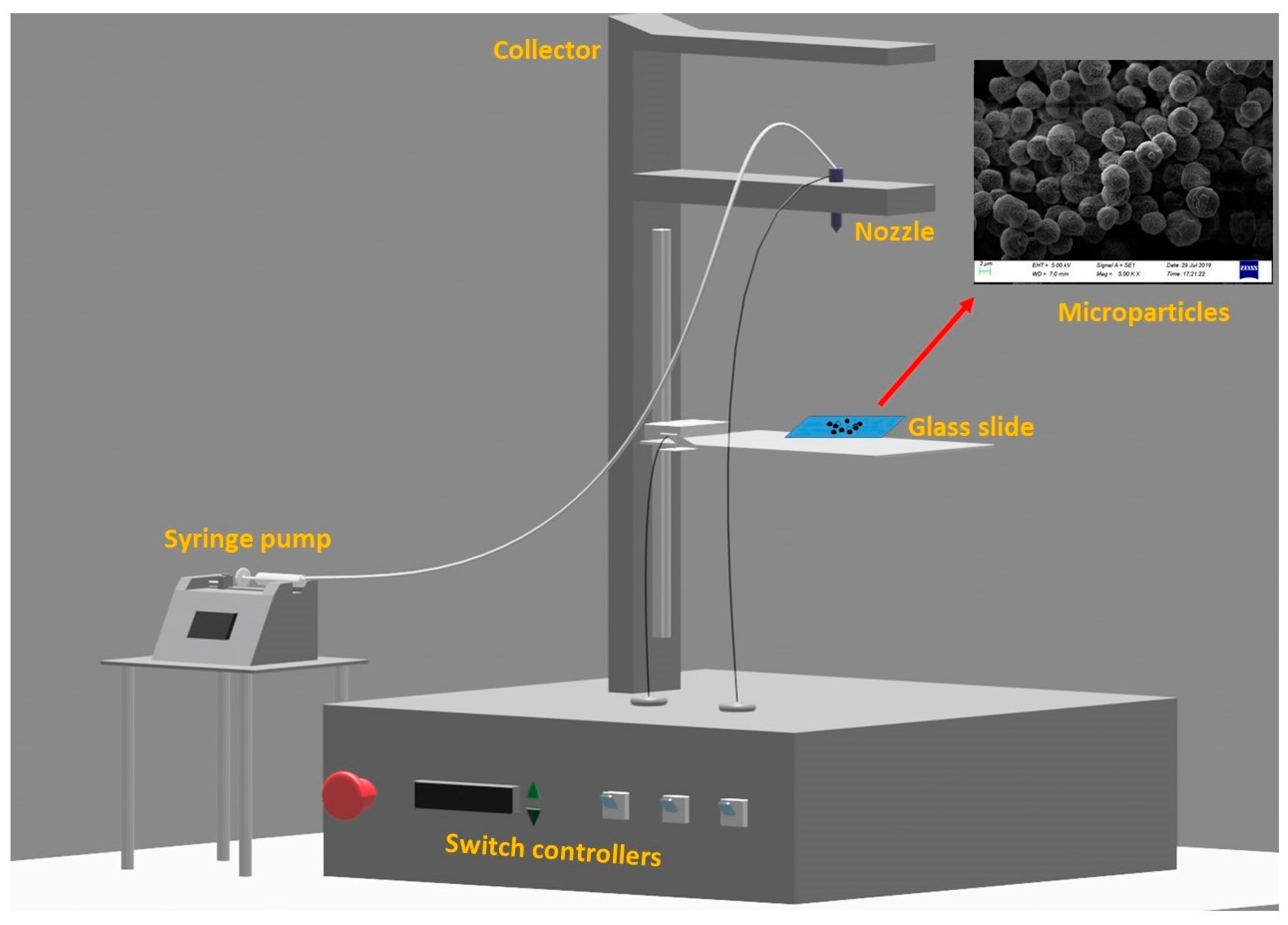
2.3. Fabrication of the Microparticles via the Electrospray Method
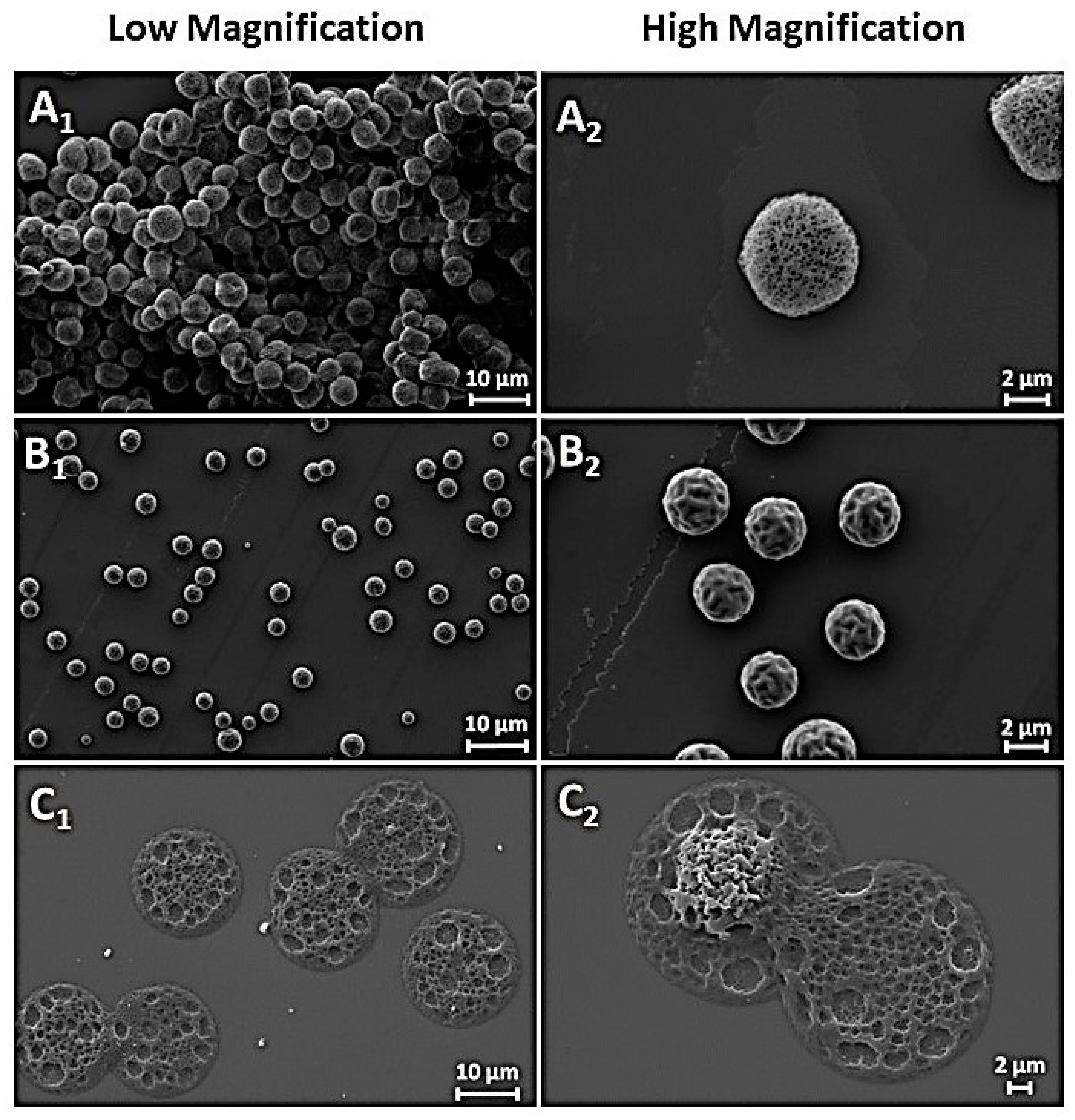
2.4. Optical Microscopy and Scanning Electron Microscopy (SEM)
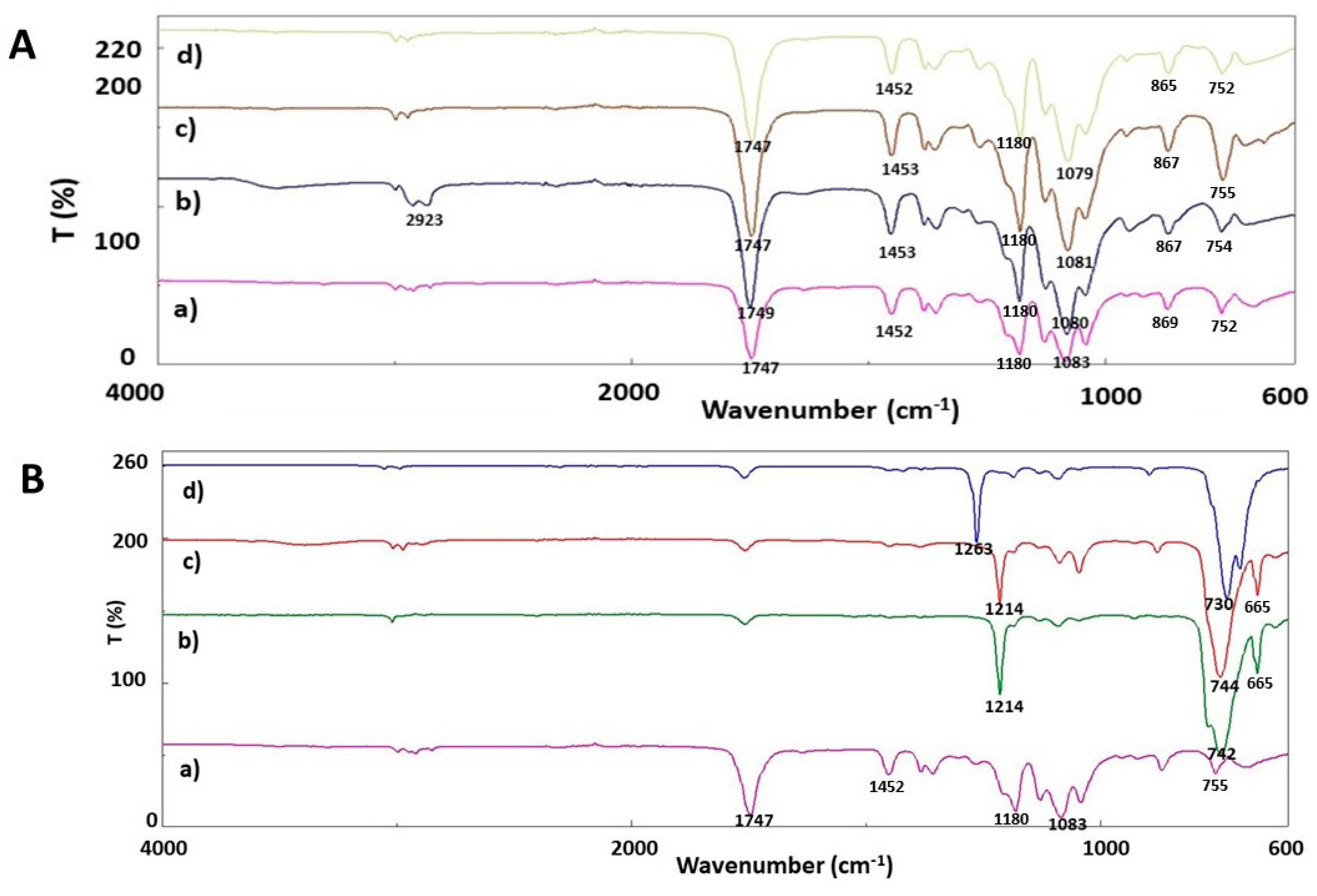
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
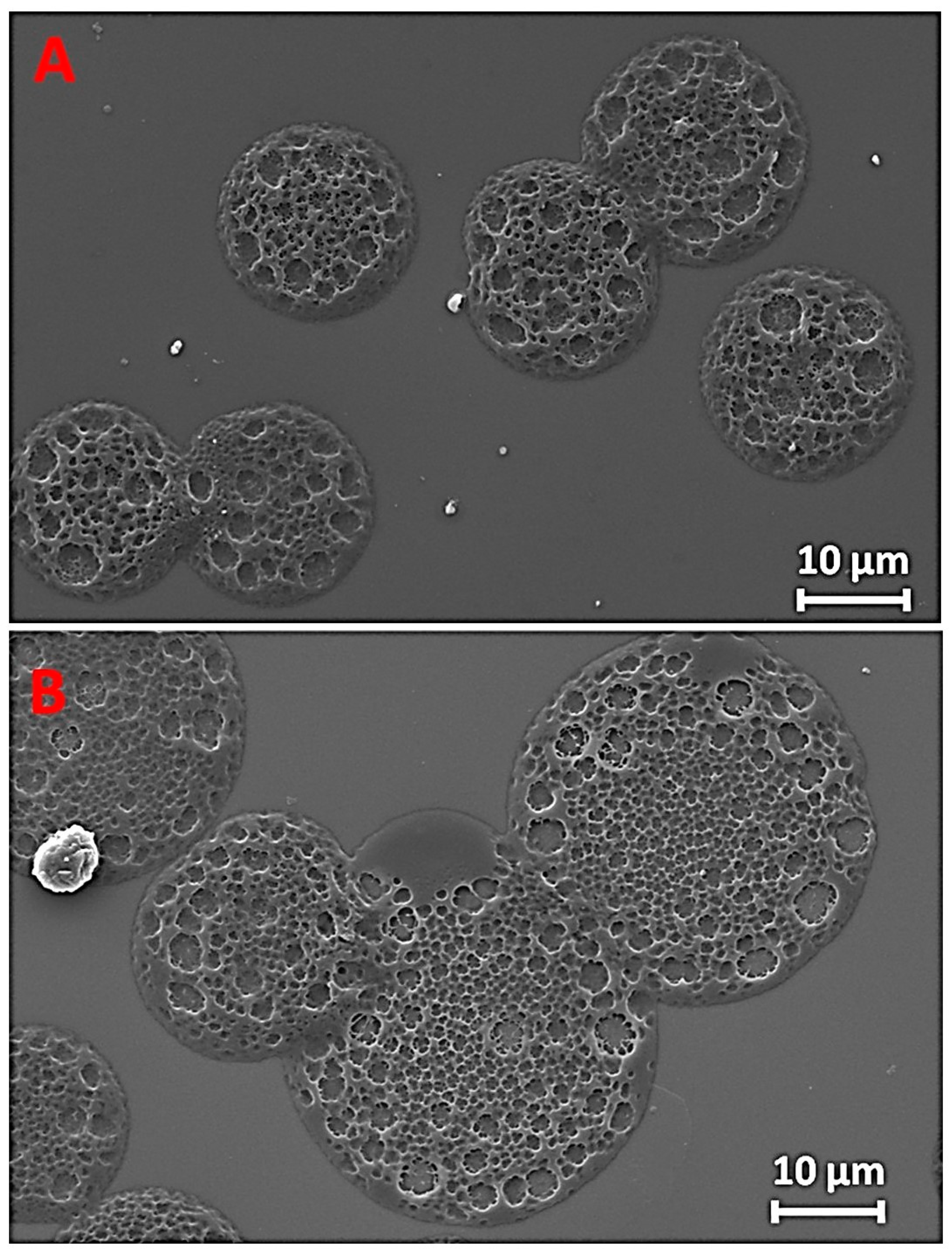
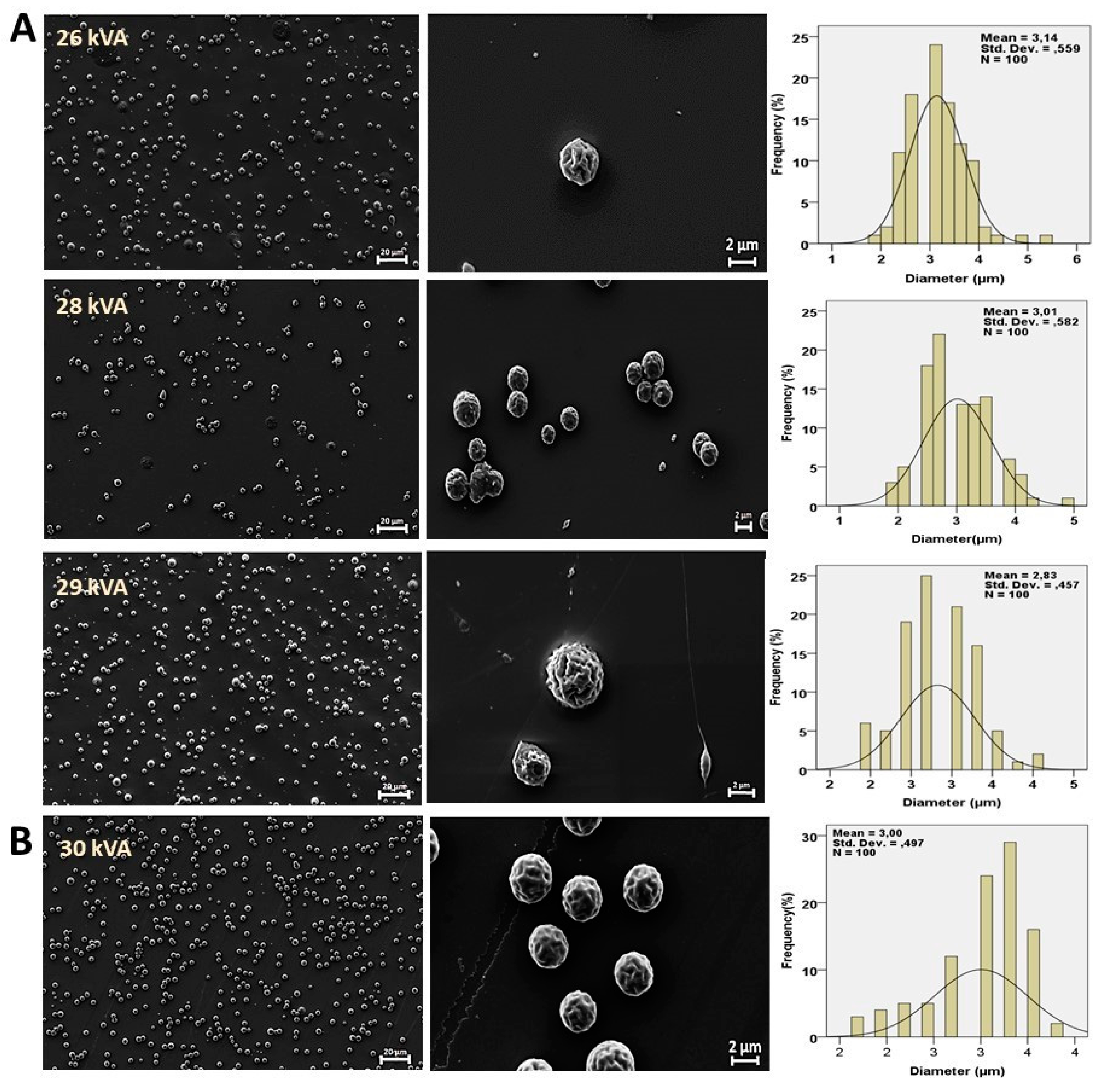
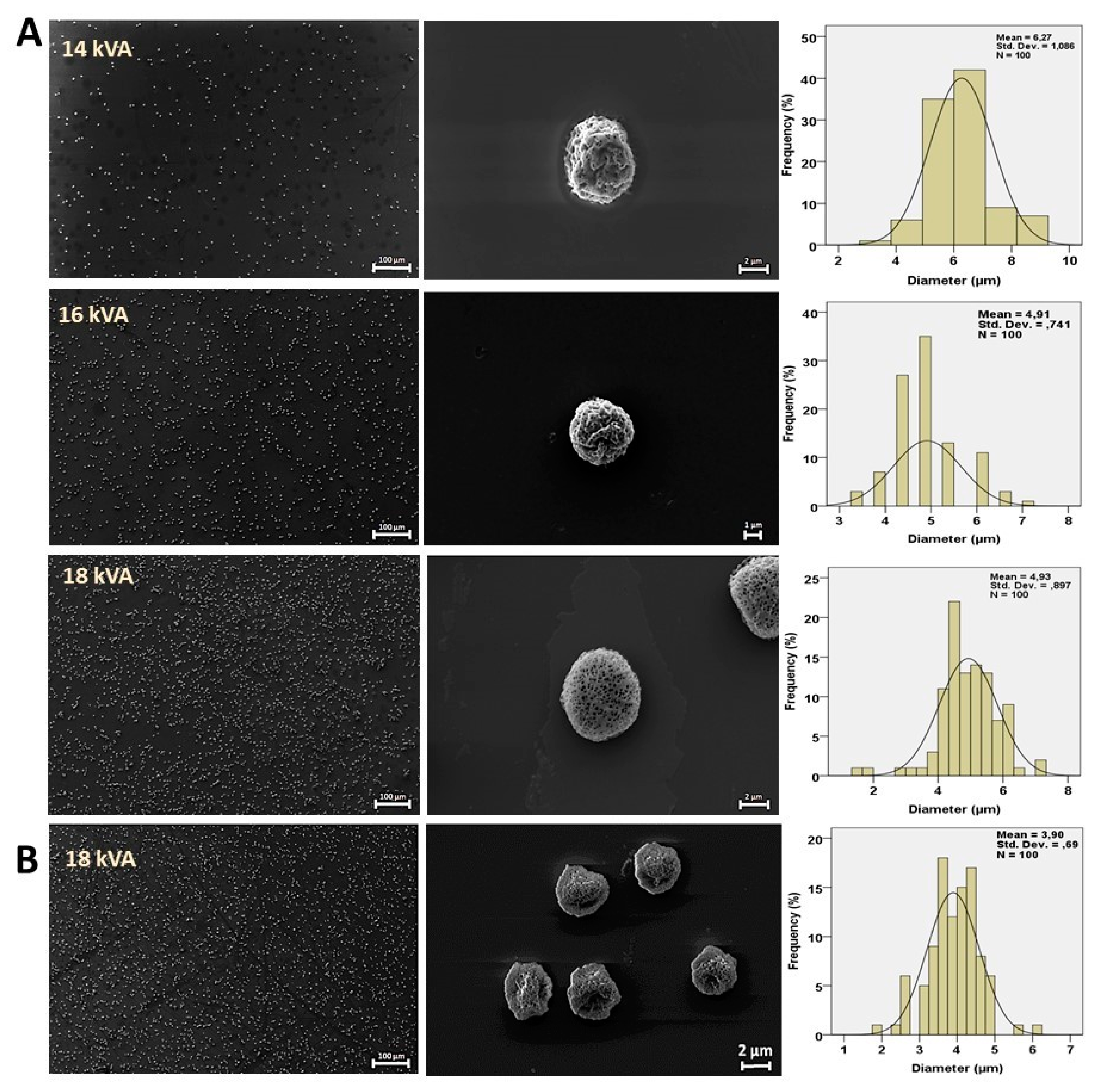
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campos, E.; Branquinho, J.; Carreira, A.S.; Carvalho, A.; Coimbra, P.; Ferreira, P.; Gil, M.H. Designing polymeric microparticles for biomedical and industrial applications. Eur. Polym. J. 2016, 49, 2005–2021. [Google Scholar] [CrossRef]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ε-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef]
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef]
- Soares, R.M.D.; Siqueira, N.M.; Prabhakaram, M.P. Materials Science & Engineering C Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef]
- Fantini, D.; Zanetti, M.; Costa, L. Polystyrene microspheres and nanospheres produced by electrospray. Macromol. Rapid Commun. 2006, 27, 2038–2042. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Torres-Chávez, P.I.; Ramírez-Wong, B.; Rascón-Chu, A.; Plascencia-Jatomea, M.; Barreras-Urbina, C.G.; Rangel-Vázquez, N.A.; Rodríguez-Félix, F. Micro- and Nanoparticles by Electrospray: Advances and Applications in Foods. J. Agric. Food Chem. 2015, 63, 4699–4707. [Google Scholar] [CrossRef] [PubMed]
- Dole, M.; Mack, L.L.; Hines, R.L.; Mobley, R.C.; Ferguson, L.D. Molecular Beams of Macroions. J. Chem. Phys. 1968, 49, 2240–2249. [Google Scholar] [CrossRef]
- Notz, P.K.; Basaran, O.A. Dynamics of drop formation in an electric field. J. Colloid Interface Sci. 1999. [Google Scholar] [CrossRef]
- Zakeri, M.; Moghadam, H.; Samimi, A.; Mohebbi-Kalhori, D. Optimization of calcium alginate beads production by electrospray using response surface methodology. Mater. Res. Express 2019, 6, 095412. [Google Scholar] [CrossRef]
- Xu, Y.; Hanna, M.A. Electrospray encapsulation of water-soluble protein with polylactide. Effects of formulations on morphology, encapsulation efficiency and release profile of particles. Int. J. Pharm. 2006, 320, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, S.S. Synthesis of biodegradable triple-layered capsules using a triaxial electrospray method. Polymer 2011, 52, 3325–3336. [Google Scholar] [CrossRef]
- Valo, H.; Peltonen, L.; Vehviläinen, S.; Karjalainen, M.; Kostiainen, R.; Laaksonen, T.; Hirvonen, J. Electrospray encapsulation of hydrophilic and hydrophobic drugs in poly(L-lactic acid) nanoparticles. Small 2009, 5, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, L.; Cui, Y.; Tu, K.; Wang, H.; Wang, L. Colloids and Surfaces B: Biointerfaces The exploration of endocytic mechanisms of PLA-PEG nanoparticles prepared by coaxialtri-capillary electrospray-template removal method. Colloids Surf. B Biointerfaces 2018, 161, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Cam, M.E.; Zhang, Y.; Edirisinghe, M. Electrosprayed microparticles: A novel drug delivery method. Expert Opin. Drug Deliv. 2019, 16, 895–901. [Google Scholar] [CrossRef]
- Ardila, N.; Ajji, Z.; Heuzey, M.C.; Ajji, A. Chitosan electrospraying: Mapping of process stability and micro and nanoparticle formation. J. Aerosol Sci. 2018, 126, 85–98. [Google Scholar] [CrossRef]
- Amoyav, B.; Benny, O. Microfluidic based fabrication and characterization of highly porous polymeric microspheres. Polymers (Basel) 2019, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Dastidar, D.; Saha, S.; Chowdhury, M. Porous microspheres: Synthesis, characterisation and applications in pharmaceutical & medical fields. Int. J. Pharm. 2018, 548, 34–48. [Google Scholar] [PubMed]
- Ilhan, E.; Ulag, S.; Sahin, A.; Yilmaz, B.K.; Ekren, N.; Kilic, O.; Sengor, M.; Kalaskar, D.M.; Oktar, F.N.; Gunduz, O. Fabrication of tissue-engineered tympanic membrane patches using 3D-Printing technology. J. Mech. Behav. Biomed. Mater. 2020, 114, 104219. [Google Scholar] [CrossRef]
- Cam, M.E.; Cesur, S.; Taskin, T.; Erdemir, G.; Kuruca, D.S.; Sahin, Y.M.; Kabasakal, L.; Gunduz, O. Fabrication, characterization and fibroblast proliferative activity of electrospun Achillea lycaonica-loaded nanofibrous mats. Eur. Polym. J. 2019. [Google Scholar] [CrossRef]
- Cesur, S.; Oktar, F.N.; Ekren, N.; Kilic, O.; Alkaya, D.B.; Seyhan, S.A.; Ege, Z.R.; Lin, C.-C.; Kuruca, S.E.; Erdemir, G.; et al. Preparation and characterization of electrospun polylactic acid/sodium alginate/orange oyster shell composite nanofiber for biomedical application. J. Aust. Ceram. Soc. 2019. [Google Scholar] [CrossRef]
- Ilhan, E.; Ulag, S.; Sahin, A.; Ekren, N.; Kilic, O.; Oktar, F.N.; Gunduz, O. Production of 3D-Printed Tympanic Membrane Scaffolds as a Tissue Engineering Application. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer Nature Switzerland AG: Cham, Switerland, 2020. [Google Scholar]
- Lassalle, V.; Ferreira, M.L.; Aires, B. PLA Nano- and Microparticles for Drug Delivery: An Overview of the Methods of Preparation. Macromol. Biosci. 2007, 767–783. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Chen, J.; He, T.; Hu, Y.; Dong, X.; Zhang, H.; Huang, W.; Ko, F.; Zhou, W. Electrospray biodegradable microcapsules loaded with curcumin for drug delivery systems with high bioactivity. RSC Adv. 2017, 7, 1724–1734. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Tane, R.; Ikuta, K. Electrospray deposition and direct patterning of polylactic acid nanofibrous microcapsules for tissue engineering. Biomed. Microdevices 2012, 14, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Megelski, S.; Stephens, J.S.; Bruce Chase, D.; Rabolt, J.F. Micro- and nanostructured surface morphology on electrospun polymer fibers. Macromolecules 2002. [Google Scholar] [CrossRef]
- Viet Linh, N.V. Fabrication of biodegradable polyester microspheres by electrospraying methods for drug carrier application. Vietnam J. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Gupta, P.; Elkins, C.; Long, T.E.; Wilkes, G.L. Electrospinning of linear homopolymers of poly(methyl methacrylate): Exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent. Polymer 2005. [Google Scholar] [CrossRef]
- Freitas, S.; Merkle, H.P.; Gander, B. Microencapsulation by solvent extraction/evaporation: Reviewing the state of the art of microsphere preparation process technology. J. Control. Release 2005, 102, 313–332. [Google Scholar] [CrossRef]
- Xu, Y.; Skotak, M.; Hanna, M. Electrospray encapsulation of water-soluble protein with polylactide. I. Effects of formulations and process on morphology and particle size. J. Microencapsul. 2006. [Google Scholar] [CrossRef] [PubMed]
- Saadipour, M.; Karkhaneh, A.; Haghbin Nazarpak, M. An investigation into curcumin release from PLA particles loaded in PCL-GELATIN fibers for skin application. Int. J. Polym. Mater. Polym. Biomater. 2020. [Google Scholar] [CrossRef]
- Xie, J.; Ng, W.J.; Lee, L.Y.; Wang, C.H. Encapsulation of protein drugs in biodegradable microparticles by co-axial electrospray. J. Colloid Interface Sci. 2008. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Gomez, A. Monodisperse electrosprays of low electric conductivity liquids in the cone-jet mode. J. Colloid Interface Sci. 1996. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Nagai, M.; Omi, S. Preparation of uniform poly(lactide) microspheres by employing the Shirasu Porous Glass (SPG) emulsification technique. Colloids Surf. A Physicochem. Eng. Asp. 1999, 153, 383–394. [Google Scholar] [CrossRef]
- Cam, M.E.; Hazar-Yavuz, A.N.; Cesur, S.; Ozkan, O.; Alenezi, H.; Turkoglu Sasmazel, H.; Sayip Eroglu, M.; Brako, F.; Ahmed, J.; Kabasakal, L.; et al. A novel treatment strategy for preterm birth: Intra-vaginal progesterone-loaded fibrous patches. Int. J. Pharm. 2020. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Yuan, X.; Chu, K.; Zhang, H.; Ji, W.; Rui, M. Preparation and optimization of poly (lactic acid) nanoparticles loaded with fisetin to improve anti-cancer therapy. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Cesur, S.; Ulag, S.; Ozak, L.; Gumussoy, A.; Arslan, S.; Yilmaz, B.K.; Ekren, N.; Agirbasli, M.; Kalaskar, D.M.; Gunduz, O. Production and characterization of elastomeric cardiac tissue-like patches for Myocardial Tissue Engineering. Polym. Test. 2020, 90, 106613. [Google Scholar] [CrossRef]
- Nishida, J.; Shigeto, S.; Yabumoto, S.; Hamaguchi, H. Anharmonic coupling of the CH-stretch and CH-bend vibrations of chloroform as studied by near-infrared electroabsorption spectroscopy. J. Chem. Phys. 2012, 137, 234501. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, R.; Zhao, Y.; Weng, S.; Xu, Y.; Noda, I. Investigation on the intermolecular interaction between diethyl ether and dichloromethane in gaseous phase by using the DAOSD approach. J. Mol. Struct. 2016, 1124, 244–248. [Google Scholar] [CrossRef]
| Solvent | PLA Concentration (%) | Flow Rate (µL/min) | Voltage (kVA) * Distance (cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Chloroform | 1–4 | 40 | 60 | 65 | 10.2–17.5 | 12 | 15 | 18 | |
| Ethanol–Chloroform | 1–3 | 12 | 15 | 40 | 50 | 12–30 | 12 | 15 | 18 |
| DCM | 3 | 15 | 20 | 11–30 | 12 | 15 | 18 | ||
| Solution | Density (g/dL) | Viscosity (cP) | Surface Tension (Mn/m) |
|---|---|---|---|
| 3 % PLA – chloroform | 1.48 | 153 | 18.85 |
| 3 % PLA – 5 % ethanol– chloroform | 1.41 | 510 | 23.31 |
| 3 % PLA – DCM | 1.32 | 151.8 | 26.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasci, M.E.; Dede, B.; Tabak, E.; Gur, A.; Sulutas, R.B.; Cesur, S.; Ilhan, E.; Lin, C.-C.; Paik, P.; Ficai, D.; et al. Production, Optimization and Characterization of Polylactic Acid Microparticles Using Electrospray with Porous Structure. Appl. Sci. 2021, 11, 5090. https://doi.org/10.3390/app11115090
Tasci ME, Dede B, Tabak E, Gur A, Sulutas RB, Cesur S, Ilhan E, Lin C-C, Paik P, Ficai D, et al. Production, Optimization and Characterization of Polylactic Acid Microparticles Using Electrospray with Porous Structure. Applied Sciences. 2021; 11(11):5090. https://doi.org/10.3390/app11115090
Chicago/Turabian StyleTasci, Muhammed Enes, Berna Dede, Eray Tabak, Aybuke Gur, Rabia Betul Sulutas, Sumeyye Cesur, Elif Ilhan, Chi-Chang Lin, Pradip Paik, Denisa Ficai, and et al. 2021. "Production, Optimization and Characterization of Polylactic Acid Microparticles Using Electrospray with Porous Structure" Applied Sciences 11, no. 11: 5090. https://doi.org/10.3390/app11115090
APA StyleTasci, M. E., Dede, B., Tabak, E., Gur, A., Sulutas, R. B., Cesur, S., Ilhan, E., Lin, C.-C., Paik, P., Ficai, D., Ficai, A., & Gunduz, O. (2021). Production, Optimization and Characterization of Polylactic Acid Microparticles Using Electrospray with Porous Structure. Applied Sciences, 11(11), 5090. https://doi.org/10.3390/app11115090











