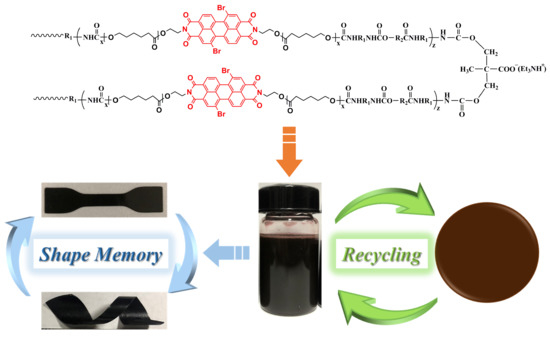
Recyclable Shape-Memory Waterborne Polyurethane Films Based on Perylene Bisimide Modified Polycaprolactone Diol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PWPU-X and BWPU-X Emulsions
2.3. Fabrication of Films
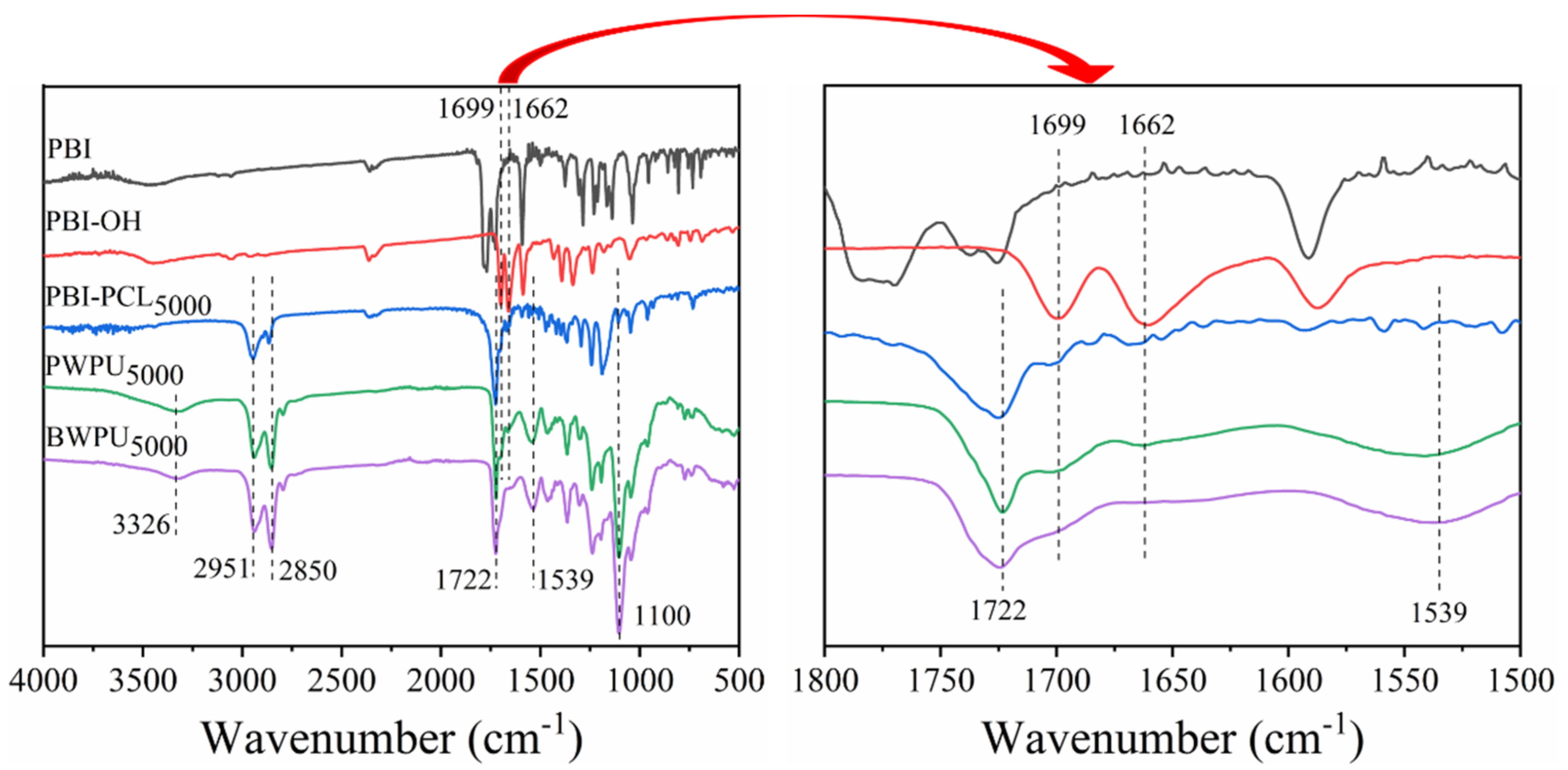
2.4. Characterization
3. Results and Discussion
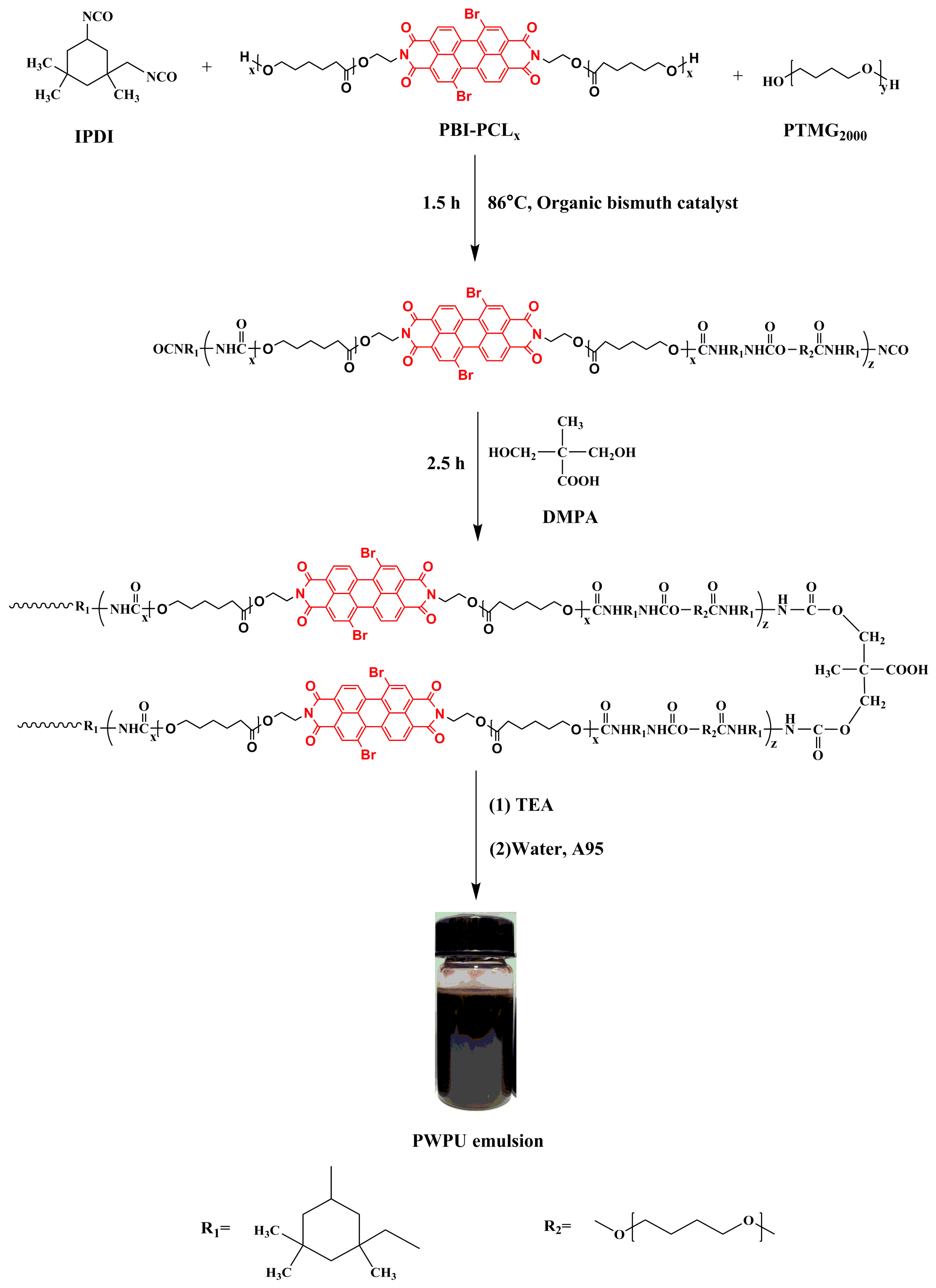
3.1. Synthesis of PWPU-X and BWPU-X Emulsions
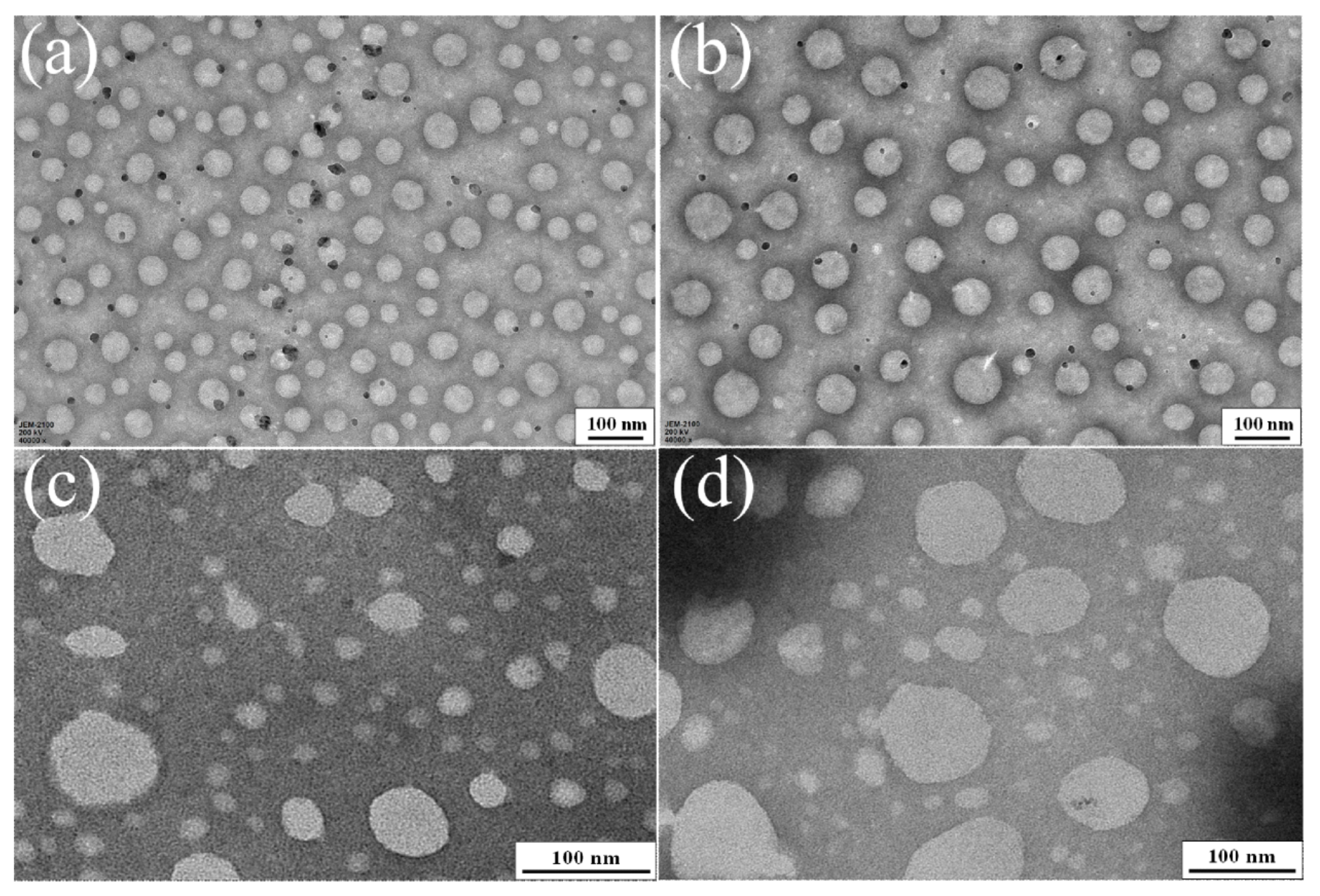
3.2. Properties of PWPU-X and BWPU-X Emulsions
3.3. Mechanical and Thermal Performance
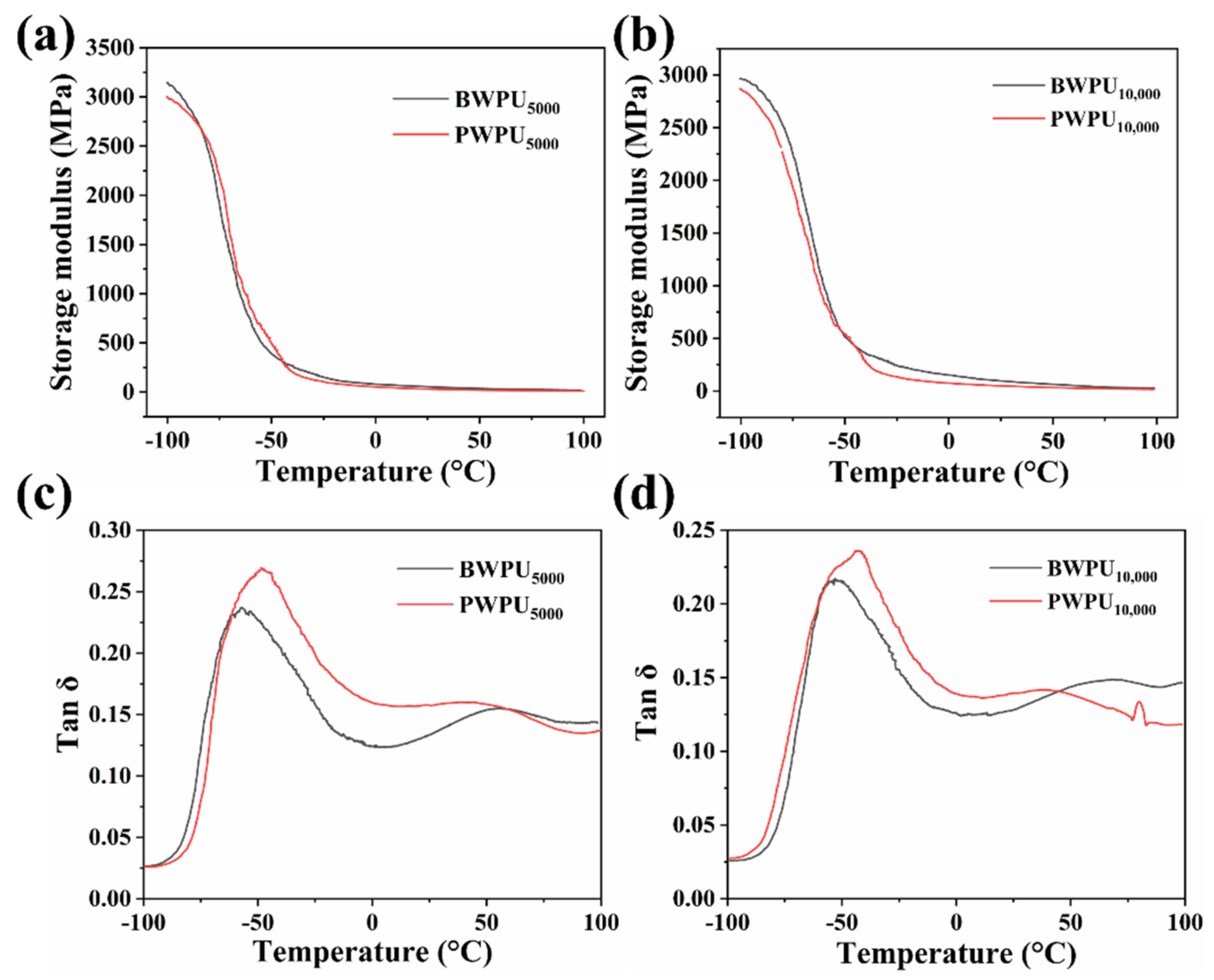
3.4. AFM and DMA Analysis
3.4.1. AFM Analysis
3.4.2. DMA Analysis
3.5. Recyclable Performance Analysis
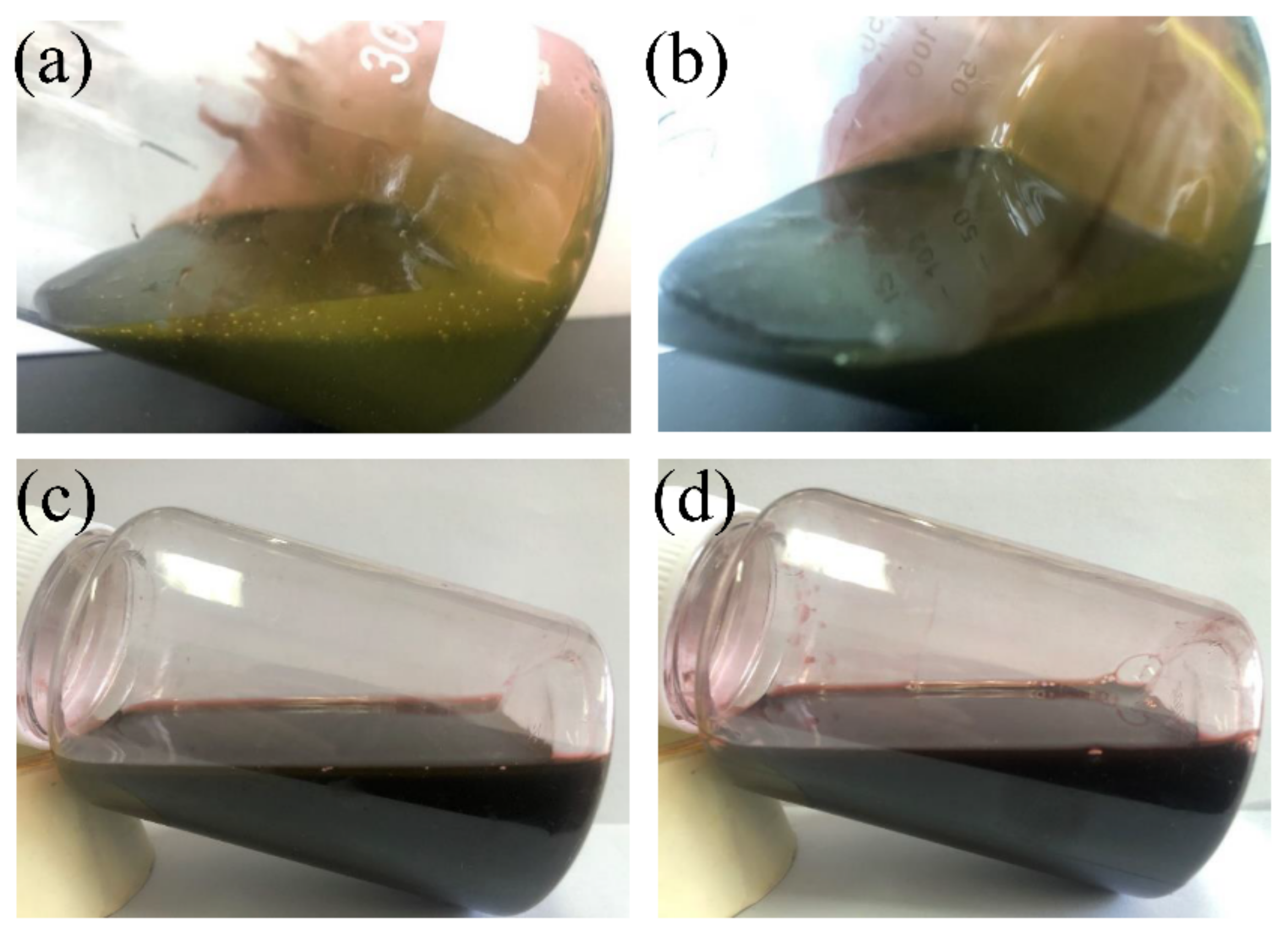
3.5.1. Green Recycling Process of PWPU-X Films
3.5.2. Properties of Recycled PWPU-X Emulsion
3.5.3. Mechanical and Thermal Performance of Recycled PWPU-X Film
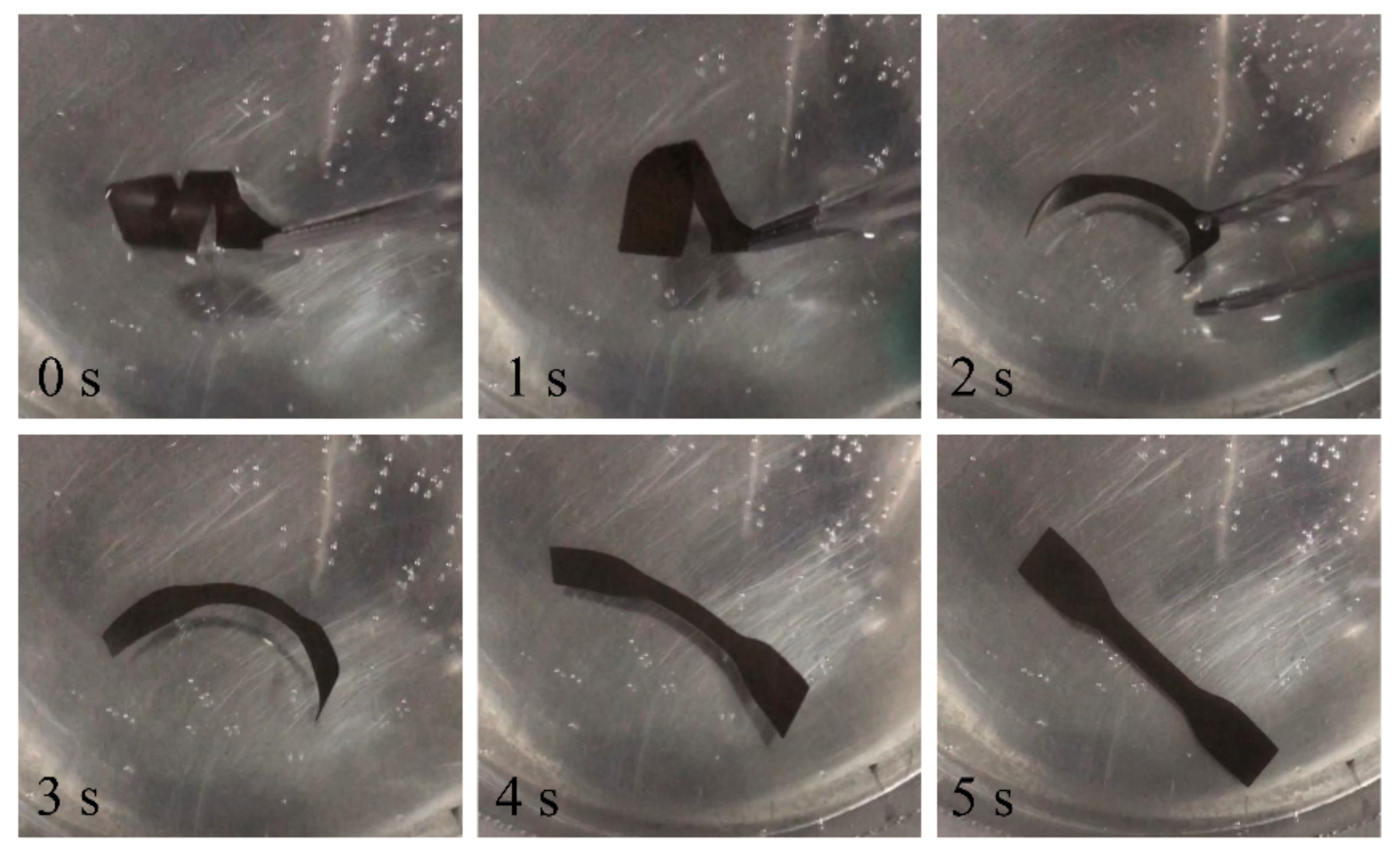
3.6. Shape-Memory Performance of PWPU-X
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, Y.; Miao, D.; Kong, L.; Jiang, J.; Guo, Z. Preparation and performance test of the super-hydrophobic polyurethane coating based on waste cooking oil. Coatings 2019, 9, 861. [Google Scholar] [CrossRef]
- Chen, S.Y.; Zhuang, R.Q.; Chuang, F.S.; Rwei, S.P. Synthetic scheme to increase the abrasion resistance of waterborne polyurethane–urea by controlling micro-phase separation. J. Appl. Polym. Sci. 2021, 138. [Google Scholar] [CrossRef]
- Shaygan, M.; Aliehyaei, M.; Ahmadi, A.; Assad, M.E.H.; Silveira, J.L. Energy, exergy, advanced exergy and economic analyses of hybrid polymer electrolyte membrane (PEM) fuel cell and photovoltaic cells to produce hydrogen and electricity. J. Clean. Prod. 2019, 234, 1082–1093. [Google Scholar] [CrossRef]
- Hong, S.; Min, K.D.; Nam, B.U.; Park, O.O. High molecular weight bio furan-based co-polyesters for food packaging applications: Synthesis, characterization and solid-state polymerization. Green Chem. 2016, 18, 412–416. [Google Scholar] [CrossRef]
- Kang, S.Y.; Ji, Z.; Tseng, L.F.; Turner, S.A.; Villanueva, D.A.; Johnson, R.; Albano, A.; Langer, R.J.A.M. Design and synthesis of waterborne polyurethanes. Adv. Mater. 2018, 30, e1706237. [Google Scholar] [CrossRef]
- Pinto, D.G.; Bernardo, L.; Amaro, A.; Lopes, S.J.C.; Materials, B. Polymer nanocomposites using titanium as reinforcement-a review. Constr. Build. Mater. 2015, 69, 506–524. [Google Scholar] [CrossRef]
- Yang, W.; Dong, Q.; Liu, S.; Xie, H.; Liu, L.; Li, J. Recycling and disposal methods for polyurethane foam wastes. Proce. Environ. Sci. 2012, 16, 167–175. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Srivastava, S.; Mahanwar, P.A.; Gadekar, P.T. Recycling and disposal methods for polyurethane wastes: A review. J. Polym. Chem. 2019, 9, 39–51. [Google Scholar] [CrossRef]
- Liu, X.; Pang, Y.; Cui, T.; Li, Y.; Mao, A. Recycling polyurethane materials to improve properties of wood composite panels. Am. J. Agric. For. 2019, 7, 146. [Google Scholar] [CrossRef]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef]
- Fang, Y.; Du, X.; Jiang, Y.; Du, Z.; Pan, P.; Cheng, X.; Wang, H. Thermal-driven self-Healing and recyclable waterborne polyurethane films based on reversible covalent interaction. ACS Sustain. Chem. Eng. 2018, 6, 14490–14500. [Google Scholar] [CrossRef]
- Nikje, M.M.A.; Garmarudi, A.B.; Idris, A.B. Polyurethane waste reduction and recycling: From bench to pilot scales. Des. Monomers Polym. 2012, 14, 395–421. [Google Scholar] [CrossRef]
- Hsu, S.; Hsieh, C.-T.; Sun, Y.-M. Synthesis and characterization of waterborne polyurethane containing poly(3-hydroxybutyrate) as new biodegradable elastomers. J. Mater. Chem. B 2015, 3, 9089–9097. [Google Scholar] [CrossRef]
- Liu, W.-K.; Zhao, Y.; Wang, R.; Luo, F.; Li, J.-S.; Li, J.-H.; Tan, H. Effect of chain extender on hydrogen bond and microphase structure of biodegradable thermoplastic polyurethanes. Chin. J. Polym. Sci. 2017, 36, 514–520. [Google Scholar] [CrossRef]
- Czifrak, K.; Lakatos, C.; Arpad Kordovan, M.; Nagy, L.; Daroczi, L.; Zsuga, M.; Keki, S. Block copolymers of poly(omega-pentadecalactone) in segmented polyurethanes: Novel biodegradable shape memory polyurethanes. Polymers 2020, 12, 1928. [Google Scholar] [CrossRef]
- Yang, J.; Wen, H.; Zhuo, H.; Chen, S.; Ban, J. A new type of photo-thermo staged-responsive shape-memory polyurethanes network. Polymers 2017, 9, 287. [Google Scholar] [CrossRef]
- Naddeo, M.; Sorrentino, A.; Pappalardo, D. Thermo-rheological and shape memory properties of block and random copolymers of lactide and epsilon-caprolactone. Polymers 2021, 13, 627. [Google Scholar] [CrossRef]
- Zeng, C.; Lu, S.; Xiao, X.; Gao, J.; Pan, L.; He, Z.; Yu, J. Enhanced thermal and mechanical properties of epoxy composites by mixing noncovalently functionalized graphene sheets. Polym. Bull. 2014, 72, 453–472. [Google Scholar] [CrossRef]
- Li, Y.; Gao, J.; Li, X.; Xu, X.; Lu, S. High mechanical and thermal properties of epoxy composites with liquid crystalline polyurethane modified graphene. Polymers 2018, 10, 485. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, T.; Chi, H.; Li, T. Nanostructured polyurethane perylene bisimide ester assemblies with tuneable morphology and enhanced stability. R. Soc. Open Sci. 2018, 5, 171686. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Xiong, Z.; Yang, J.; Pan, L.; Wu, Y.; Wei, C.; Lu, S. Preparation and model of high-performance shape-memory polyurethane with hydroxylated perylene bisimide. RSC Adv. 2016, 6, 110329–110336. [Google Scholar] [CrossRef]
- Mirhosseini, M.M.; Haddadi-Asl, V.; Jouibari, I.S. How the soft segment arrangement influences the microphase separation kinetics and mechanical properties of polyurethane block polymers. Mater. Res. Express 2019, 6, 1591–2053. [Google Scholar] [CrossRef]
- Jang, T.; Kim, H.J.; Jang, J.B.; Kim, T.H.; Lee, W.; Seo, B.; Ko, W.B.; Lim, C.S. Synthesis of waterborne polyurethane using phosphorus-modified rigid polyol and its physical properties. Polymers 2021, 13, 432. [Google Scholar] [CrossRef]
- Li, J.W.; Tsen, W.C.; Tsou, C.H.; Suen, M.C.; Chiu, C.W. Synthetic environmentally friendly castor oil based-polyurethane with carbon black as a microphase separation promoter. Polymers 2019, 11, 1333. [Google Scholar] [CrossRef]
- Wang, X.; Zhan, S.; Lu, Z.; Li, J.; Yang, X.; Qiao, Y.; Men, Y.; Sun, J. Healable, Recyclable, and Mechanically Tough Polyurethane Elastomers with Exceptional Damage Tolerance. Adv. Mater. 2020, 32, e2005759. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Du, B.; Li, P.; Lai, X.; Niu, Y. Preparation and properties of a novel waterborne fluorinated polyurethane–acrylate hybrid emulsion. Colloid Polym. Sci. 2013, 292, 579–587. [Google Scholar] [CrossRef]
- Parashar, P.; Tripathi, C.B.; Arya, M.; Kanoujia, J.; Singh, M.; Yadav, A.; Saraf, S.A. A facile approach for fabricating CD44-targeted delivery of hyaluronic acid-functionalized PCL nanoparticles in urethane-induced lung cancer: Bcl-2, MMP-9, caspase-9, and BAX as potential markers. Drug Deliv. Transl. Res. 2019, 9, 37–52. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, F. Self-assembled fabrication and flame-retardant properties of reduced graphene oxide/waterborne polyurethane nanocomposites. J. Therm. Anal. Calorim. 2014, 118, 1561–1568. [Google Scholar] [CrossRef]
- Han, Y.; Hu, J.; Xin, Z. In-Situ Incorporation of Alkyl-Grafted Silica into Waterborne Polyurethane with High Solid Content for Enhanced Physical Properties of Coatings. Polymers 2018, 10, 514. [Google Scholar] [CrossRef]
- Wolska, A.; Goździkiewicz, M.; Ryszkowska, J. Thermal and mechanical behaviour of flexible polyurethane foams modified with graphite and phosphorous fillers. J. Mater. Sci. 2012, 47, 5627–5634. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, L.; Dong, D.; Wang, Y. Enhancement of water and organic solvent resistances of a waterborne polyurethane film by incorporating liquid polysulfide. RSC Adv. 2016, 6, 17163–17171. [Google Scholar] [CrossRef]
- Roy, A.; Yu, X.; Dunn, S.; McGrath, J.E. Influence of microstructure and chemical composition on proton exchange membrane properties of sulfonated–fluorinated, hydrophilic–hydrophobic multiblock copolymers. J. Membr. Sci. 2009, 327, 118–124. [Google Scholar] [CrossRef]
- Luo, Q.; Chen, J.; Gnanasekar, P.; Ma, X.; Qin, D.; Na, H.; Zhu, J.; Yan, N. A facile preparation strategy of polycaprolactone (PCL)-based biodegradable polyurethane elastomer with a highly efficient shape memory effect. New J. Chem. 2020, 44, 658–662. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Hou, L.; Chen, Y.; Xu, T. Effect of polyether/polyester polyol ratio on properties of waterborne two-component polyurethane coatings. Prog. Org. Coat. 2020, 141, 105545. [Google Scholar] [CrossRef]
- Rueda, L.; Saralegui, A.; Fernandez d’Arlas, B.; Zhou, Q.; Berglund, L.A.; Corcuera, M.A.; Mondragon, I.; Eceiza, A. Cellulose nanocrystals/polyurethane nanocomposites. Study from the viewpoint of microphase separated structure. Carbohydr. Polym. 2013, 92, 751–757. [Google Scholar] [CrossRef]
- Crawford, D.M.; Escarsega, J.A.J.T.A. Dynamic mechanical analysis of novel polyurethane coating for military applications. Thermochim. Acta 2000, 357, 161–168. [Google Scholar] [CrossRef]
- Lin, C.; Ge, H.; Wang, T.; Huang, M.; Ying, P.; Zhang, P.; Wu, J.; Ren, S.; Levchenko, V. A self-healing and recyclable polyurethane/halloysite nanocomposite based on thermoreversible Diels-Alder reaction. Polymers 2020, 206, 122894. [Google Scholar] [CrossRef]
- Yang, S.; Du, X.; Deng, S.; Qiu, J.; Du, Z.; Cheng, X.; Wang, H. Recyclable and self-healing polyurethane composites based on Diels-Alder reaction for efficient solar-to-thermal energy storage. Chem. Eng. J. 2020, 398, 125654. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, S.; Sun, P.; Tang, B.; Yin, Z.; Cao, X.; Chen, Q.; Xu, J.F.; Zhang, X. Tough and multi-recyclable cross-linked supramolecular polyureas via incorporating noncovalent bonds into main-chains. Adv. Mater. 2020, 32, e2000096. [Google Scholar] [CrossRef]
- Hasan, S.M.; Raymond, J.E.; Wilson, T.S.; Keller, B.K.; Maitland, D.J. Effects of isophorone diisocyanate on the thermal and mechanical properties of shape-memory polyurethane foams. Macromol. Chem. Phys. 2014, 215, 2420–2429. [Google Scholar] [CrossRef]
- Chien, Y.C.; Chuang, W.T.; Jeng, U.S.; Hsu, S.H. Preparation, characterization, and mechanism for biodegradable and biocompatible polyurethane shape memory elastomers. ACS Appl. Mater. Interfaces 2017, 9, 5419–5429. [Google Scholar] [CrossRef]











| Sample | IPDI (mmol) | PTMG2000 (mmol) | PBI-PCL5000 (mmol) | BDO-PCL5000 (mmol) | PBI-PCL10,000 (mmol) | BDO-PCL10,000 (mmol) | DMPA (mmol) | A95 (mmol) |
|---|---|---|---|---|---|---|---|---|
| PWPU5000 | 60 | 19.2 | 0.8 | 0 | 0 | 0 | 20 | 28 |
| BWPU5000 | 60 | 19.2 | 0 | 0.8 | 0 | 0 | 20 | 28 |
| PWPU10,000 | 60 | 19.2 | 0 | 0 | 0.8 | 0 | 20 | 28 |
| BWPU10,000 | 60 | 19.2 | 0 | 0 | 0 | 0.8 | 20 | 28 |
| Samples | Dz a (nm) | PDI b | Zeta Potential c (mV) |
|---|---|---|---|
| BWPU5000 | 80.99 | 0.232 | −54.5 |
| PWPU5000 | 40.26 | 0.131 | −60.7 |
| BWPU10,000 | 153.3 | 0.254 | −50.1 |
| PWPU10,000 | 49.05 | 0.216 | −54.0 |
| Samples | T5%/°C | T10%/°C | T50%/°C | T80%/°C |
|---|---|---|---|---|
| BWPU5000 | 261.2 | 296.1 | 390.3 | 418.3 |
| PWPU5000 | 281.0 | 315.9 | 402.7 | 424.4 |
| BWPU10,000 | 262.2 | 303.1 | 384.3 | 415.0 |
| PWPU10,000 | 280.1 | 314.3 | 401.2 | 428.5 |
| Samples | T5%/°C | T20%/°C | T50%/°C | T80%/°C |
|---|---|---|---|---|
| PWPU5000 | 281.0 | 357.4 | 402.7 | 424.4 |
| 3rd Cycle-PWPU5000 | 274.8 | 350.6 | 397.4 | 418.5 |
| PWPU10,000 | 280.1 | 341.6 | 401.2 | 428.5 |
| 3rd Cycle-PWPU10,000 | 279.8 | 341.6 | 400.9 | 428.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, K.; Zhang, H.; Qu, J.; Wang, J.; Bai, Y.; Sai, F. Recyclable Shape-Memory Waterborne Polyurethane Films Based on Perylene Bisimide Modified Polycaprolactone Diol. Polymers 2021, 13, 1755. https://doi.org/10.3390/polym13111755
Wei K, Zhang H, Qu J, Wang J, Bai Y, Sai F. Recyclable Shape-Memory Waterborne Polyurethane Films Based on Perylene Bisimide Modified Polycaprolactone Diol. Polymers. 2021; 13(11):1755. https://doi.org/10.3390/polym13111755
Chicago/Turabian StyleWei, Kang, Haitao Zhang, Jianbo Qu, Jianyong Wang, Yang Bai, and Futao Sai. 2021. "Recyclable Shape-Memory Waterborne Polyurethane Films Based on Perylene Bisimide Modified Polycaprolactone Diol" Polymers 13, no. 11: 1755. https://doi.org/10.3390/polym13111755
APA StyleWei, K., Zhang, H., Qu, J., Wang, J., Bai, Y., & Sai, F. (2021). Recyclable Shape-Memory Waterborne Polyurethane Films Based on Perylene Bisimide Modified Polycaprolactone Diol. Polymers, 13(11), 1755. https://doi.org/10.3390/polym13111755