Evaluation on the Mechanical Properties of Ground Granulated Blast Slag (GGBS) and Fly Ash Stabilized Soil via Geopolymer Process
Abstract
:1. Introduction
2. Materials and Experimental Method
2.1. Sources of Raw Materials
2.2. Characterization of Raw Materials
2.3. Method
2.3.1. Unconfined Compression Test
2.3.2. Liquid Limit
2.3.3. Plastic Limit
2.3.4. X-ray Diffraction (XRD)
2.3.5. Scanning Electron Microscope (SEM)
2.3.6. Fourier Transformation Infrared Spectroscopy (FTIR)
3. Results and Discussion
3.1. Raw Materials Characterization
3.1.1. Physical Properties
3.1.2. Chemical Properties
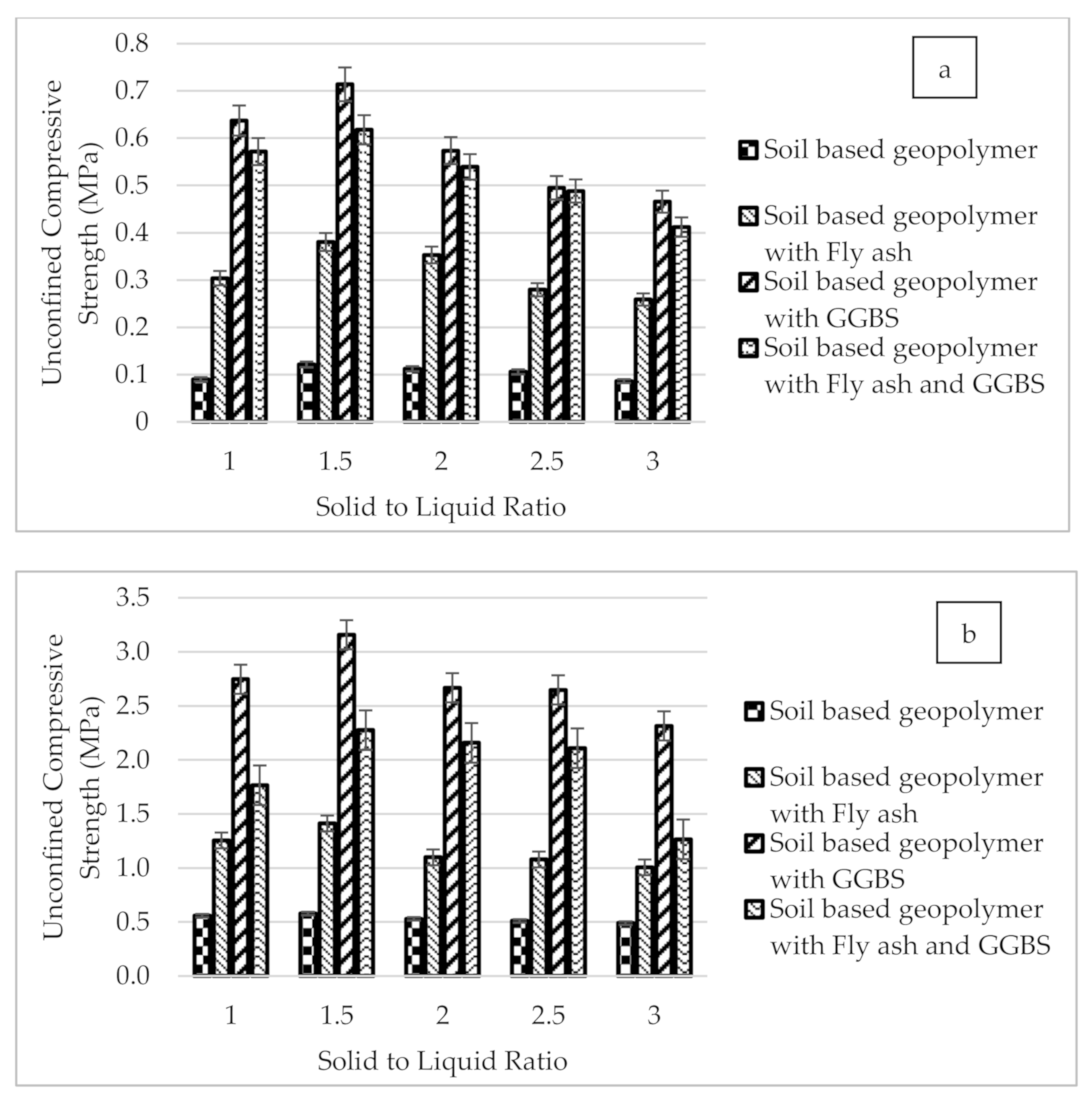
3.2. Unconfined Compression Test
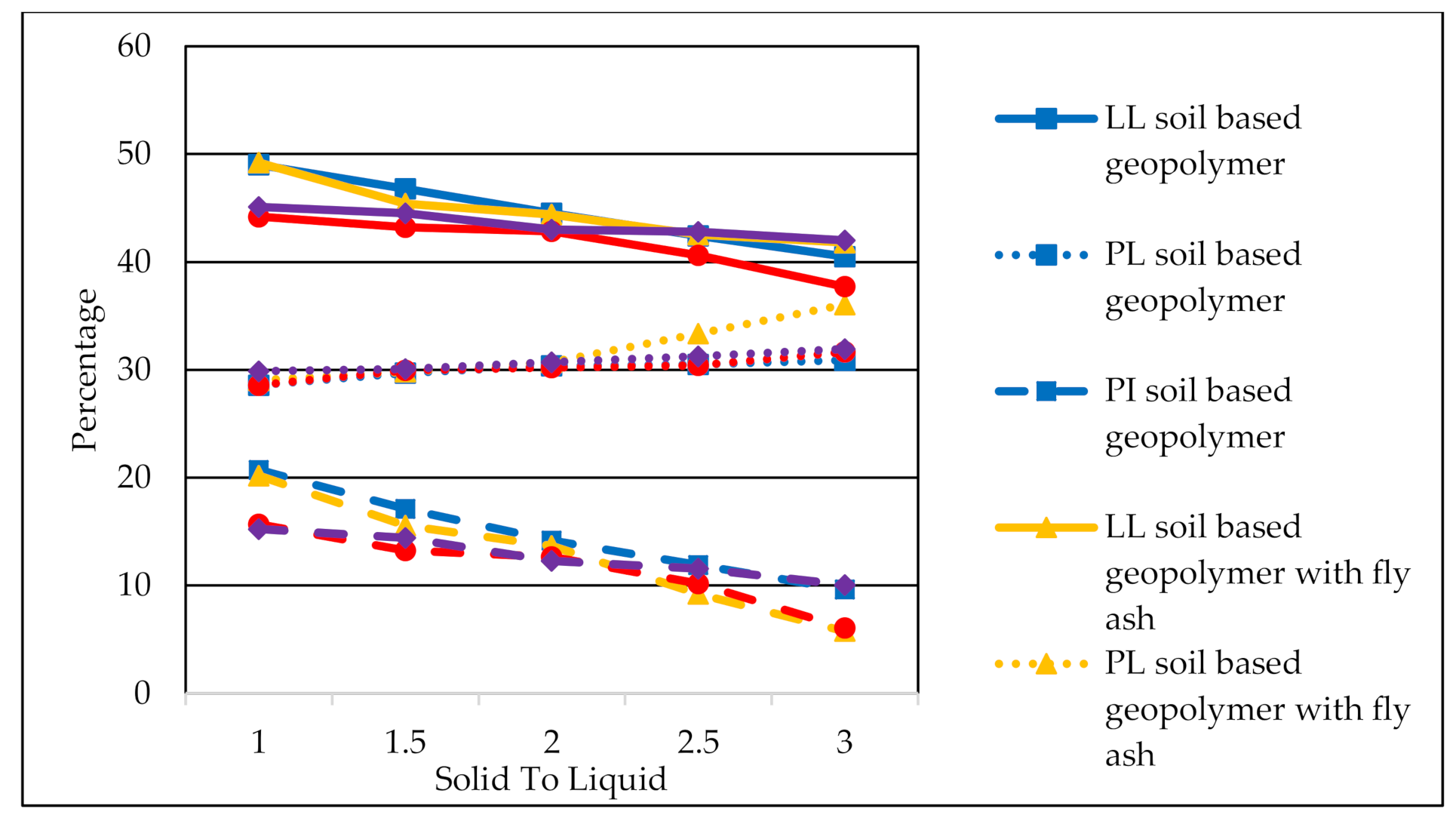
3.3. Atterberg Limit Test
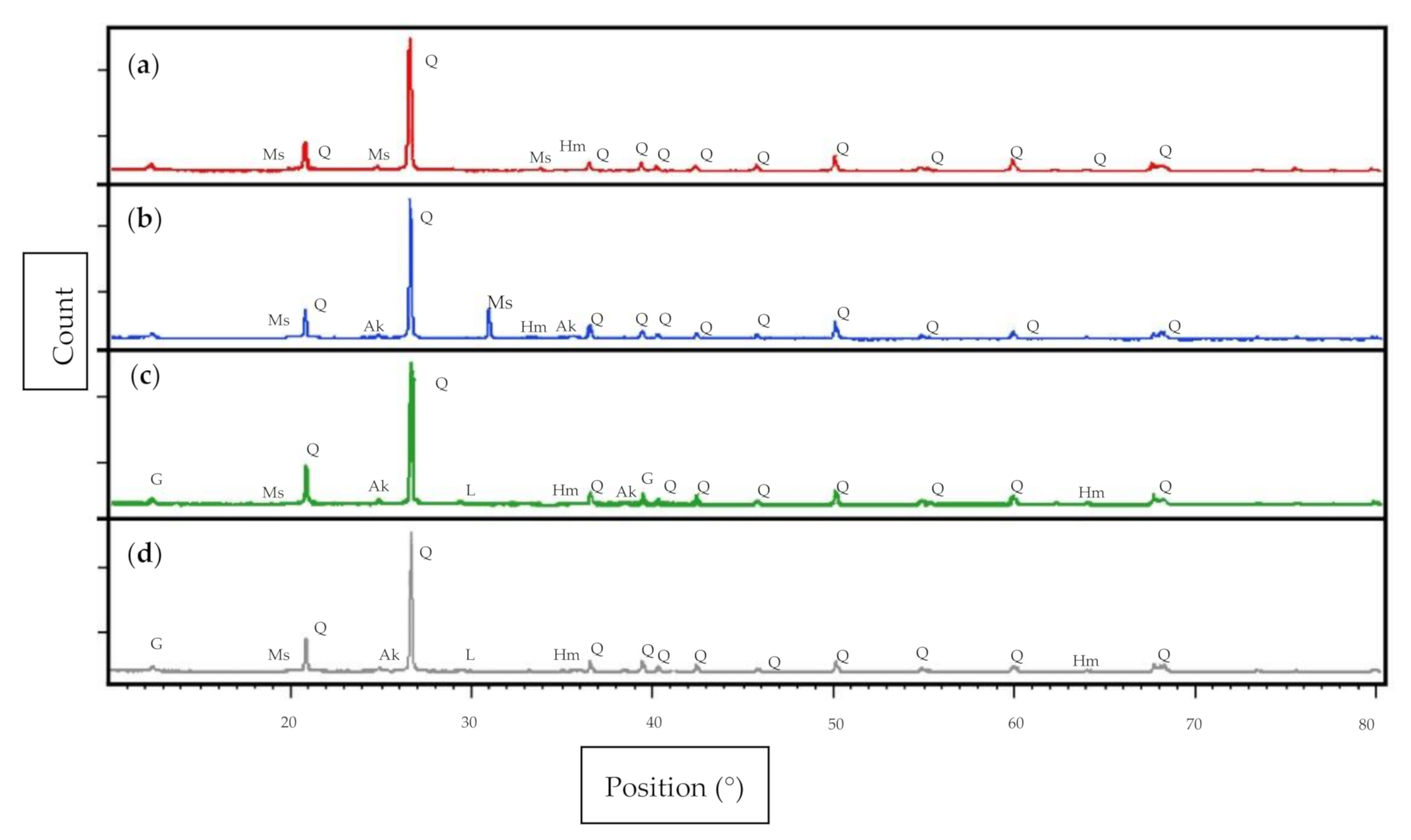
3.4. Phase Analysis
3.5. Morphology Analysis
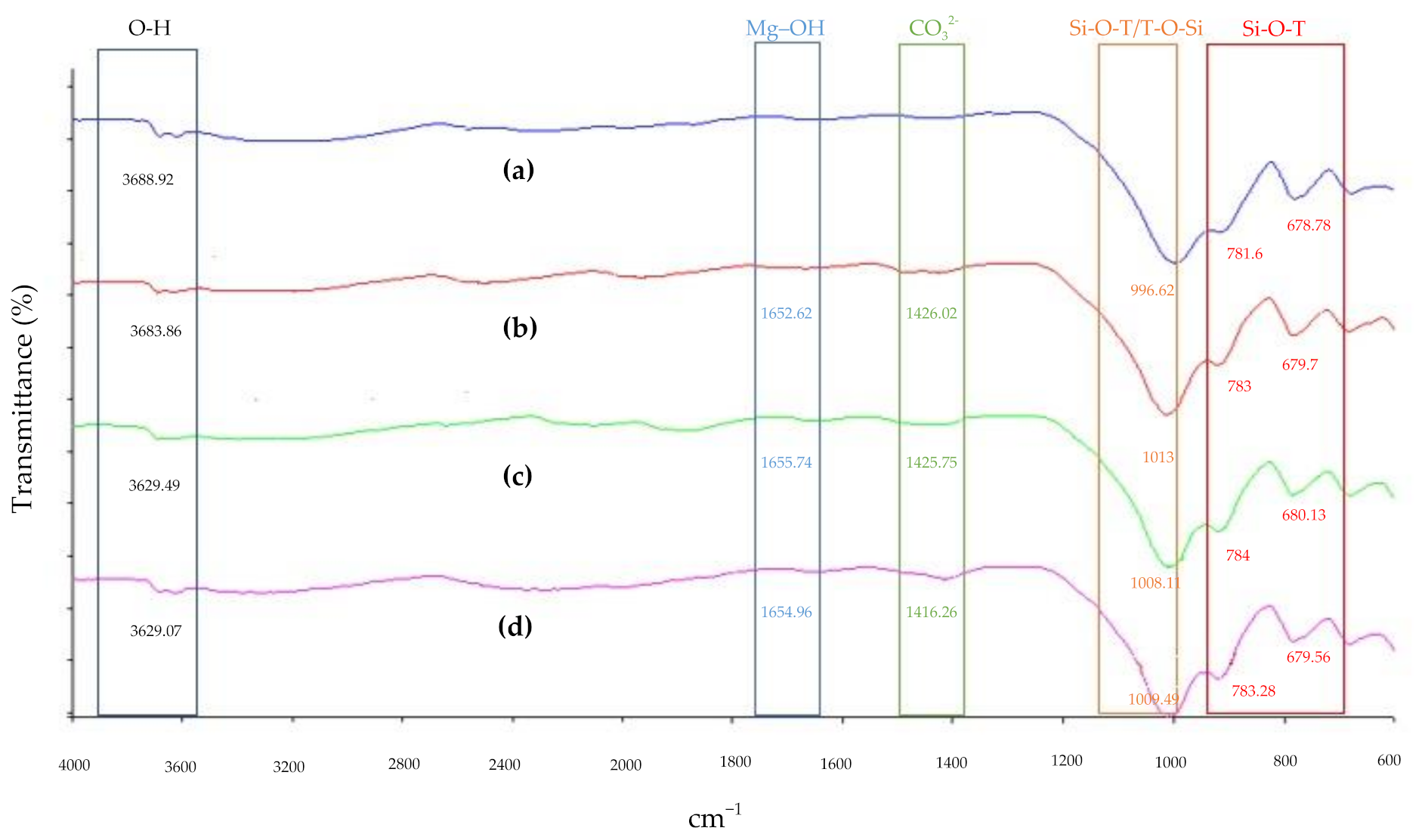
3.6. Functional Group Analysis
4. Conclusions
- The effect of the S/L ratio and the optimum S/L ratio was obtained at the ratio of 1.5 for all geopolymer soil samples. This ratio was found to have produced workability for stabilized geopolymer soil in the mixing process, thus increased the unconfined compression test of the geopolymer soil.
- Based on the compression test results, the geopolymer soil with GGBS and fly ash could be used as the road subgrade since the values achieved were more than 0.8 MPa. This indicates that the soil stabilization using fly ash and GGBS based geopolymer has proven effective in increasing the strength of the soil according to the ASTM D4609 standard and Design Guideline for Alternative Pavement Structures (Low Volume Roads) of Malaysia Public Work Department (PWD).
- The XRD diffractogram indicates the presence of gypsum (CaSO4·2H2O), akermanite (2CaO·MgO·2SiO2), and larnite (Ca2SiO4) of geopolymer soil with fly ash and GGBS, which had improved the strength and reduced the plastic limit and liquid limit of the clay soil.
- The morphology showed that the soil and GGBS particles were fully reacted; more homogeneous intervening geopolymer matrix, dense and fairly smooth. The existence of additional GGBS and fly ash particles filled the void between clay soil particles resulting in the stable and compact structure of clay soil, thus increasing the compression strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davidson, E.A.; Verchot, L.V.; Cattanio, J.H.; Ackerman, I.L.; Carvalho, J.E.M. Effects of soil water content on soil respiration in forests and cattle pastures of Eastern Amazonia. Biogeochemistry 2000, 48, 53–69. [Google Scholar] [CrossRef]
- Eberwein, J.R.; Oikawa, P.Y.; Allsman, L.A.; Jenerette, G.D. Carbon availability regulates soil respiration response to nitrogen and temperature. Soil Biol. Biochem. 2015, 88, 158–164. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, W.; Jiang, X.; Huang, Z. Effects of initial water content on microstructure and mechanical properties of lean clay soil stabilized by compound calcium-based stabilizer. Materials 2018, 11, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Xu, Y.; Chen, J.; Wang, Y. Study on the stabilization of a new type of waste solidifying agent for soft soil. Materials 2019, 12, 826. [Google Scholar] [CrossRef] [Green Version]
- José Luis, P.; Roberto, T.; Miguel, C.; Adrián, R.; Erick, G. Evaluation of the improvement effect of limestone powder waste in the stabilization of swelling clayey soil. Sustainability 2019, 11, 679. [Google Scholar] [CrossRef] [Green Version]
- Khan, T.A.; Taha, M.R.; Khan, M.M.; Shah, S.A.R.; Aslam, M.A.; Waqar, A.; Khan, A.R.; Waseem, M. Strength and volume change characteristics of clayey soils: Performance evaluation of enzymes. Minerals 2020, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Di Marta, S.; Di Bruno, B.; Fratalocchi, E.; Länsivaara, T. Lime treatment of a soft sensitive clay: A sustainable reuse option. Geoscience 2020, 10, 182. [Google Scholar] [CrossRef]
- Vuki’cevi’c, M.; Marjanovi´c, M.; Pujevi´c, V.; Jockovi´c, S. The alternatives to traditional materials for subsoil stabilization and embankments. Materials 2019, 12, 3018. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, N.; Satyam, N. Experimental study on the influence of polypropylene fiber on the swelling pressure expansion attributes of silica fume stabilized clayey soil. Geosciences 2019, 9, 377. [Google Scholar] [CrossRef] [Green Version]
- She, J.; Lu, Z.; Yao, H.; Fang, R.; Xian, S. Experimental study on the swelling behaviour of expansive soil at different depths under unidirectional seepage. Appl. Sci. 2019, 9, 1233. [Google Scholar] [CrossRef] [Green Version]
- Emarah, D.A.; Safwat, A.S. Swelling soils treatment using lime and sea water for roads construction. Alex. Eng. J. 2018, 57, 2357–2365. [Google Scholar] [CrossRef]
- Long, Z.; Cheng, Y.; Yang, G. Study on triaxial creep test and constitutive model of compacted red clay. Int. J. Civ. Eng. 2021, 19, 517–531. [Google Scholar] [CrossRef]
- Jeff, B.; Khademi, F. Research papers expansive soil: Causes and treatments. Manag. J. Civ. Eng. 2018, 6, 3. [Google Scholar] [CrossRef]
- Phummiphan, I.; Horpibulsuk, S.; Rachan, R.; Arulrajah, A.; Shen, S.L.; Chindaprasirt, P. Corrigendum to “High calcium fly ash geopolymer stabilized lateritic soil and granulated blast furnace slag blends as a pavement base material”. J. Hazard. Mater. 2021, 412, 125205. [Google Scholar] [CrossRef]
- Alam, S.; Das, S.K.; Rao, H. Strength and durability characteristic of alkali activated GGBS stabilized red mud as geo-material. Constr. Build. Mater. 2019, 211, 932–942. [Google Scholar] [CrossRef]
- Martins, A.C.P.; Carvalho, J.M.F.; Costa, L.C.B.; Andrade, H.D.; Melo, T.V.; Ribeiro, J.C.L.; Pedroti, L.G.; Peixoto, R.A.F. Steel slags in cement-based composites: An ultimate review on characterization, applications and performance. Constr. Build. Mater. 2021, 291, 123265. [Google Scholar] [CrossRef]
- Salimi, M.; Ghorbani, A. Mechanical and compressibility characteristics of a soft clay stabilized by slag-based mixtures and geopolymers. Appl. Clay Sci. 2020, 184, 105390. [Google Scholar] [CrossRef]
- Sharman, A.K.; Sivapullaiah, P.V. Ground granulated blast furnace slag amended fly ash as an expansive soil stabilizer. Soils Found. 2016, 56, 205–212. [Google Scholar] [CrossRef]
- ASTM Standards D 4609. Standard Guide for Evaluating Effectiveness of Admixtures for Soil Stabilization; ASTM Standards: Conshohocken, PA, USA, 2008. [Google Scholar]
- National Cooperative Highway Research Program (NCHRP). Guide for Mechanistic-Empirical Design of New and Rehabilitated Pavement Structures; NCHRP: Washington, DC, USA, 2001. [Google Scholar]
- Jabatan, K.R. Design Guide for Alternative Pavement Structures (Low Volume Roads); Cawangan Kejuruteraan jalan & Geoteknik, Jabatan kerja raya Malaysia: Kuala Lumpur, Malaysia, 2012. [Google Scholar]
- Thomas, A.; Tripathi, R.K.; Yadu, L.K. A laboratory investigation of soil stabilization using enzyme and alkali-activated ground granulated blast-furnace slag. J. Sci. Eng. 2018, 43, 5193–5202. [Google Scholar] [CrossRef]
- Tigue, A.A.S.; April, A.S.; Roy, A.J.; Malenab, R.A.J. Chemical stability and leaching behavior of one-part geopolymer from soil and coal fly ash mixtures. Minerals 2018, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Canakci, H.; Güllü, H.; Alhashemy, A. Performances of using geopolymers made with various stabilizers for deep mixing. Materials 2019, 12, 2542. [Google Scholar] [CrossRef] [Green Version]
- Singhi, B.; Laskar, A.I.; Ahmed, A. Investigation on soil–geopolymer with slag, fly ash, and their blending. Arab. J. Sci. Eng. 2016, 41, 393–400. [Google Scholar] [CrossRef]
- Zaliha, S.Z.S.; Al Bakri, A.M.M.; Kamarudin, H.; Fauziah, A. Characterization of soils as potential raw materials for soil stabilization application using geopolymerization method. Mater. Sci. Forum 2015, 803, 135–143. [Google Scholar] [CrossRef]
- Tigue, A.A.S.; April, A.S.; Jonathan, R.D. Synthesis of a one-part geopolymer system for soil stabilizer using fly ash and volcanic ash. MATEC Web Conf. 2018, 5, 05017. [Google Scholar] [CrossRef] [Green Version]
- Wong, B.Y.F.; Kwong, S.W.; Ignatius, R.K.P. A review on geopolymerisation in soil stabilization. Mater. Sci. Eng. 2019, 495, 012070. [Google Scholar] [CrossRef]
- Trinh, S.H.; Quynh, A.; Thi, B. Influencing of clay and binder content on compression strength of soft soil stabilized by geopolymer based fly ash. Int. J. Appl. Eng. Res. 2018, 13, 7954–7958. [Google Scholar]
- Candamano, S.; Sgambitterra, E.; Lamuta, C.; Pagnotta, L.; Sudip, C.; Crea, F. Graphene nanoplatelets in geopolymeric systems: A new dimention of Nanocomposites. Mater. Lett. 2018, 236, 550–553. [Google Scholar] [CrossRef]
- Abdullah, M.S.; Fauziah, A. Effect of alkaline activator to fly ash ratio for geopolymer stabilized soil. MATEC Web Conf. 2017, 97, 01021. [Google Scholar] [CrossRef] [Green Version]
- ASTM D422-63(2007)e2. Standard Test Method for Particle-Size Analysis of Soils (Withdrawn 2016); ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- ASTM D854-00. Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer; ASTM International: West Conshohocken, PA, USA, 2000. [Google Scholar]
- British Standard BS 1377-2. Methods of Test for Soils for Civil Engineering Purposes. Classification Tests; British Standard: London, UK, 1990. [Google Scholar]
- ASTM D698-12e2. Standard Test Methods for Laboratory Compaction Characteristics of Soil Using Standard Effort (12 400 ft-lbf/ft3 (600 kN-m/m3)); ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Saputra, O.R.; Wardhono, A. The effect of NaOH variation on Na2SiO3 on the press value of dry geopolymer mortar dry mixing method on fly ash ratio condition to 5:1 activator. J. Unesa 2019, 2, 1. [Google Scholar]
- ASTM Standards D 2166. Standard Test Method for Unconfined Compression Strength of Cohesive Soil; ASTM Standards: Conshohocken, PA, USA, 2006. [Google Scholar]
- Ige, J.A.; Ajamu, S.O. Unconfined compressive strength test of a fly ash stabilized sandy soil. Inter. J. Latest Res Eng. Technol. 2015, 3, 1–11. [Google Scholar]
- ASTM Standards D 2487. Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System); ASTM Standards: Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM Standards C 618. Standard Practice for Classification of Fly Ash; ASTM Standards: Conshohocken, PA, USA, 2012. [Google Scholar]
- Fernandez Jimenez, A.; Puertas, F. The alkali-silica reaction in alkali-activated granulated slag mortars with reactive aggregate. Cem. Concr. Res. 2002, 32, 1019–1024. [Google Scholar] [CrossRef]
- Lee, W.H.; Cheng, T.W.; Lin, K.Y.; Lin, K.L.; Wu, C.C.; Tsai, C.T. Geopolymer Technologies for Stabilization of Basic Oxygen Furnace Slags and Sustainable Application as Construction Materials. Sustainability 2020, 12, 5002. [Google Scholar] [CrossRef]
- Mehta, A.; Kumar, K. Strength and Durability Characteristics of Fly Ash and Slag Based Geopolymer Concrete. Int. J. Civ. Eng. Technol. 2016, 7, 305–314. [Google Scholar]
- Azad, N.M.; Samarakoon, S.M.S.M.K. Utilization of Industrial By-Products/Waste to Manufacture Geopolymer Cement/Concrete. Sustainability 2021, 13, 873. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Cement a Review; Geopolymer Science and Technics Publisher: Saint-Quentin, France, 2013; 11p. [Google Scholar]
- Timakul, P.; Thanaphatwetphisit, K.; Angkavattana, P. Effect silica to alumina ratio on the compressive strength of class c fly ash based geopolymers. Eng. Mater. 2015, 659, 80–84. [Google Scholar] [CrossRef]
- Abdila, S.R.; Abdullah, M.M.A.B.; Tahir, M.F.M.; Ahmad, R.; Syafwandi; Isradi, M. Characterization of fly ash and ground granulated blast slag for soil stabilization application using geopolymerization method. Mater. Sci. Eng. 2020, 864, 012013. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2017, 9, 40–48. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Candamano, S.; Crea, F.; Gazzaniga, G.; Pastore, T. The combined use of admixtures for shrinkage reduction in one-part alkali activated slag-based mortars and pastes. Constr. Build. Mater. 2020, 248, 118682. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Mohd Salleh, M.A.A.; Azimi, E.A.; Chaiprapa, J.; Sandu, A.V. Strength development of solely ground granulated blast furnace slag geopolymers. Constr. Build. Mater. 2020, 250, 118720. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Heah, C.Y.; Liew, Y.M. Behaviour changes of ground granulated blast furnace slag geopolymers at high temperature. Adv. Cem. Res. 2018, 465–475. [Google Scholar] [CrossRef]
- Parhi, P.S.; Garanayak, L.; Mahamaya, M.; Das, S.K. Stabilization of Expansive Soils Using Alkali Activated Fly Ash. In Handbook of Advances in Characterization and Analysis of Expansive Soils and Rocks; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9783319619316. [Google Scholar]
- Sargent, P. Geopolymer. In Handbook of Alkali-Activated Cements, Mortars and Concretes the Development of Alkali-Activated Mixtures for Soil Stabilisation; Woodhead Publishing Limited: Cambridge, UK, 2014; ISBN 9781782422761. [Google Scholar]
- ASTM Standards D 3282. Standard Practice for Classification of Soils and Soil-Aggregate Mixtures for Highway Construction Purposes; ASTM Standards: Conshohocken, PA, USA, 2004. [Google Scholar]
- Subaer, S. Geopolymer Physics. In Handbook Introduction to Physics Geopolymer; DP2M Dikti: Kota Gorontalo, Indonesia, 2012; ISBN 9789791082792. [Google Scholar]
- Aziz, I.H.; Zulkifly, K.; Sakkas, K.; Panias, D.; Tsaousi, G.M.; Bakri, M.M.A.A.; Heah Cheng, Y. The characterization of steel slag by alkali activation. OALib J. 2017, 4, 1. [Google Scholar] [CrossRef]
- Tajdini, M.; Hajialilue, B.M.; Golmohamadi, S. An experimental investigation on effect of adding natural and synthetic fibres on mechanical and behavioural parameters of soil–cement materials. Int. J. Civ. Eng. 2018, 16, 353–370. [Google Scholar] [CrossRef]
- Latifi, N.; Ahmad Safuan, A.R.; Aminaton, M.; Mahmood, M.T. Effect of magnesium chloride solution on the physico-chemical characteristics of tropical peat. Environ. Earth Sci. 2016, 75, 220. [Google Scholar] [CrossRef]
- Arioz, E.; Arioz, Ö.; Koçkar, Ö.M. The effect of curing conditions on the properties of geopolymer samples. Int. J. Chem. Eng. 2013, 4, 423–426. [Google Scholar] [CrossRef] [Green Version]
- Sedira, N.; João, C.-G. Alkali-activated binders based on tungsten mining waste and electric-arc-furnace slag: Compressive strength and microstructure properties. CivilEng 2020, 1, 10. [Google Scholar] [CrossRef]




| Sample | Percentage Blended Mix Proportion | NaOH Concentration (M) | S/L Ratio | Na2SiO3/NaOH Ratio |
|---|---|---|---|---|
| Soil based geopolymer-1 | 100% Soil | 10 | 1.0 | 2.0 |
| Soil based geopolymer-2 | 100% Soil | 10 | 1.5 | 2.0 |
| Soil based geopolymer-3 | 100% Soil | 10 | 2.0 | 2.0 |
| Soil based geopolymer-4 | 100% Soil | 10 | 2.5 | 2.0 |
| Soil based geopolymer-5 | 100% Soil | 10 | 3.0 | 2.0 |
| Soil based geopolymer with fly ash-1 | 80% soil and 20% Fly ash | 10 | 1.0 | 2.0 |
| Soil based geopolymer with fly ash-2 | 80% soil and 20% Fly ash | 10 | 1.5 | 2.0 |
| Soil based geopolymer with fly ash-3 | 80% soil and 20% Fly ash | 10 | 2.0 | 2.0 |
| Soil based geopolymer with fly ash-4 | 80% soil and 20% Fly ash | 10 | 2.5 | 2.0 |
| Soil based geopolymer with fly ash-5 | 80% soil and 20% Fly ash | 10 | 3.0 | 2.0 |
| Soil based geopolymer with GGBS-1 | 80% Soil and 20% GGBS | 10 | 1.0 | 2.0 |
| Soil based geopolymer with GGBS-2 | 80% Soil and 20% GGBS | 10 | 1.5 | 2.0 |
| Soil based geopolymer with GGBS-3 | 80% Soil and 20% GGBS | 10 | 2.0 | 2.0 |
| Soil based geopolymer with GGBS-4 | 80% Soil and 20% GGBS | 10 | 2.5 | 2.0 |
| Soil based geopolymer with GGBS-5 | 80% Soil and 20% GGBS | 10 | 3.0 | 2.0 |
| Soil based geopolymer with GGBS and fly ash-1 | 80% Soil 10% GGBS and 10% Fly ash | 10 | 1.0 | 2.0 |
| Soil based geopolymer with GGBS and fly ash-2 | 80% Soil 10% GGBS and 10% Fly ash | 10 | 1.5 | 2.0 |
| Soil based geopolymer with GGBS and fly ash-3 | 80% Soil 10% GGBS and 10% Fly ash | 10 | 2.0 | 2.0 |
| Soil based geopolymer with GGBS and fly ash-4 | 80% Soil 10% GGBS and 10% Fly ash | 10 | 2.5 | 2.0 |
| Soil based geopolymer with GGBS and fly ash-5 | 80% Soil 10% Fly ash and 10% GGBS | 10 | 3.0 | 2.0 |
| Properties | Unit | Soil | Fly Ash | GGBS |
|---|---|---|---|---|
| i. Particle Analysis: | ||||
| ii. Fine Particle | % | 52.00 | 94.00 | 96.00 |
| iii. Course Particle | % | 48.00 | 6.00 | 4.00 |
| Specific Gravity | - | 2.686 | - | |
| i. Atterberg Limit: | - | - | - | - |
| ii. Liquid Limit | % | 51.20 | 23.40 | 40.73 |
| iii. Plastic Limit | % | 28.48 | Non-plastic | Non plastic |
| iv. Index Plasticity | % | 22.72 | Non-plastic | Non plastic |
| USCS Classification | - | (CH) | - | - |
| Inorganic clay and high plasticity | - | - | ||
| Optimum Water Content | % | 15 | - | - |
| Optimum Dry Density | gram/cm3 | 1.66 | - | - |
| Element | Clay Soil (%) | Fly Ash (%) | GGBS (%) |
|---|---|---|---|
| SiO2 | 73.30 | 30.70 | 30.40 |
| Al2O3 | 17.00 | 13.30 | 10.50 |
| Fe2O3 | 6.15 | 23.92 | - |
| CaO | - | 22.40 | 50.37 |
| MgO | - | 3.6 | 3.2 |
| Others | 3.55 | 6.08 | 5.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdila, S.R.; Abdullah, M.M.A.B.; Ahmad, R.; Rahim, S.Z.A.; Rychta, M.; Wnuk, I.; Nabiałek, M.; Muskalski, K.; Tahir, M.F.M.; Syafwandi; et al. Evaluation on the Mechanical Properties of Ground Granulated Blast Slag (GGBS) and Fly Ash Stabilized Soil via Geopolymer Process. Materials 2021, 14, 2833. https://doi.org/10.3390/ma14112833
Abdila SR, Abdullah MMAB, Ahmad R, Rahim SZA, Rychta M, Wnuk I, Nabiałek M, Muskalski K, Tahir MFM, Syafwandi, et al. Evaluation on the Mechanical Properties of Ground Granulated Blast Slag (GGBS) and Fly Ash Stabilized Soil via Geopolymer Process. Materials. 2021; 14(11):2833. https://doi.org/10.3390/ma14112833
Chicago/Turabian StyleAbdila, Syafiadi Rizki, Mohd Mustafa Al Bakri Abdullah, Romisuhani Ahmad, Shayfull Zamree Abd Rahim, Małgorzata Rychta, Izabela Wnuk, Marcin Nabiałek, Krzysztof Muskalski, Muhammad Faheem Mohd Tahir, Syafwandi, and et al. 2021. "Evaluation on the Mechanical Properties of Ground Granulated Blast Slag (GGBS) and Fly Ash Stabilized Soil via Geopolymer Process" Materials 14, no. 11: 2833. https://doi.org/10.3390/ma14112833








