Involvement of mTOR Pathways in Recovery from Spinal Cord Injury by Modulation of Autophagy and Immune Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Spinal Cord Injury
2.3. Western Blot Analysis
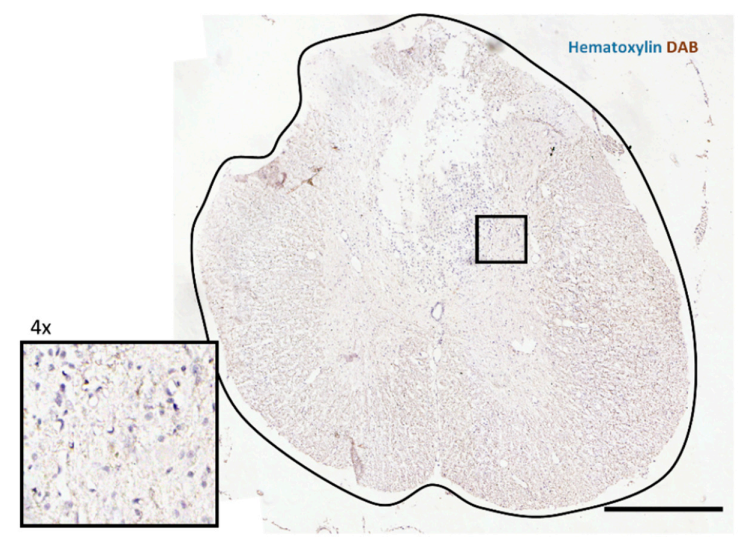
2.4. Immunohistochemistry
2.5. Cytokines
2.6. Behavioral Test
2.7. Statistical Analysis
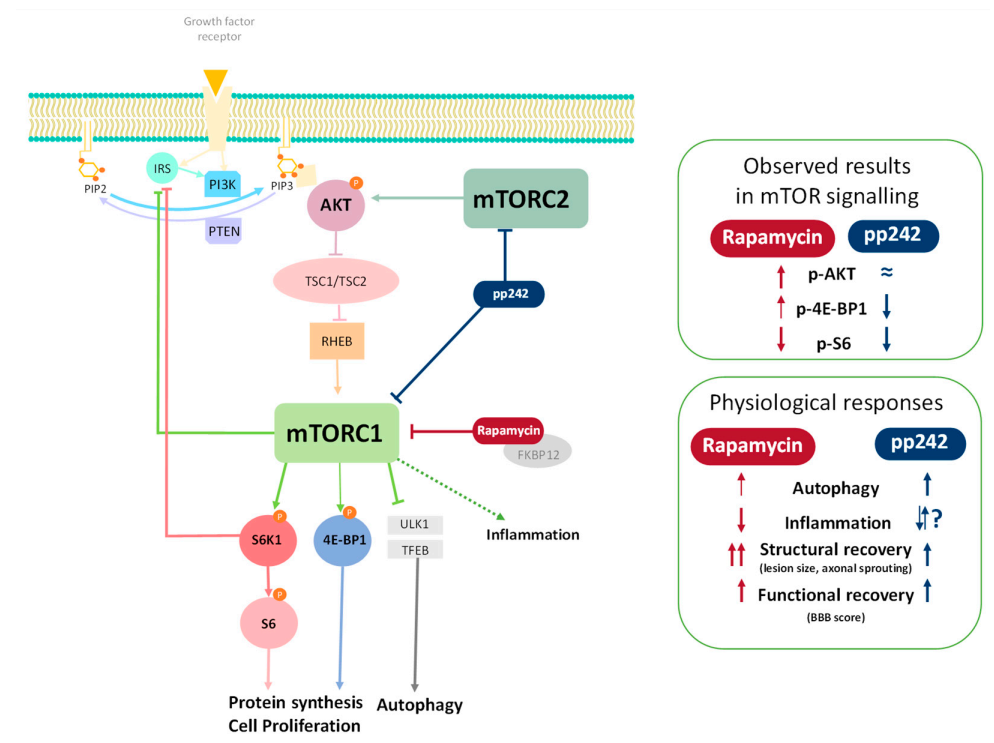
3. Results
3.1. Effect of RAPA and pp242 on the Recovery of SCI by Inhibition of mTOR Pathway
p-S6 Levels Are Downregulated by mTOR Inhibitors
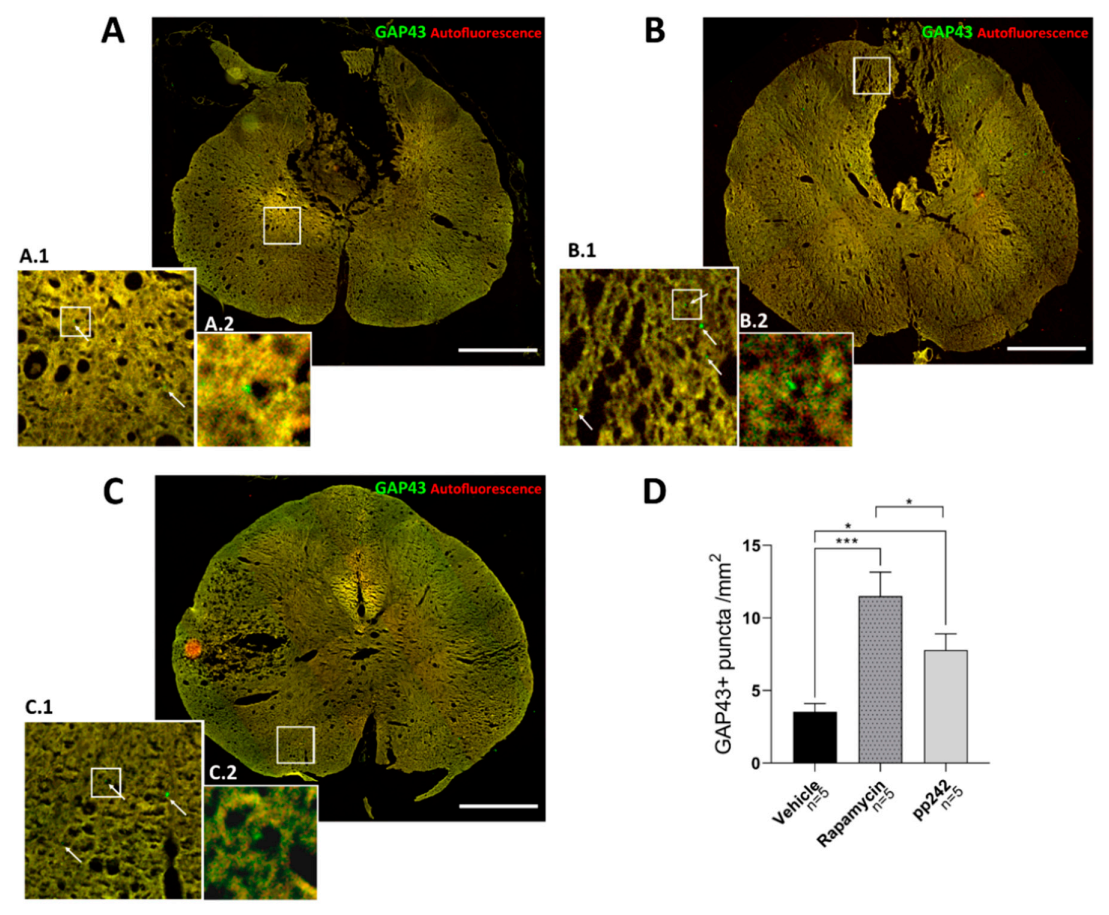
3.2. Inhibition of mTOR Pathway by RAPA or pp242 Enhances Autophagy in SCI
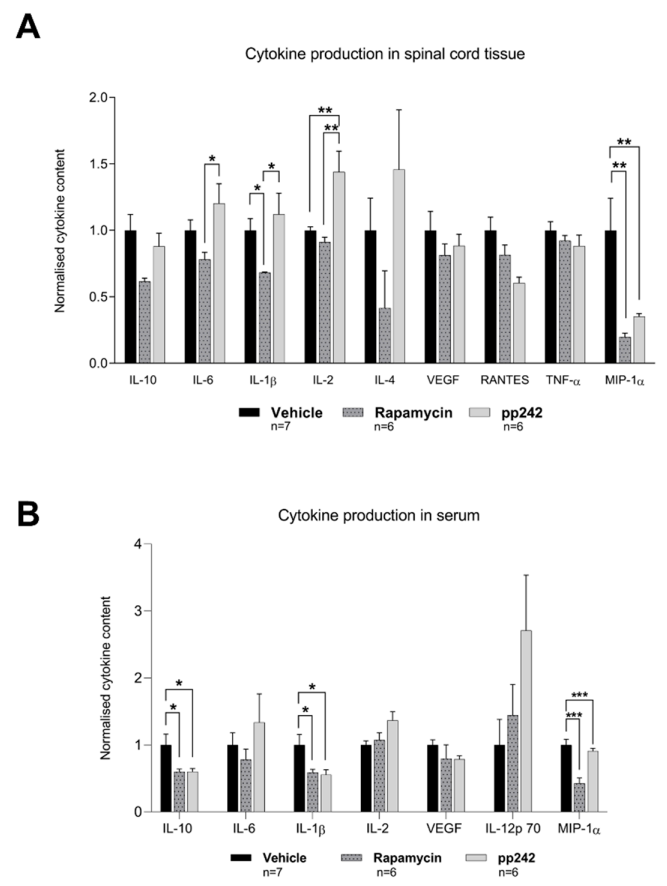
3.3. Suppression of mTOR Pathway by RAPA or pp242 Alters Cytokine Production in SCI
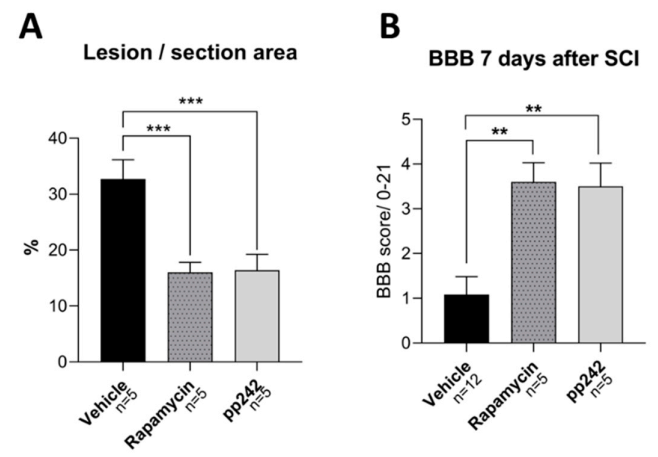
3.4. mTOR Inhibition Leads to Structural and Functional Recovery in Acute SCI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Dumont, R.J.; Okonkwo, D.O.; Verma, S.; Hurlbert, R.J.; Boulos, P.T.; Ellegala, D.B.; Dumont, A.S. Acute Spinal Cord Injury, Part I: Pathophysiologic Mechanisms. Clin. Neuropharmacol. 2001, 24, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Oyinbo, C.A. Secondary Injury Mechanisms in Traumatic Spinal Cord Injury: A Nugget of This Multiply Cascade. Acta Neurobiol. Exp. 2011, 71, 281–299. [Google Scholar]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic Spinal Cord Injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Satkunendrarajah, K.; Nassiri, F.; Karadimas, S.K.; Lip, A.; Yao, G.; Fehlings, M.G. Riluzole Promotes Motor and Respiratory Recovery Associated with Enhanced Neuronal Survival and Function Following High Cervical Spinal Hemisection. Exp. Neurol. 2016, 276, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Norimatsu, Y.; Ohmori, T.; Kimura, A.; Madoiwa, S.; Mimuro, J.; Seichi, A.; Yatomi, Y.; Hoshino, Y.; Sakata, Y. FTY720 Improves Functional Recovery after Spinal Cord Injury by Primarily Nonimmunomodulatory Mechanisms. Am. J. Pathol. 2012, 180, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Wang, Z.; Shi, H.; Wu, F.; Lin, B.; Xu, X.; Wang, X.; Fu, X.; Li, Z.; et al. Exogenous Basic Fibroblast Growth Factor Inhibits ER Stress–Induced Apoptosis and Improves Recovery from Spinal Cord Injury. CNS Neurosci. Ther. 2012, 19, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Machova Urdzikova, L.; Karova, K.; Ruzicka, J.; Kloudova, A.; Shannon, C.; Dubisova, J.; Murali, R.; Kubinova, S.; Sykova, E.; Jhanwar-Uniyal, M.; et al. The Anti-Inflammatory Compound Curcumin Enhances Locomotor and Sensory Recovery after Spinal Cord Injury in Rats by Immunomodulation. Int. J. Mol. Sci. 2015, 17, 49. [Google Scholar] [CrossRef]
- Machova Urdzikova, L.; Ruzicka, J.; Karova, K.; Kloudova, A.; Svobodova, B.; Amin, A.; Dubisova, J.; Schmidt, M.; Kubinova, S.; Jhanwar-Uniyal, M.; et al. A Green Tea Polyphenol Epigallocatechin-3-Gallate Enhances Neuroregeneration after Spinal Cord Injury by Altering Levels of Inflammatory Cytokines. Neuropharmacology 2017, 126, 213–223. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and Medical Implications of Mammalian Autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Nixon, R.A. The Role of Autophagy in Neurodegenerative Disease. Nat. Med. 2013, 19, 983–997. [Google Scholar] [CrossRef]
- Friedman, L.G.; Lachenmayer, M.L.; Wang, J.; He, L.; Poulose, S.M.; Komatsu, M.; Holstein, G.R.; Yue, Z. Disrupted Autophagy Leads to Dopaminergic Axon and Dendrite Degeneration and Promotes Presynaptic Accumulation of -Synuclein and LRRK2 in the Brain. J. Neurosci. 2012, 32, 7585–7593. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyama, Y.; Kominami, E.; et al. Loss of Autophagy in the Central Nervous System Causes Neurodegeneration in Mice. Nature 2006, 441, 880–884. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Codogno, P.; Levine, B. Autophagy Modulation as a Potential Therapeutic Target for Diverse Diseases. Nat. Rev. Drug Discov. 2012, 11, 709–730. [Google Scholar] [CrossRef]
- Kinarivala, N.; Patel, R.; Boustany, R.-M.; Al-Ahmad, A.; Trippier, P.C. Discovery of Aromatic Carbamates That Confer Neuroprotective Activity by Enhancing Autophagy and Inducing the Anti-Apoptotic Protein B-Cell Lymphoma 2 (Bcl-2). J. Med. Chem. 2017, 60, 9739–9756. [Google Scholar] [CrossRef]
- Kanno, H.; Ozawa, H.; Sekiguchi, A.; Yamaya, S.; Itoi, E. Induction of Autophagy and Autophagic Cell Death in Damaged Neural Tissue after Acute Spinal Cord Injury in Mice. Spine 2011, 36, E1427–E1434. [Google Scholar] [CrossRef]
- Liu, S.; Sarkar, C.; Dinizo, M.; Faden, A.I.; Koh, E.Y.; Lipinski, M.M.; Wu, J. Disrupted Autophagy after Spinal Cord Injury Is Associated with ER Stress and Neuronal Cell Death. Cell Death Dis. 2015, 6, e1582. [Google Scholar] [CrossRef]
- Leiva-Rodríguez, T.; Romeo-Guitart, D.; Marmolejo-Martínez-Artesero, S.; Herrando-Grabulosa, M.; Bosch, A.; Forés, J.; Casas, C. ATG5 Overexpression Is Neuroprotective and Attenuates Cytoskeletal and Vesicle-Trafficking Alterations in Axotomized Motoneurons. Cell Death Dis. 2018, 9, 626. [Google Scholar] [CrossRef]
- Sekiguchi, A.; Kanno, H.; Ozawa, H.; Yamaya, S.; Itoi, E. Rapamycin Promotes Autophagy and Reduces Neural Tissue Damage and Locomotor Impairment after Spinal Cord Injury in Mice. J. Neurotrauma 2012, 29, 946–956. [Google Scholar] [CrossRef]
- Tang, P.; Hou, H.; Zhang, L.; Lan, X.; Mao, Z.; Liu, D.; He, C.; Du, H.; Zhang, L. Autophagy Reduces Neuronal Damage and Promotes Locomotor Recovery via Inhibition of Apoptosis after Spinal Cord Injury in Rats. Mol. Neurobiol. 2014, 49, 276–287. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. MTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and MTOR Regulate Autophagy through Direct Phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, S.; He, L.; Rong, Y.; Brier, L.W.; Sun, Q.; Liu, R.; Fan, W.; Chen, S.; Yue, Z.; et al. MTORC1-Mediated NRBF2 Phosphorylation Functions as a Switch for the Class III PtdIns3K and Autophagy. Autophagy 2017, 13, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Rabanal-Ruiz, Y.; Otten, E.G.; Korolchuk, V.I. MTORC1 as the Main Gateway to Autophagy. Essays Biochem. 2017, 61, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Spilman, P.; Podlutskaya, N.; Hart, M.J.; Debnath, J.; Gorostiza, O.; Bredesen, D.; Richardson, A.; Strong, R.; Galvan, V. Inhibition of MTOR by Rapamycin Abolishes Cognitive Deficits and Reduces Amyloid-Beta Levels in a Mouse Model of Alzheimer’s Disease. PLoS ONE 2010, 5, e9979. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, A.S.; Miller, J.; Arrasate, M.; Wong, J.S.; Pleiss, M.A.; Finkbeiner, S. A Small-Molecule Scaffold Induces Autophagy in Primary Neurons and Protects against Toxicity in a Huntington Disease Model. Proc. Natl. Acad. Sci. USA 2010, 107, 16982–16987. [Google Scholar] [CrossRef]
- Li, X.-G.; Du, J.-H.; Lu, Y.; Lin, X.-J. Neuroprotective Effects of Rapamycin on Spinal Cord Injury in Rats by Increasing Autophagy and Akt Signaling. Neural Regen. Res. 2019, 14, 721. [Google Scholar] [CrossRef]
- Srivastava, I.N.; Shperdheja, J.; Baybis, M.; Ferguson, T.; Crino, P.B. MTOR Pathway Inhibition Prevents Neuroinflammation and Neuronal Death in a Mouse Model of Cerebral Palsy. Neurobiol. Dis. 2016, 85, 144–154. [Google Scholar] [CrossRef]
- Xie, L.; Sun, F.; Wang, J.; Mao, X.; Xie, L.; Yang, S.-H.; Su, D.; Simpkins, J.W.; Greenberg, D.A.; Jin, K. MTOR Signaling Inhibition Modulates Macrophages/Microglia-Mediated Neuroinflammation and Secondary Injury via Regulatory T Cells after Focal Ischemia. J. Immunol. 2014, 192, 6009–6019. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Lin, J.-H.; Muharram, A.; Liu, W.-G. Beclin-1-Mediated Autophagy Protects Spinal Cord Neurons against Mechanical Injury-Induced Apoptosis. Apoptosis 2014, 19, 933–945. [Google Scholar] [CrossRef]
- Jhanwar-Uniyal, M.; Amin, A.G.; Cooper, J.B.; Das, K.; Schmidt, M.H.; Murali, R. Discrete Signaling Mechanisms of MTORC1 and MTORC2: Connected yet Apart in Cellular and Molecular Aspects. Adv. Biol. Regul. 2017, 64, 39–48. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.-H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a Novel Binding Partner of MTOR, Defines a Rapamycin-Insensitive and Raptor-Independent Pathway That Regulates the Cytoskeleton. Curr. Biol. CB 2004, 14, 1296–1302. [Google Scholar] [CrossRef]
- Neil, J.; Shannon, C.; Mohan, A.; Laurent, D.; Murali, R.; Jhanwar-Uniyal, M. ATP-Site Binding Inhibitor Effectively Targets MTORC1 and MTORC2 Complexes in Glioblastoma. Int. J. Oncol. 2016, 48, 1045–1052. [Google Scholar] [CrossRef]
- Feng, H.; Yang, Z.; Bai, X.; Yang, M.; Fang, Y.; Zhang, X.; Guo, Q.; Ning, H. Therapeutic Potential of a Dual MTORC1/2 Inhibitor for the Prevention of Posterior Capsule Opacification: An in Vitro Study. Int. J. Mol. Med. 2018, 41, 2099–2107. [Google Scholar] [CrossRef]
- Vanický, I.; Urdzíková, L.; Saganová, K.; Čízková, D.; Gálik, J. A Simple and Reproducible Model of Spinal Cord Injury Induced by Epidural Balloon Inflation in the Rat. J. Neurotrauma 2001, 18, 1399–1407. [Google Scholar] [CrossRef]
- Urdzíková, L.; Vanický, I. Post-Traumatic Moderate Systemic Hyperthermia Worsens Behavioural Outcome after Spinal Cord Injury in the Rat. Spinal Cord 2006, 44, 113–119. [Google Scholar] [CrossRef]
- Banerjee, S.; Gianino, S.M.; Gao, F.; Christians, U.; Gutmann, D.H. Interpreting Mammalian Target of Rapamycin and Cell Growth Inhibition in a Genetically-Engineered Mouse Model of Nf1-Deficient Astrocytes. Mol. Cancer Ther. 2011, 10. [Google Scholar] [CrossRef]
- Md Rashid, M.; Lee, H.; Jung, B.H. Metabolite Identification and Pharmacokinetic Profiling of PP242, an ATP-Competitive Inhibitor of MTOR Using Ultra High-Performance Liquid Chromatography and Mass Spectrometry. J. Chromatogr. B 2018, 1072, 244–251. [Google Scholar] [CrossRef]
- Rivero-Gutiérrez, B.; Anzola, A.; Martínez-Augustin, O.; de Medina, F.S. Stain-Free Detection as Loading Control Alternative to Ponceau and Housekeeping Protein Immunodetection in Western Blotting. Anal. Biochem. 2014, 467, 1–3. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Urdzíková, L.M.; Růžička, J.; LaBagnara, M.; Kárová, K.; Kubinová, Š.; Jiráková, K.; Murali, R.; Syková, E.; Jhanwar-Uniyal, M.; Jendelová, P. Human Mesenchymal Stem Cells Modulate Inflammatory Cytokines after Spinal Cord Injury in Rat. Int. J. Mol. Sci. 2014, 15, 11275–11293. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Kanno, H.; Ozawa, H.; Sekiguchi, A.; Yamaya, S.; Tateda, S.; Yahata, K.; Itoi, E. The Role of MTOR Signaling Pathway in Spinal Cord Injury. Cell Cycle 2012, 11, 3175. [Google Scholar] [CrossRef]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR Signaling in Growth and Metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef]
- Chen, H.-C.; Fong, T.-H.; Lee, A.-W.; Chiu, W.-T. Autophagy Is Activated in Injured Neurons and Inhibited by Methylprednisolone After Experimental Spinal Cord Injury. Spine 2012, 37, 470–475. [Google Scholar] [CrossRef]
- Chen, C.-H.; Sung, C.-S.; Huang, S.-Y.; Feng, C.-W.; Hung, H.-C.; Yang, S.-N.; Chen, N.-F.; Tai, M.-H.; Wen, Z.-H.; Chen, W.-F. The Role of the PI3K/Akt/MTOR Pathway in Glial Scar Formation Following Spinal Cord Injury. Exp. Neurol. 2016, 278, 27–41. [Google Scholar] [CrossRef]
- Codeluppi, S.; Svensson, C.I.; Hefferan, M.P.; Valencia, F.; Silldorff, M.D.; Oshiro, M.; Marsala, M.; Pasquale, E.B. The Rheb-MTOR Pathway Is Upregulated in Reactive Astrocytes of the Injured Spinal Cord. J. Neurosci. 2009, 29, 1093–1104. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Huang, B.; Ma, S. Blocking Mammalian Target of Rapamycin (MTOR) Improves Neuropathic Pain Evoked by Spinal Cord Injury. Transl. Neurosci. 2016, 7. [Google Scholar] [CrossRef]
- Chen, H.-C.; Fong, T.-H.; Hsu, P.-W.; Chiu, W.-T. Multifaceted Effects of Rapamycin on Functional Recovery after Spinal Cord Injury in Rats through Autophagy Promotion, Anti-Inflammation, and Neuroprotection. J. Surg. Res. 2013, 179, e203–e210. [Google Scholar] [CrossRef]
- Du, J.; Li, X.; Lin, X.; Lu, Y.; Chen, B. A Rapamycin-Enhanced Autophagy Reduces Neural Apoptosis by Blocking Bax Mitochondral Translation and Cytochrome C Release in Acute Spinal Cord Injury in Rats. Med. Case Rep. 2017, 3. [Google Scholar] [CrossRef]
- O’Reilly, K.E.; Rojo, F.; She, Q.-B.; Solit, D.; Mills, G.B.; Smith, D.; Lane, H.; Hofmann, F.; Hicklin, D.J.; Ludwig, D.L.; et al. MTOR Inhibition Induces Upstream Receptor Tyrosine Kinase Signaling and Activates Akt. Cancer Res. 2006, 66, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D. Phosphorylation and Regulation of Akt/PKB by the Rictor-MTOR Complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Paterniti, I.; Siracusa, R.; Impellizzeri, D.; Esposito, E.; Cuzzocrea, S. KU0063794, a Dual MTORC1 and MTORC2 Inhibitor, Reduces Neural Tissue Damage and Locomotor Impairment After Spinal Cord Injury in Mice. Mol. Neurobiol. 2017, 54, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.J.; Beal, P.A.; Keith, C.T.; Chen, J.; Bum Shin, T.; Schreiber, S.L. Control of P70 S6 Kinase by Kinase Activity of FRAP in Vivo. Nature 1995, 377, 441–446. [Google Scholar] [CrossRef]
- Gingras, A.C.; Kennedy, S.G.; O’Leary, M.A.; Sonenberg, N.; Hay, N. 4E-BP1, a Repressor of MRNA Translation, Is Phosphorylated and Inactivated by the Akt(PKB) Signaling Pathway. Genes Dev. 1998, 12, 502–513. [Google Scholar] [CrossRef]
- Feldman, M.E.; Apsel, B.; Uotila, A.; Loewith, R.; Knight, Z.A.; Ruggero, D.; Shokat, K.M. Active-Site Inhibitors of MTOR Target Rapamycin-Resistant Outputs of MTORC1 and MTORC2. PLoS Biol. 2009, 7. [Google Scholar] [CrossRef]
- Thoreen, C.C.; Kang, S.A.; Chang, J.W.; Liu, Q.; Zhang, J.; Gao, Y.; Reichling, L.J.; Sim, T.; Sabatini, D.M.; Gray, N.S. An ATP-Competitive Mammalian Target of Rapamycin Inhibitor Reveals Rapamycin-Resistant Functions of MTORC1. J. Biol. Chem. 2009, 284, 8023–8032. [Google Scholar] [CrossRef]
- Jiang, Y.P.; Ballou, L.M.; Lin, R.Z. Rapamycin-Insensitive Regulation of 4e-BP1 in Regenerating Rat Liver. J. Biol. Chem. 2001, 276, 10943–10951. [Google Scholar] [CrossRef]
- Choo, A.Y.; Yoon, S.-O.; Kim, S.G.; Roux, P.P.; Blenis, J. Rapamycin Differentially Inhibits S6Ks and 4E-BP1 to Mediate Cell-Type-Specific Repression of MRNA Translation. Proc. Natl. Acad. Sci. USA 2008, 105, 17414–17419. [Google Scholar] [CrossRef]
- Perlson, E.; Hanz, S.; Ben-Yaakov, K.; Segal-Ruder, Y.; Seger, R.; Fainzilber, M. Vimentin-Dependent Spatial Translocation of an Activated MAP Kinase in Injured Nerve. Neuron 2005, 45, 715–726. [Google Scholar] [CrossRef]
- Willis, D.E.; Twiss, J.L. The Evolving Roles of Axonally Synthesized Proteins in Regeneration. Curr. Opin. Neurobiol. 2006, 16, 111–118. [Google Scholar] [CrossRef]
- Michaelevski, I.; Medzihradszky, K.F.; Lynn, A.; Burlingame, A.L.; Fainzilber, M. Axonal Transport Proteomics Reveals Mobilization of Translation Machinery to the Lesion Site in Injured Sciatic Nerve. Mol. Cell. Proteom. MCP 2010, 9, 976–987. [Google Scholar] [CrossRef]
- Petrova, V.; Eva, R. The Virtuous Cycle of Axon Growth: Axonal Transport of Growth-Promoting Machinery as an Intrinsic Determinant of Axon Regeneration. Dev. Neurobiol. 2018, 78, 898–925. [Google Scholar] [CrossRef]
- Goldshmit, Y.; Kanner, S.; Zacs, M.; Frisca, F.; Pinto, A.R.; Currie, P.D.; Pinkas-Kramarski, R. Rapamycin Increases Neuronal Survival, Reduces Inflammation and Astrocyte Proliferation after Spinal Cord Injury. Mol. Cell. Neurosci. 2015, 68, 82–91. [Google Scholar] [CrossRef]
- Kang, S.A.; Pacold, M.E.; Cervantes, C.L.; Lim, D.; Lou, H.J.; Ottina, K.; Gray, N.S.; Turk, B.E.; Yaffe, M.B.; Sabatini, D.M. MTORC1 Phosphorylation Sites Encode Their Sensitivity to Starvation and Rapamycin. Science 2013, 341, 1236566. [Google Scholar] [CrossRef]
- Hsieh, A.C.; Liu, Y.; Edlind, M.P.; Ingolia, N.T.; Janes, M.R.; Sher, A.; Shi, E.Y.; Stumpf, C.R.; Christensen, C.; Bonham, M.J.; et al. The Translational Landscape of MTOR Signalling Steers Cancer Initiation and Metastasis. Nature 2012, 485, 55–61. [Google Scholar] [CrossRef]
- Yan, P.; Bai, L.; Lu, W.; Gao, Y.; Bi, Y.; Lv, G. Regulation of Autophagy by AMP-Activated Protein Kinase/Sirtuin 1 Pathway Reduces Spinal Cord Neurons Damage. Iran. J. Basic Med. Sci. 2017, 20, 1029–1036. [Google Scholar] [CrossRef]
- Dossou, A.S.; Basu, A. The Emerging Roles of MTORC1 in Macromanaging Autophagy. Cancers 2019, 11, 1422. [Google Scholar] [CrossRef]
- Peña-Llopis, S.; Vega-Rubin-de-Celis, S.; Schwartz, J.C.; Wolff, N.C.; Tran, T.A.T.; Zou, L.; Xie, X.-J.; Corey, D.R.; Brugarolas, J. Regulation of TFEB and V-ATPases by MTORC1. EMBO J. 2011, 30, 3242–3258. [Google Scholar] [CrossRef]
- Gordeev, S.A.; Bykova, T.V.; Zubova, S.G.; Bystrova, O.A.; Martynova, M.G.; Pospelov, V.A.; Pospelova, T.V. MTOR Kinase Inhibitor Pp242 Causes Mitophagy Terminated by Apoptotic Cell Death in E1A-Ras Transformed Cells. Oncotarget 2015, 6, 44905–44926. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.-L. MTOR: A Pharmacologic Target for Autophagy Regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ding, Y.; Chu, C.; Tang, J.; Xiao, Q.; Luo, Z.-G. Autophagy Induction Stabilizes Microtubules and Promotes Axon Regeneration after Spinal Cord Injury. Proc. Natl. Acad. Sci. USA 2016, 113, 11324–11329. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Nie, L.; Chen, L.; Sun, Y.; Li, G. Rapamycin Relieves Inflammation of Experimental Autoimmune Encephalomyelitis by Altering the Balance of Treg/Th17 in a Mouse Model. Neurosci. Lett. 2019, 705, 39–45. [Google Scholar] [CrossRef]
- Liu, S.; Xu, G.-Y.; Johnson, K.M.; Echetebu, C.; Ye, Z.; Hulsebosch, C.E.; McAdoo, D.J. Regulation of Interleukin-1β by the Interleukin-1 Receptor Antagonist in the Glutamate-Injured Spinal Cord: Endogenous Neuroprotection. Brain Res. 2008, 1231, 63–74. [Google Scholar] [CrossRef]
- Boato, F.; Rosenberger, K.; Nelissen, S.; Geboes, L.; Peters, E.M.; Nitsch, R.; Hendrix, S. Absence of IL-1β Positively Affects Neurological Outcome, Lesion Development and Axonal Plasticity after Spinal Cord Injury. J. Neuroinflamm. 2013, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Pelisch, N.; Rosas Almanza, J.; Stehlik, K.E.; Aperi, B.V.; Kroner, A. CCL3 Contributes to Secondary Damage after Spinal Cord Injury. J. Neuroinflamm. 2020, 17, 362. [Google Scholar] [CrossRef] [PubMed]
- Stammers, A.T.; Liu, J.; Kwon, B.K. Expression of Inflammatory Cytokines Following Acute Spinal Cord Injury in a Rodent Model. J. Neurosci. Res. 2012, 90, 782–790. [Google Scholar] [CrossRef]
- Mukaino, M.; Nakamura, M.; Yamada, O.; Okada, S.; Morikawa, S.; Renault-Mihara, F.; Iwanami, A.; Ikegami, T.; Ohsugi, Y.; Tsuji, O.; et al. Anti-IL-6-Receptor Antibody Promotes Repair of Spinal Cord Injury by Inducing Microglia-Dominant Inflammation. Exp. Neurol. 2010, 224, 403–414. [Google Scholar] [CrossRef]
- Okada, S.; Nakamura, M.; Mikami, Y.; Shimazaki, T.; Mihara, M.; Ohsugi, Y.; Iwamoto, Y.; Yoshizaki, K.; Kishimoto, T.; Toyama, Y.; et al. Blockade of Interleukin-6 Receptor Suppresses Reactive Astrogliosis and Ameliorates Functional Recovery in Experimental Spinal Cord Injury. J. Neurosci. Res. 2004, 76, 265–276. [Google Scholar] [CrossRef]
- Yang, G.; Tang, W.-Y. Resistance of Interleukin-6 to the Extracellular Inhibitory Environment Promotes Axonal Regeneration and Functional Recovery Following Spinal Cord Injury. Int. J. Mol. Med. 2017, 39, 437–445. [Google Scholar] [CrossRef]
- Gao, W.; Li, F.; Zhou, Z.; Xu, X.; Wu, Y.; Zhou, S.; Yin, D.; Sun, D.; Xiong, J.; Jiang, R.; et al. IL-2/Anti-IL-2 Complex Attenuates Inflammation and BBB Disruption in Mice Subjected to Traumatic Brain Injury. Front. Neurol. 2017, 8. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, Y.; Ye, Q.; Yu, F.; Zhu, W.; Li, P.; Wei, Z.; Yang, Y.; Shi, Y.; Thomson, A.W.; et al. In Vivo Expansion of Regulatory T Cells with IL-2/IL-2 Antibody Complex Protects against Transient Ischemic Stroke. J. Neurosci. 2018, 38, 10168–10179. [Google Scholar] [CrossRef]
- Walsh, J.T.; Zheng, J.; Smirnov, I.; Lorenz, U.; Tung, K.; Kipnis, J. Regulatory T Cells in Central Nervous System Injury: A Double-Edged Sword. J. Immunol. 2014, 193, 5013–5022. [Google Scholar] [CrossRef]
- Karova, K.; Wainwright, J.V.; Machova-Urdzikova, L.; Pisal, R.V.; Schmidt, M.; Jendelova, P.; Jhanwar-Uniyal, M. Transplantation of Neural Precursors Generated from Spinal Progenitor Cells Reduces Inflammation in Spinal Cord Injury via NF-ΚB Pathway Inhibition. J. Neuroinflamm. 2019, 16, 12. [Google Scholar] [CrossRef]
- Mukhamedshina, Y.O.; Akhmetzyanova, E.R.; Martynova, E.V.; Khaiboullina, S.F.; Galieva, L.R.; Rizvanov, A.A. Systemic and Local Cytokine Profile Following Spinal Cord Injury in Rats: A Multiplex Analysis. Front. Neurol. 2017, 8. [Google Scholar] [CrossRef]
- Kwiecien, J.M.; Dabrowski, W.; Dąbrowska-Bouta, B.; Sulkowski, G.; Oakden, W.; Kwiecien-Delaney, C.J.; Yaron, J.R.; Zhang, L.; Schutz, L.; Marzec-Kotarska, B.; et al. Prolonged Inflammation Leads to Ongoing Damage after Spinal Cord Injury. PLoS ONE 2020, 15, e0226584. [Google Scholar] [CrossRef]
- Thompson, C.D.; Zurko, J.C.; Hanna, B.F.; Hellenbrand, D.J.; Hanna, A. The Therapeutic Role of Interleukin-10 after Spinal Cord Injury. J. Neurotrauma 2013, 30, 1311–1324. [Google Scholar] [CrossRef]


| Experimental Group | Procedure | Treatment | Transcardial Perfusion with 4% Paraformaldehyde | Freshly Isolated Tissues | Total |
|---|---|---|---|---|---|
| RAPA | SCI | Rapamycin 5 mg/kg | 5 | 6 | 11 |
| pp242 | SCI | pp242 5 mg/kg | 5 | 6 | 11 |
| Vehicle | SCI | Saline, DMSO, triton | 5 | 7 | 12 |
| No lesion | n/a | n/a | n/a | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargova, I.; Machova Urdzikova, L.; Karova, K.; Smejkalova, B.; Sursal, T.; Cimermanova, V.; Turnovcova, K.; Gandhi, C.D.; Jhanwar-Uniyal, M.; Jendelova, P. Involvement of mTOR Pathways in Recovery from Spinal Cord Injury by Modulation of Autophagy and Immune Response. Biomedicines 2021, 9, 593. https://doi.org/10.3390/biomedicines9060593
Vargova I, Machova Urdzikova L, Karova K, Smejkalova B, Sursal T, Cimermanova V, Turnovcova K, Gandhi CD, Jhanwar-Uniyal M, Jendelova P. Involvement of mTOR Pathways in Recovery from Spinal Cord Injury by Modulation of Autophagy and Immune Response. Biomedicines. 2021; 9(6):593. https://doi.org/10.3390/biomedicines9060593
Chicago/Turabian StyleVargova, Ingrid, Lucia Machova Urdzikova, Kristyna Karova, Barbora Smejkalova, Tolga Sursal, Veronika Cimermanova, Karolina Turnovcova, Chirag D. Gandhi, Meena Jhanwar-Uniyal, and Pavla Jendelova. 2021. "Involvement of mTOR Pathways in Recovery from Spinal Cord Injury by Modulation of Autophagy and Immune Response" Biomedicines 9, no. 6: 593. https://doi.org/10.3390/biomedicines9060593
APA StyleVargova, I., Machova Urdzikova, L., Karova, K., Smejkalova, B., Sursal, T., Cimermanova, V., Turnovcova, K., Gandhi, C. D., Jhanwar-Uniyal, M., & Jendelova, P. (2021). Involvement of mTOR Pathways in Recovery from Spinal Cord Injury by Modulation of Autophagy and Immune Response. Biomedicines, 9(6), 593. https://doi.org/10.3390/biomedicines9060593







