Promoting Neuronal Outgrowth Using Ridged Scaffolds Coated with Extracellular Matrix Proteins
Abstract
1. Introduction
2. Material and Methods
2.1. OPF Synthesis and OPF+ Scaffold Fabrication
2.2. Swelling Ratio
2.3. OPF+ Sheet Culture Preparation and ECM Protein Coating
2.4. Whole DRG Explants
2.5. DRG Neuronal Cultures
2.6. Immunocytochemistry
2.7. Image Analysis of DRG Neurons
2.8. Schwann Cell Cultures
2.9. Neuronal–Schwann Cell Co-Culture
2.10. Statistics
3. Results
3.1. Ridged OPF+ Sheet Characterization
3.2. Whole DRG Neurite Outgrowth Is Enhanced on Laminin-Coated Sheets
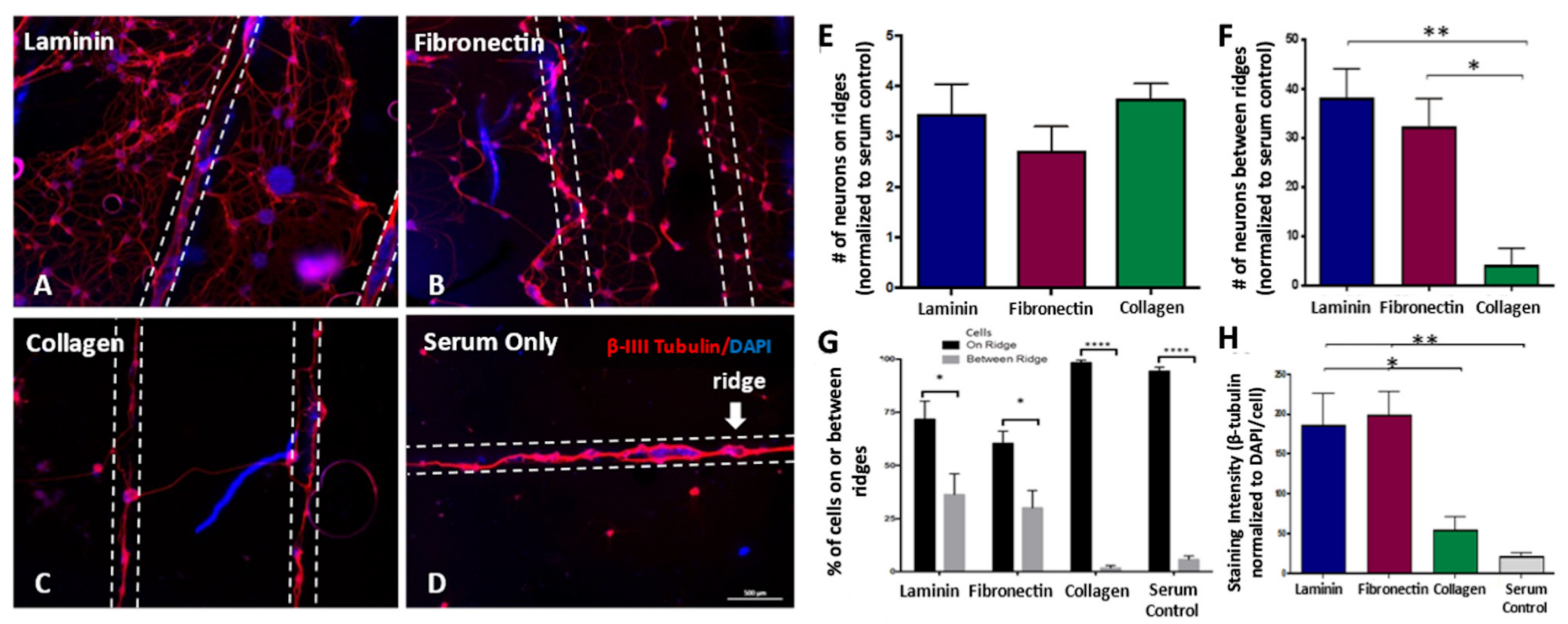
3.3. Dissociated DRG Neuron Neurite Outgrowth Is Enhanced on Laminin-Coated Sheets
3.4. Increasing the Number of Ridges Improves Neuronal Cell Attachment, Alignment, and Neurite Density
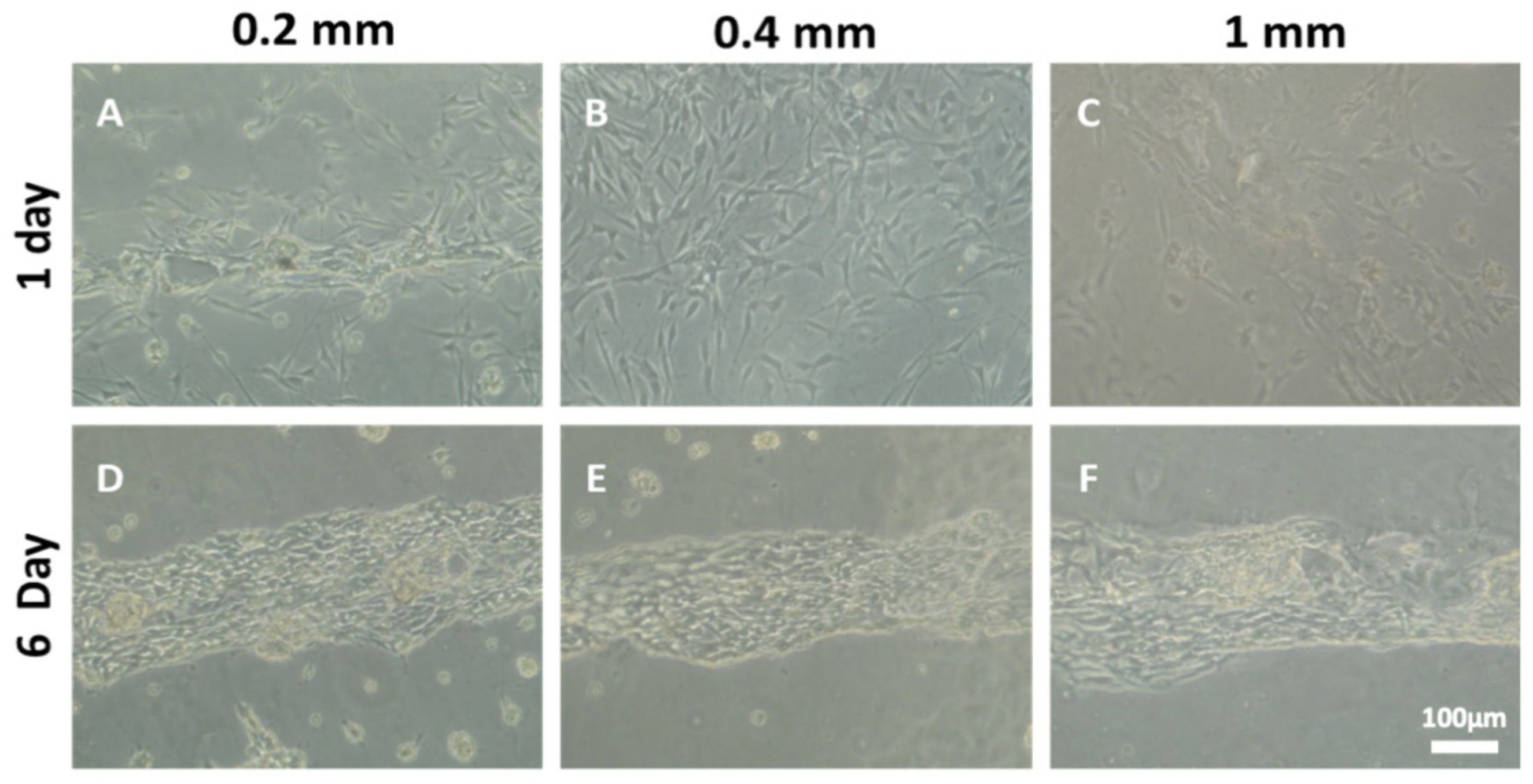
3.5. The Number of Ridges Influences Schwann Cell Organization and Axonal Myelination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, A.M.; Khazaei, M.; Fehlings, M.G. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog. Brain Res. 2015, 218, 15–54. [Google Scholar]
- Badner, A.; Siddiqui, A.M.; Fehlings, M.G. Spinal cord injuries: How could cell therapy help? Expert Opin. Biol. Ther. 2017, 17, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Madigan, N.N.; McMahon, S.; O’Brien, T.; Yaszemski, M.J.; Windebank, A.J. Current tissue engineering and novel therapeutic approaches to axonal regeneration following spinal cord injury using polymer scaffolds. Respir. Physiol. Neurobiol. 2009, 169, 183–199. [Google Scholar] [CrossRef]
- Hakim, J.S.; Esmaeili Rad, M.; Grahn, P.J.; Chen, B.K.; Knight, A.M.; Schmeichel, A.M.; Isaq, N.A.; Dadsetan, M.; Yaszemski, M.J.; Windebank, A.J. Positively charged oligo [poly (ethylene glycol) fumarate] scaffold implantation results in a permissive lesion environment after spinal cord injury in rat. Tissue Eng. Part A 2015, 21, 2099–2114. [Google Scholar] [CrossRef]
- Chen, B.K.; Madigan, N.N.; Hakim, J.S.; Dadsetan, M.; McMahon, S.S.; Yaszemski, M.J.; Windebank, A.J. GDNF Schwann cells in hydrogel scaffolds promote regional axon regeneration, remyelination and functional improvement after spinal cord transection in rats. J. Tissue Eng. Regen. Med. 2018, 12, e398–e407. [Google Scholar] [CrossRef]
- Harris, G.M.; Madigan, N.N.; Lancaster, K.Z.; Enquist, L.W.; Windebank, A.J.; Schwartz, J.; Schwarzbauer, J.E. Nerve Guidance by a Decellularized Fibroblast Extracellular Matrix. Matrix Biol. 2017, 60, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Knight, A.M.; Madigan, N.N.; Gross, L.; Dadsetan, M.; Nesbitt, G.E.; Rooney, J.J.; Currier, B.L.; Yaszemski, M.J.; Spinner, R.J. Comparison of polymer scaffolds in rat spinal cord: A step toward quantitative assessment of combinatorial approaches to spinal cord repair. Biomaterials 2011, 32, 8077–8086. [Google Scholar] [CrossRef] [PubMed]
- Dadsetan, M.; Szatkowski, J.P.; Yaszemski, M.J.; Lu, L. Characterization of photo-cross-linked oligo [poly (ethylene glycol) fumarate] hydrogels for cartilage tissue engineering. Biomacromolecules 2007, 8, 1702–1709. [Google Scholar] [CrossRef]
- Dadsetan, M.; Knight, A.M.; Lu, L.; Windebank, A.J.; Yaszemski, M.J. Stimulation of neurite outgrowth using positively charged hydrogels. Biomaterials 2009, 30, 3874–3881. [Google Scholar] [CrossRef] [PubMed]
- Madigan, N.N.; Chen, B.K.; Knight, A.M.; Rooney, G.E.; Sweeney, E.; Kinnavane, L.; Yaszemski, M.J.; Dockery, P.; O’Brien, T.; McMahon, S.S. Comparison of cellular architecture, axonal growth, and blood vessel formation through cell-loaded polymer scaffolds in the transected rat spinal cord. Tissue Eng. Part A 2014, 20, 2985–2997. [Google Scholar] [CrossRef]
- Clark, P.; Connolly, P.; Curtis, A.; Dow, J.; Wilkinson, C. Cell guidance by ultrafine topography in vitro. J. Cell Sci. 1991, 99, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, S.; Cui, W.; Sherlock, R.; O’Connell, C.; Damodaran, G.; Gorman, A.; Windebank, A.; Pandit, A. Effect of functionalized micropatterned PLGA on guided neurite growth. Acta Biomater. 2009, 5, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Barillé, R.; Janik, R.; Kucharski, S.; Eyer, J.; Letournel, F. Photo-responsive polymer with erasable and reconfigurable micro- and nano-patterns: An in vitro study for neuron guidance. Colloids Surf. B Biointerfaces 2011, 88, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Goldner, J.S.; Bruder, J.M.; Li, G.; Gazzola, D.; Hoffman-Kim, D. Neurite bridging across micropatterned grooves. Biomaterials 2006, 27, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, A.E.; Marlow, M.M.; Oudega, M. Extracellular matrix components as therapeutics for spinal cord injury. Neurosci. Lett. 2017, 652, 50–55. [Google Scholar] [CrossRef]
- Yao, L.; Damodaran, G.; Nikolskaya, N.; Gorman, A.M.; Windebank, A.; Pandit, A. The effect of laminin peptide gradient in enzymatically cross-linked collagen scaffolds on neurite growth. J. Biomed. Mater. Res. Part A 2010, 92, 484–492. [Google Scholar] [CrossRef]
- Adams, D.N.; Kao, E.Y.C.; Hypolite, C.L.; Distefano, M.D.; Hu, W.S.; Letourneau, P.C. Growth cones turn and migrate up an immobilized gradient of the laminin IKVAV peptide. Dev. Neurobiol. 2005, 62, 134–147. [Google Scholar] [CrossRef]
- Baron-Van Evercooren, A.; Kleinman, H.K.; Seppä, H.; Rentier, B.; Dubois-Dalcq, M. Fibronectin promotes rat Schwann cell growth and motility. J. Cell Biol. 1982, 93, 211–216. [Google Scholar] [CrossRef]
- Mosahebi, A.; Wiberg, M.; Terenghi, G. Addition of fibronectin to alginate matrix improves peripheral nerve regeneration in tissue-engineered conduits. Tissue Eng. 2003, 9, 209–218. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lee, Y.-S.; Lin, V.W.; Silver, J. Fibronectin inhibits chronic pain development after spinal cord injury. J. Neurotrauma 2012, 29, 589–599. [Google Scholar] [CrossRef]
- Novikova, L.N.; Novikov, L.N.; Kellerth, J.-O. Biopolymers and biodegradable smart implants for tissue regeneration after spinal cord injury. Curr. Opin. Neurol. 2003, 16, 711–715. [Google Scholar] [CrossRef]
- Conti, A.M.; Fischer, S.J.; Windebank, A.J. Inhibition of axonal growth from sensory neurons by excess nerve growth factor. Ann. Neurol. 1997, 42, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Ta, L.E.; Espeset, L.; Podratz, J.; Windebank, A.J. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum–DNA binding. Neurotoxicology 2006, 27, 992–1002. [Google Scholar] [CrossRef]
- Podratz, J.L.; Windebank, A.J. NGF rescues DRG neurons in vitro from oxidative damage produced by hemodialyzers. Neurotoxicology 2005, 26, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Knight, A.M.; De Ruiter, G.C.; Spinner, R.J.; Yaszemski, M.J.; Currier, B.L.; Windebank, A.J. Axon regeneration through scaffold into distal spinal cord after transection. J. Neurotrauma 2009, 26, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Olson, H.E.; Rooney, G.E.; Gross, L.; Nesbitt, J.J.; Galvin, K.E.; Knight, A.; Chen, B.; Yaszemski, M.J.; Windebank, A.J. Neural stem cell–and schwann cell–loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng. Part A 2009, 15, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Hakim, J.; Rodysill, B.; Chen, B.K.; Schmeichel, A.; Yaszemski, M.J.; Windebank, A.; Madigan, N. Combinatorial Tissue Engineering Partially Restores Function after Spinal Cord Injury. J. Tissue Eng. Regen. Med. 2019, 13, 857–873. [Google Scholar] [CrossRef]
- Liu, L.; Wang, N.; Han, Y.; Li, Y.; Liu, W. Redox-Triggered Self-Rolling Robust Hydrogel Tubes for Cell Encapsulation. Macromol. Rapid Commun. 2014, 35, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Vasiev, I.; Greer, A.I.M.; Khokhar, A.Z.; Stormonth-Darling, J.; Tanner, K.E.; Gadegaard, N. Self-folding nano- and micropatterned hydrogel tissue engineering scaffolds by single step photolithographic process. Microelectron. Eng. 2013, 108, 76–81. [Google Scholar] [CrossRef]
- Zhang, Q.; Wommer, J.; O’Rourke, C.; Teitelman, J.; Tang, Y.; Robison, J.; Lin, G.; Yin, J. Origami and kirigami inspired self-folding for programming three-dimensional shape shifting of polymer sheets with light. Extrem. Mech. Lett. 2017, 11, 111–120. [Google Scholar] [CrossRef]
- Thérien-Aubin, H.l.; Wu, Z.L.; Nie, Z.; Kumacheva, E. Multiple shape transformations of composite hydrogel sheets. J. Am. Chem. Soc. 2013, 135, 4834–4839. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Q.; Liu, W.; Nie, Z. Light-Mediated Shape Transformation of a Self-Rolling Nanocomposite Hydrogel Tube. ACS Appl. Mater. Interfaces 2020, 12, 13521–13528. [Google Scholar] [CrossRef] [PubMed]
- Gombotz, W.R.; Wang, G.H.; Horbett, T.A.; Hoffman, A.S. Protein adsorption to poly(ethylene oxide) surfaces. J. Biomed. Mater. Res. 1991, 25, 1547–1562. [Google Scholar] [CrossRef]
- Dodla, R.V.; Bellamkonda, M.C. Anisotropic scaffolds facilitate enhanced neurite extension in vitro. J. Biomed. Mater. Res. A 2006, 78, 213–221. [Google Scholar] [CrossRef]
- Besser, R.R.; Bowles, A.C.; Alassaf, A.; Carbonero, D.; Claure, I.; Jones, E.; Reda, J.; Wubker, L.; Batchelor, W.; Ziebarth, N.; et al. Enzymatically crosslinked gelatin–laminin hydrogels for applications in neuromuscular tissue engineering. Biomater. Sci. 2020, 8, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, C.A.; Dalecki, D.; Hocking, D.C. Extracellular matrix fibronectin stimulates the self-assembly of microtissues on native collagen gels. Tissue Eng. Part A 2010, 16, 3805–3819. [Google Scholar] [CrossRef]
- Deister, C.; Aljabari, S.; Schmidt, C.E. Effects of collagen 1, fibronectin, laminin and hyaluronic acid concentration in multi-component gels on neurite extension. J. Biomater. Sci. Polym. Ed. 2007, 18, 983–997. [Google Scholar] [CrossRef]
- Miller, C.; Jeftinija, S.; Mallapragada, S. Synergistic effects of physical and chemical guidance cues on neurite alignment and outgrowth on biodegradable polymer substrates. Tissue Eng. 2002, 8, 367–378. [Google Scholar] [CrossRef]
- Ma, Z.; Mao, Z.; Gao, C. Surface modification and property analysis of biomedical polymers used for tissue engineering. Colloids Surf. B Biointerfaces 2007, 60, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Levesque, S.G.; Shoichet, M.S. Synthesis of cell-adhesive dextran hydrogels and macroporous scaffolds. Biomaterials 2006, 27, 5277–5285. [Google Scholar] [CrossRef] [PubMed]
- Dodla, M.C.; Bellamkonda, R.V. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials 2008, 29, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Koser, D.E.; Thompson, A.J.; Foster, S.K.; Dwivedy, A.; Pillai, E.K.; Sheridan, G.K.; Svoboda, H.; Viana, M.; da, F. Costa, L.; Guck, J. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 2016, 19, 1592. [Google Scholar] [CrossRef]
- Lei, W.-L.; Xing, S.-G.; Deng, C.-Y.; Ju, X.-C.; Jiang, X.-Y.; Luo, Z.-G. Laminin/β1 integrin signal triggers axon formation by promoting microtubule assembly and stabilization. Cell Res. 2012, 22, 954. [Google Scholar] [CrossRef] [PubMed]
- Hatten, M.E. Riding the glial monorail: A common mechanism for glialguided neuronal migration in different regions of the developing mammalian brain. Trends Neurosci. 1990, 13, 179–184. [Google Scholar] [CrossRef]
- Siddiqui, A.M.; Ahuja, C.S.; Tator, C.H.; Fehlings, M.G. Chapter 3: Spinal cord protective and regenerative therapies. In Neurotrauma and Critical Care of the Spine, 2nd ed.; Jallo, J., Vaccaro, A., Eds.; Thieme: New York, NY, USA, 2018; p. 238. [Google Scholar]
- Rooney, G.E.; Knight, A.M.; Madigan, N.N.; Gross, L.; Chen, B.; Giraldo, C.V.; Seo, S.; Nesbitt, J.J.; Dadsetan, M.; Yaszemski, M.J. Sustained delivery of dibutyryl cyclic adenosine monophosphate to the transected spinal cord via oligo [(polyethylene glycol) fumarate] hydrogels. Tissue Eng. Part A 2011, 17, 1287–1302. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Guénard, V.; Kleitman, N.; Bunge, M.B. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J. Comp. Neurol. 1995, 351, 145–160. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, A.M.; Brunner, R.; Harris, G.M.; Miller, A.L., II; Waletzki, B.E.; Schmeichel, A.M.; Schwarzbauer, J.E.; Schwartz, J.; Yaszemski, M.J.; Windebank, A.J.; et al. Promoting Neuronal Outgrowth Using Ridged Scaffolds Coated with Extracellular Matrix Proteins. Biomedicines 2021, 9, 479. https://doi.org/10.3390/biomedicines9050479
Siddiqui AM, Brunner R, Harris GM, Miller AL II, Waletzki BE, Schmeichel AM, Schwarzbauer JE, Schwartz J, Yaszemski MJ, Windebank AJ, et al. Promoting Neuronal Outgrowth Using Ridged Scaffolds Coated with Extracellular Matrix Proteins. Biomedicines. 2021; 9(5):479. https://doi.org/10.3390/biomedicines9050479
Chicago/Turabian StyleSiddiqui, Ahad M., Rosa Brunner, Gregory M. Harris, Alan Lee Miller, II, Brian E. Waletzki, Ann M. Schmeichel, Jean E. Schwarzbauer, Jeffrey Schwartz, Michael J. Yaszemski, Anthony J. Windebank, and et al. 2021. "Promoting Neuronal Outgrowth Using Ridged Scaffolds Coated with Extracellular Matrix Proteins" Biomedicines 9, no. 5: 479. https://doi.org/10.3390/biomedicines9050479
APA StyleSiddiqui, A. M., Brunner, R., Harris, G. M., Miller, A. L., II, Waletzki, B. E., Schmeichel, A. M., Schwarzbauer, J. E., Schwartz, J., Yaszemski, M. J., Windebank, A. J., & Madigan, N. N. (2021). Promoting Neuronal Outgrowth Using Ridged Scaffolds Coated with Extracellular Matrix Proteins. Biomedicines, 9(5), 479. https://doi.org/10.3390/biomedicines9050479







