Interaction between Biofilm Formation, Surface Material and Cleanability Considering Different Materials Used in Pig Facilities—An Overview
Abstract
:1. Introduction
2. Material Types in Pig Facilities
3. Influence of the Surface Type in the Bacteria Colonization
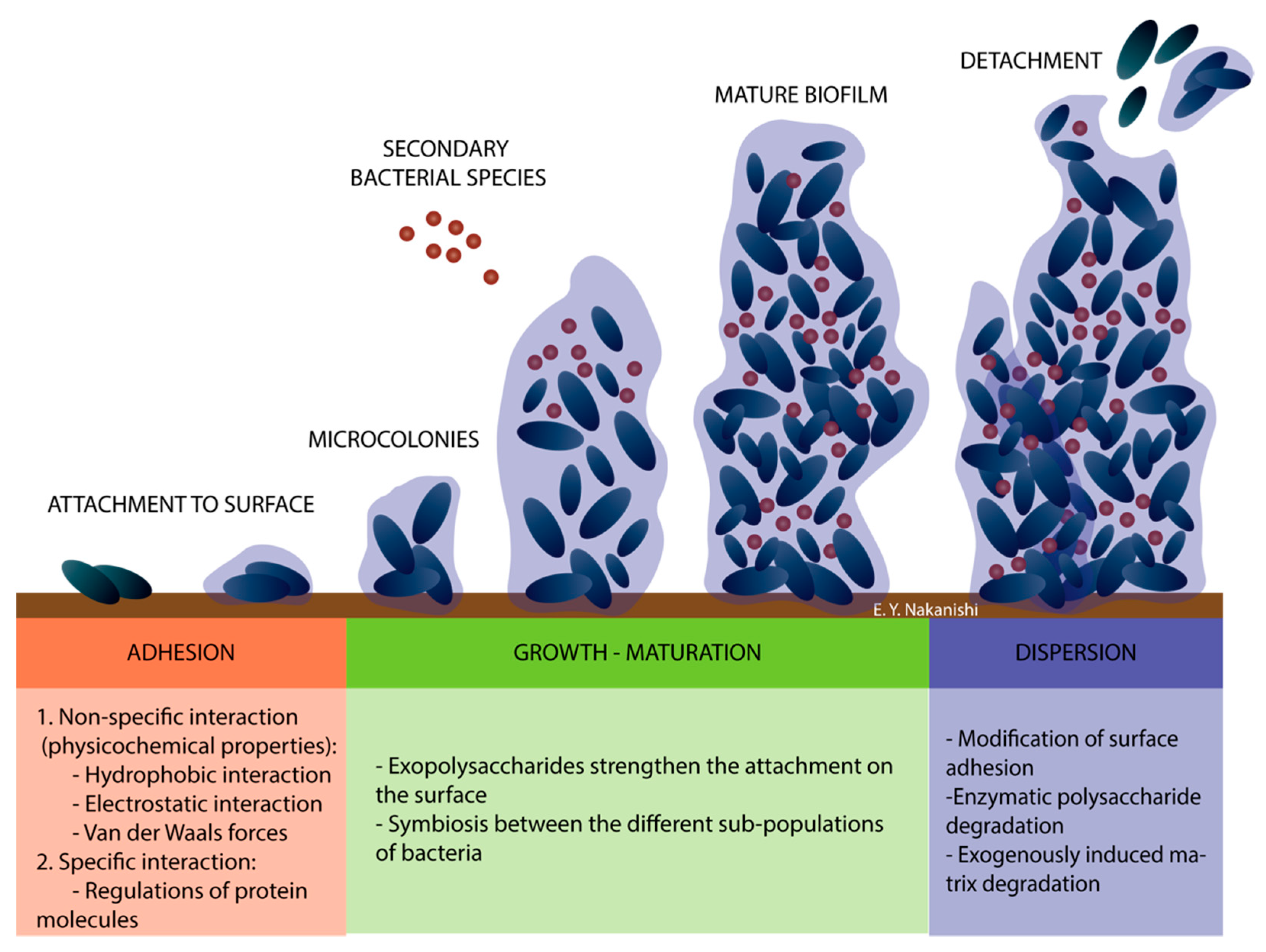
3.1. Biofilm and Formation Phases
3.2. Surface Roughness Properties
3.2.1. Importance of Surface Roughness in Pig Facilities
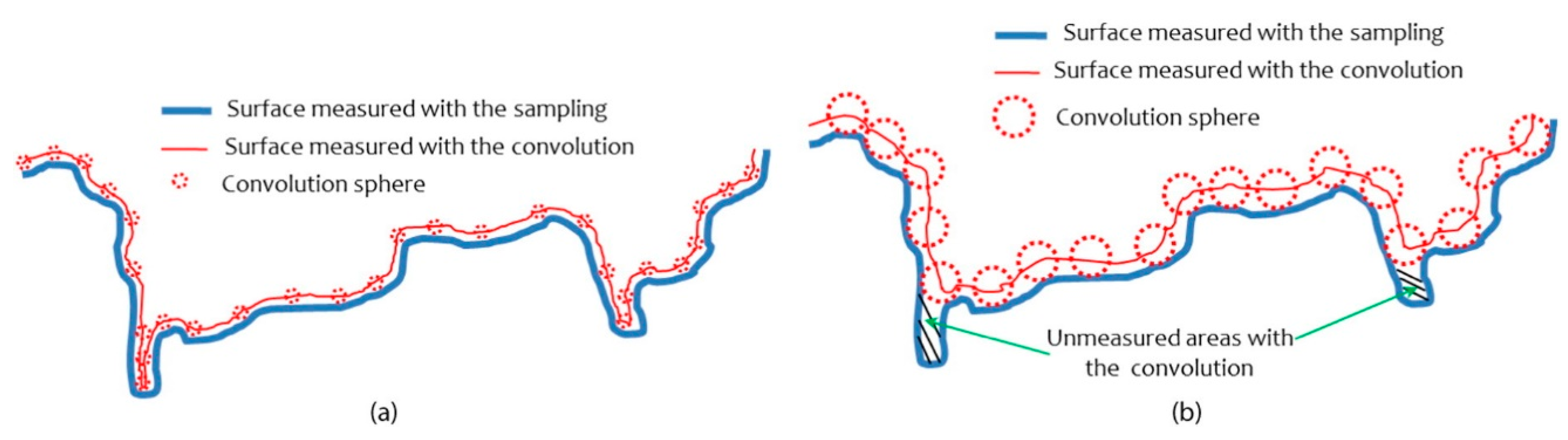
3.2.2. Surface Roughness of Materials
3.2.3. The Impact of Roughness on Bacteria Colonization
3.3. Surface-Free Energy Properties
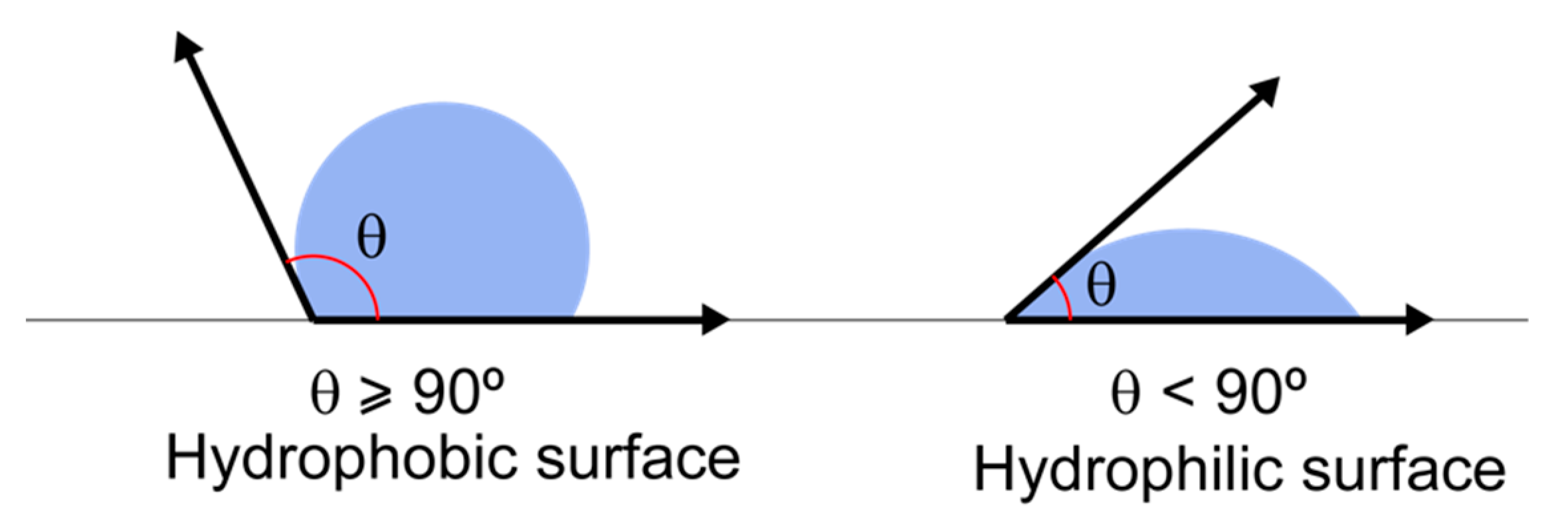
3.3.1. Surface-Free Energy Materials
3.3.2. Influence of SFE on Bacterial Colonization
4. Impact of Washing Procedures on the Materials’ Sanitary Characteristics
4.1. Material Cleanability Evaluation Methods and Parameters
- Mycoplasma Hyopneumoniae: Up to 7 days in organic matter
- Actinobacillus Pleuropneumoniae: Few days in organic matter
- Pasteurella Multocida: 8 days in water or 6 days in liquid manure
- Streptococcus suis: 25 days (9 °C) or 100 days (0 °C)
- Salmonella ssp.: Years in manure, 115 days in water and 120 days in soil
- Escherichia coli: 11 weeks in manure
4.2. Effect of the Washing Procedure on Biofilm Destruction on Different Materials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Les Éleveurs de porcs du Québec-Guide de Lavage, Désinfection et Séchage Des Porcheries. 2011. Available online: http://www.accesporcqc.ca/nsphp/portail/publications/pub_dl.php?dir=364&download=guidedeldsdesporcheries.pdf (accessed on 17 May 2021).
- Nantel-Fortier, N.; Lachapelle, V.; Letellier, A.; L’Homme, Y.; Brassard, J. Kobuvirus Shedding Dynamics in a Swine Production System and Their Association with Diarrhea. Vet. Microbiol. 2019, 235, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.; Grenier, D. Understanding the Virulence of Streptococcus Suis: A Veterinary, Medical, and Economic Challenge. Med. Mal. Infect. 2018, 48, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Letellier, A.; Messier, S.; Paré, J.; Ménard, J.; Quessy, S. Distribution of Salmonella in Swine Herds in Quebec. Vet. Microbiol. 1999, 67, 299–306. [Google Scholar] [CrossRef]
- Smith, B.A.; Meadows, S.; Meyers, R.; Parmley, E.J.; Fazil, A. Seasonality and Zoonotic Foodborne Pathogens in Canada: Relationships between Climate and Campylobacter, E. Coli and Salmonella in Meat Products. Epidemiol. Infect. 2019, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perri, A.M.; Poljak, Z.; Dewey, C.; Harding, J.C.S.; O’Sullivan, T.L. Network Analyses Using Case-Control Data to Describe and Characterize the Initial 2014 Incursion of Porcine Epidemic Diarrhea (PED) in Canadian Swine Herds. Prev. Vet. Med. 2019, 162, 18–28. [Google Scholar] [CrossRef]
- Post, K.W. Overview of Bacteria. In Diseases of Swine; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 743–748. [Google Scholar]
- Wang, Y.; Liao, J.; Mehmood, K.; Chang, Y.F.; Tang, Z.; Zhang, H. Escherichia Coli Isolated in Pigs, Guangdong, China: Emergence of Extreme Drug Resistance (XDR) Bacteria. J. Infect. 2020, 81, 318–356. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.; Akdeniz, N. A Review of the Animal Disease Outbreaks and Biosecure Animal Mortality Composting Systems. Waste Manag. 2019, 90, 121–131. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food Outlook–Biannual Report on Global Food Markets; FAO: Rome, Italy, 2019. [Google Scholar]
- Gallardo, C.; Fernández-Pinero, J.; Arias, M. African Swine Fever (ASF) Diagnosis, an Essential Tool in the Epidemiological Investigation. Virus Res. 2019, 271. [Google Scholar] [CrossRef]
- FAO. The Global Platform for African Swine Fever and Other Important Diseases of Swine; Animal Production and Health Report No. 4; FAO: Rome, Italy, 2014. [Google Scholar]
- Lekagul, A.; Tangcharoensathien, V.; Yeung, S. Patterns of Antibiotic Use in Global Pig Production: A Systematic Review. Vet. Anim. Sci. 2019, 7, 100058. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Witney, A.A.; Gould, K.A.; Moodley, A.; Guardabassi, L.; Voss, A.; Denis, O.; Broens, E.M.; Hinds, J.; Lindsay, J.A. The Distribution of Mobile Genetic Elements (MGEs) in MRSA CC398 Is Associated with Both Host and Country. Genome Biol. Evol. 2011, 3, 1164–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Foy, C. Évaluation des Matériaux en Fonction de la Contamination Bactérienne de Surface, des Émissions d’odeurs et des Caractéristiques Physiques afin de Réduire la Dérive Sanitaire des Bâtiments Porcins. Master’s Thesis, Laval University, Québec City, QC, Canada, 2005. [Google Scholar]
- Colclasure, V.J.; Soderquist, T.J.; Lynch, T.; Schubert, N.; McCormick, D.S.; Urrutia, E.; Knickerbocker, C.; McCord, D.; Kavouras, J.H. Coliform Bacteria, Fabrics, and the Environment. Am. J. Infect. Control 2015, 43, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Krishna, V.D.; Torremorell, M.; Goyal, S.M.; Cheeran, M.C.J. Stability of Porcine Epidemic Diarrhea Virus on Fomite Materials at Different Temperatures. Vet. Sci. 2018, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Kramer, A.; Schwebke, I.; Kampf, G. How Long Do Nosocomial Pathogens Persist on Inanimate Surfaces? A Systematic Review. BMC Infect. Dis. 2006, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Krippendorff, K. Content Analysis: An Introduction to Its Methodology, 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 2004; ISBN 0-7619-1544-3. [Google Scholar]
- Melmer, D.J.; O’Sullivan, T.L.; Poljak, Z. A Descriptive Analysis of Swine Movements in Ontario (Canada) as a Contributor to Disease Spread. Prev. Vet. Med. 2018, 159, 211–219. [Google Scholar] [CrossRef]
- USDA. The United States Department of Agriculture-Foreign Animal Disease Preparedness and Response Plan; USDA: Washngton, DC, USA, 2011.
- Boon, C.R.; Wray, C. Building Design in Relation to the Control of Diseases of Intensively Housed Livestock. J. Agric. Eng. Res. 1989, 43, 149–161. [Google Scholar] [CrossRef]
- Gorman, C.; Turnbull, J.E. PLAN M-3003: Construction et Installations Techniques Des Porcheries; Canada Plan Service: Québec City, QC, Canada, 1988; Volume 1. [Google Scholar]
- MWPS-8 Midwest Plan Service. Swine Housin and Equipment Handbook, 4th ed.; Midwest Plan Service: Ames, IA, USA, 1983; ISBN 0-8937-054-8. [Google Scholar]
- AHDB. Agriculture and Horticulture Development Board-Finisher Pig Buildings Design and Build—A Blueprint for English Farms; BPEX: Warwickshire, UK, 2013; pp. 1–123. [Google Scholar]
- Pelletier, F.; Marquis, A.; Godbout, S.; Joncas, R. Gas and Odor Emiissions From Swine Building Material. Am. Soc. Agric. Eng. 2005, 48, 721–728. [Google Scholar] [CrossRef]
- Apedo, K.L.; Montgomery, P.; Serres, N.; Fond, C.; Feugeas, F. Geometrical Roughness Analysis of Cement Paste Surfaces Using Coherence Scanning Interferometry and Confocal Microscopy. Mater. Charact. 2016, 118, 212–224. [Google Scholar] [CrossRef] [Green Version]
- Brajkovic, D.; Antonijevic, D.; Milovanovic, P.; Kisic, D.; Zelic, K.; Djuric, M.; Rakocevic, Z. Surface Characterization of the Cement for Retention of Implant Supported Dental Prostheses: In Vitro Evaluation of Cement Roughness and Surface Free Energy. Appl. Surf. Sci. 2014, 311, 131–138. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro-and Nanotopography Sensitive Bacterial Attachment Mechanisms: A Review. Front. Microbiol. 2019, 10, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsikogianni, M.; Missirlis, Y.F. Concise Review of Mechanisms of Bacterial Adhesion to Biomaterials and of Techniques Used in Estimating Bacteria-Material Interactions. Eur. Cells Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Mueller, R.F.; Characklis, W.G.; Jones, W.L.; Sears, J.T. Characterization of Initial Events in Bacterial Surface Colonization by Two Pseudomonas Species Using Image Analysis. Biotechnol. Bioeng. 1992, 39, 1161–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinh, Q.T.; Bal Krishna, K.C.; Salih, A.; Listowski, A.; Sathasivan, A. Biofilm Growth on PVC and HDPE Pipes Impacts Chlorine Stability in the Recycled Water. J. Environ. Chem. Eng. 2020, 8, 104476. [Google Scholar] [CrossRef]
- Hathroubi, S. Rôle des Polysaccharides de Surface dans la Formation des Biofilms et rôle du Biofilm D’actinobacillus Pleuropneumoniae dans la Pathogénicité. Ph.D. Thesis, Université de Montréal, Montréal, QC, Canada, 2016. [Google Scholar]
- Busscher, H.J.; Norde, W.; van der Mei, H.C. Specific Molecular Recognition and Nonspecific Contributions to Bacterial Interaction Forces Downloaded From. Appl. Environ. Microbiol. 2008, 74, 2559–2564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harimawan, A.; Rajasekar, A.; Ting, Y.P. Bacteria Attachment to Surfaces-AFM Force Spectroscopy and Physicochemical Analyses. J. Colloid Interface Sci. 2011, 364, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Gharechahi, M.; Moosavi, H.; Forghani, M. Effect of Surface Roughness and Materials Composition. J. Biomater. Nanobiotechnology 2012, 3, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Berne, C.; Ducret, A.; Hardy, G.G.; Brun, Y.V. Adhesins Involved in Attachment to Abiotic Surfaces by Gram-Negative Bacteria. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Gupta, K.K.; Devi, D. Characteristics Investigation on Biofilm Formation and Biodegradation Activities of Pseudomonas Aeruginosa Strain ISJ14 Colonizing Low Density Polyethylene (LDPE) Surface. Heliyon 2020, 6, e04398. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Abdallah, M.; Campistron, P.; Moulin, E.; Callens, D.; Khelissa, S.O.; Debreyne, P.; Chihib, N.E.; Delaplace, G. Detection of Biofilm Formation by Ultrasonic Coda Wave Interferometry. J. Food Eng. 2021, 290, 110219. [Google Scholar] [CrossRef]
- Li, G.; Wu, Y.; Li, Y.; Hong, Y.; Zhao, X.; Reyes, P.I.; Lu, Y. Early Stage Detection of Staphylococcus Epidermidis Biofilm Formation Using MgZnO Dual-Gate TFT Biosensor. Biosens. Bioelectron. 2020, 151, 111993. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.M.; Pracser, N.; Thalguter, S.; Fischel, K.; Rammer, N.; Pospíšilová, L.; Alispahic, M.; Wagner, M.; Rychli, K. Identification of Biofilm Hotspots in a Meat Processing Environment: Detection of Spoilage Bacteria in Multi-Species Biofilms. International. J. Food Microbiol. 2020, 328, 108668. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, S.; Pick, H.; Oveisi, E.; Girault, H.H.; Lesch, A. Soft-Probe-Scanning Electrochemical Microscopy Reveals Electrochemical Surface Reactivity of E. Coli Biofilms. Sens. Actuators B Chem. 2021, 334, 129669. [Google Scholar] [CrossRef]
- Rumbaugh, K.P.; Sauer, K. Biofilm Dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Ficker, T.; Martišek, D. Digital Fracture Surfaces and Their Roughness Analysis: Applications to Cement-Based Materials. Cem. Concr. Res. 2012, 42, 827–833. [Google Scholar] [CrossRef]
- Santos, P.M.D.; Júlio, E.N.B.S. A State-of-the-Art Review on Roughness Quantification Methods for Concrete Surfaces. Constr. Build. Mater. 2013, 38, 912–923. [Google Scholar] [CrossRef]
- ISO. EN ISO 16610-21 Geometrical Product Specifications (GPS)—Filtration—Part 21: Linear Profile Filters: Gaussian Filters; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- ISO. EN ISO 11562 Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Metrological Characteristics of Phase Correct Filters; ISO: Geneva, Switzerland, 1996. [Google Scholar]
- Kiliç, M.; Burdurlu, E.; Aslan, S.; Altun, S.; Tümerdem, Ö. The Effect of Surface Roughness on Tensile Strength of the Medium Density Fiberboard (MDF) Overlaid with Polyvinyl Chloride (PVC). Mater. Des. 2009, 30, 4580–4583. [Google Scholar] [CrossRef]
- Luo, B.; Zhang, J.; Bao, X.; Liu, H.; Li, L. The Effect of Granularity on Surface Roughness and Contact Angle in Wood Sanding Process. Meas. J. Int. Meas. Confed. 2020, 165, 108133. [Google Scholar] [CrossRef]
- Tabarsa, T.; Ashori, A.; Gholamzadeh, M. Evaluation of Surface Roughness and Mechanical Properties of Particleboard Panels Made from Bagasse. Compos. Part B 2011, 42, 1330–1335. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, X.; Huang, J.; Li, H. Flame Sprayed Environmentally Friendly High Density Polyethylene (HDPE)–Capsaicin Composite Coatings for Marine Antifouling Applications. Mater. Lett. 2019, 238, 46–50. [Google Scholar] [CrossRef]
- Vuoristo, P. Thermal Spray Coating Processes. In Comprehensive Materials Processing; Hashmi, S., Batalha, G.F., Tyne, C.J.V., Yilbas, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 4, pp. 229–276. ISBN 9780080965338. [Google Scholar]
- Barnat-Hunek, D.; Grzegorczyk-Frańczak, M.; Suchorab, Z. Surface Hydrophobisation of Mortars with Waste Aggregate by Nanopolymer Trietoxi-Isobutyl-Silane and Methyl Silicon Resin. Constr. Build. Mater. 2020, 264. [Google Scholar] [CrossRef]
- Szafraniec, M.; Barnat-Hunek, D.; Grzegorczyk-Frańczak, M.; Trochonowicz, M. Surface Modification of Lightweight Mortars by Nanopolymers to Improve Theirwater-Repellency and Durability. Materials 2020, 13, 1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezaroba, C.; Becker, D.; Nahorny, J.; Recco, A.A.C.; Fontana, L.C. Nano-Rugosidade Gerada Em Amostras de Polímero PEAD Através de Plasma RF de N2/O2. Matéria 2018, 23. [Google Scholar] [CrossRef]
- Hill, D.; Barron, A.R.; Alexander, S. Controlling the Wettability of Plastic by Thermally Embedding Coated Aluminium Oxide Nanoparticles into the Surface. J. Colloid Interface Sci. 2020, 567, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Lin, E.S.; Uddin, M.H.; Ong, J.W.; Abid, H.A.; Xiong, Z.; Li, D.; Liew, O.W.; Ng, T.W. Temporal Evolution of Wetting Transitions of Graphene Oxide Coated on Roughened Polyvinyl Chloride Surfaces. Mater. Today Commun. 2020, 25, 101650. [Google Scholar] [CrossRef]
- Burfoot, D.; Middleton, K. Effects of Operating Conditions of High Pressure Washing on the Removal of Biofilms from Stainless Steel Surfaces. J. Food Eng. 2009, 90, 350–357. [Google Scholar] [CrossRef]
- Song, J.W.; Zeng, D.L.; Fan, L.W. Temperature Dependence of Contact Angles of Water on a Stainless Steel Surface at Elevated Temperatures and Pressures: In Situ Characterization and Thermodynamic Analysis. J. Colloid Interface Sci. 2020, 561, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Hiziroglu, S.; Jarusombuti, S.; Fueangvivat, V. Surface Characteristics of Wood Composites Manufactured in Thailand. Build. Environ. 2004, 39, 1359–1364. [Google Scholar] [CrossRef]
- Candan, Z.; Büyüksari, U.; Korkut, S.; Unsal, O.; Çakicier, N. Wettability and Surface Roughness of Thermally Modified Plywood Panels. Ind. Crops Prod. 2012, 36, 434–436. [Google Scholar] [CrossRef]
- Lazghab, M.; Saleh, K.; Pezron, I.; Guigon, P.; Komunjer, L. Wettability Assessment of Finely Divided Solids. Powder Technol. 2005, 157, 79–91. [Google Scholar] [CrossRef]
- Kisić, D.; Nenadović, M.; Barudžija, T.; Noga, P.; Vaňa, D.; Muška, M.; Rakočević, Z. Modification of Polyethylene’s Surface Properties by High Fluence Fe Implantation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 462, 143–153. [Google Scholar] [CrossRef]
- Barth, E.; Myrvik, Q.M.; Wagner, W.; Gristina, A.G. In Vitro and in Vivo Comparative Colonization of Staphylococcus Aureus and Staphylococcus Epidermidis on Orthopaedic Implant Materials. Biomaterials 1989, 10, 325–328. [Google Scholar] [CrossRef]
- Tatchou-Nyamsi-König, J.A.; Dague, E.; Mullet, M.; Duval, J.F.L.; Gaboriaud, F.; Block, J.C. Adhesion of Campylobacter Jejuni and Mycobacterium Avium onto Polyethylene Terephtalate (PET) Used for Bottled Waters. Water Res. 2008, 42, 4751–4760. [Google Scholar] [CrossRef] [PubMed]
- Norton, C.D.; LeChevallier, M.W.; Falkinham, J.O. Survival of Mycobacterium Avium in a Model Distribution System. Water Res. 2004, 38, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Daffonchio, D.; Thaveesri, J.; Verstraete, W. Contact Angle Measurement and Cell Hydrophobicity of Granular Sludge from Upflow Anaerobic Sludge Bed Reactors. Appl. Environ. Microbiol. 1995, 61, 3676–3680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pimentel-Filho, N.J.; de Martins, M.C.F.; Nogueira, G.B.; Mantovani, H.C.; Vanetti, M.C.D. Bovicin HC5 and Nisin Reduce Staphylococcus Aureus Adhesion to Polystyrene and Change the Hydrophobicity Profile and Gibbs Free Energy of Adhesion. Int. J. Food Microbiol. 2014, 190, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Braga, P.C.; Reggio, S. Correlation between Reduction of Surface Hydrophobicity of S. Aureus and the Decrease in Its Adhesiveness Induced by Subinhibitory Concentrations of Brodimoprim. Pharmacol. Res. 1995, 32, 315–319. [Google Scholar] [CrossRef]
- Absolom, D.R.; Lamberti, F.V.; Policova, Z.; Zingg, W.; van Oss, C.J.; Wilhelm Neumann, A.A. Surface Thermodynamics of Bacterial Adhesion. Appl. Environ. Microbiol. 1983, 46, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerca, N.; Pier, G.B.; Vilanova, M.; Oliveira, R.; Azeredo, J. Quantitative Analysis of Adhesion and Biofilm Formation on Hydrophilic and Hydrophobic Surfaces of Clinical Isolates of Staphylococcus Epidermidis. Res. Microbiol. 2005, 156, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmerman, C.P.; Fleer, A.; Besnier, J.M.; de Graaf, L.; Cremers, F.; Verhoef, J. Characterization of a Proteinaceous Adhesin of Staphylococcus Epidermidis Which Mediates Attachment to Polystyrene. Infect. Immun. 1991, 59, 4187–4192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.; Zhu, Y.; Shi, W.; He, K.; Xiao, X.; Xu, X.; Shi, J.; Xu, G. Mechanically Stable Superhydrophobic Surface on Cement-Based Materials. Chem. Phys. 2020, 538, 110912. [Google Scholar] [CrossRef]
- Kondyurin, A.; Naseri, P.; Fisher, K.; McKenzie, D.R.; Bilek, M.M.M. Mechanisms for Surface Energy Changes Observed in Plasma Immersion Ion Implanted Polyethylene: The Roles of Free Radicals and Oxygen-Containing Groups. Polym. Degrad. Stab. 2009, 94, 638–646. [Google Scholar] [CrossRef]
- Dias, A.C.; Carraro, B.Z.; Dallanora, D.; Coser, F.J.; Machado, G.S.; Machado, I.P.; Pinheiro, R.; Rohr, S.A. Manual Brasileiro de Boas Práticas Agropecuárias Na Produção; ABCS: Brasília, Brazil, 2011. [Google Scholar]
- Yi, S.W.; Cho, A.; Kim, E.; Oh, S.I.; Roh, J.H.; Jung, Y.H.; Choe, C.; Yoo, J.G.; Do, Y.J. Evaluation of Adenosine Triphosphate Testing for On-Farm Cleanliness Monitoring Compared to Microbiological Testing in an Empty Pig Farrowing Unit. J. Anim. Sci. Technol. 2020, 62, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, K.; Millet, S.; van Weyenberg, S.; Herman, L.; Heyndrickx, M.; Dewulf, J.; de Reu, K. Comparison of Competitive Exclusion with Classical Cleaning and Disinfection on Bacterial Load in Pig Nursery Units. BMC Vet. Res. 2016, 12, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, R.D.; Verran, J.; Hall, K.E.; Underhill, C.; Hibbert, S.; West, R. Cleanability of Stainless Steel as Determined by X-Ray Photoelectron Spectroscopy. Appl. Surf. Sci. 2001, 172, 135–143. [Google Scholar] [CrossRef]
- Kuisma, R.; Kymäläinen, H.-R.; Hellstedt, M.; Jauhiainen, P.; Määttä, J.; Sjöberg, A.-M. Properties and Cleanability of New and Traditional Surface Materials in Cattle Barns-a Field Study. Agric. Food Sci. 2008, 17, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Kymäläinen, H.-R.; Määttä, J.; Puumala, M.; Kaustell, K.O.; Mattila, T.; Joutsen, B.-L.; Kuisma, R.; Hurme, K.-R.; Uusi-Rauva, A.; Sjöberg, A.-M. A Laboratory Study of the Effect of Coating on Cleanability of Concrete Flooring for Use in Piggeries. Biosyst. Eng. 2008, 99, 88–98. [Google Scholar] [CrossRef]
- Määttä, J.; Kymäläinen, H.R.; Sjöberg, A.M. Application of Radiochemical Determination Methods in Cleanability Research of Building Materials. J. Environ. Radioact. 2011, 102, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Hurnik, D. Investigation into Optimal Washing and Disinfection Techniques for Pig Pens. In Proceedings of the London Swine Conference-Production at the Leading Edge, London, ON, Canada, 6–7 April 2005; pp. 135–138. [Google Scholar]
- Böhm, R. Disinfection and Hygiene in the Veterinary Field and Disinfection of Animal Houses and Transport Vehicles. Int. Biodeterior. Biodegrad. 1998, 41, 217–224. [Google Scholar] [CrossRef]
- Predicala, B.Z.; Alvarado, A.C. Alternatives for Animal Drinking and Barn Cleaning to Reduce Water Use in Swine Facilities. Can. Biosyst. Eng. 2014, 56, 5.7–5.15. [Google Scholar] [CrossRef]
| Type of Facility | Location/Equipment/Component | Material | Reference |
|---|---|---|---|
| Gestation | Gestation crate | Galvanized steel | [26] |
| Pens’ slatted floor | Precast concrete slats | [23,24] | |
| Farrowing | Slatted floor in farrowing crates | Plastisol or high-density polyethylene (HDPE) | [26] |
| Plastic-coated steel slats or plastic slotted flooring | [24] | ||
| Rough expanded metal, plastic coated, fiberglass reinforced t-slats, welded wire, woven wire or cast-iron grid | [23,26] | ||
| Central part of the slatted floor in farrowing crates | Cast-iron grid | [23,26] | |
| Farrowing crates (partition) | Galvanized steel | [26] | |
| Plywood, steel or concrete panels | [23] | ||
| Nursery | Slatted floor in farrowing crates | Plastisol or high-density polyethylene (HDPE) | [26] |
| Plastic-coated steel slats or plastic slotted flooring | [24] | ||
| Rough expanded metal, plastic coated, molded plastics, perforated metal planks, fiberglass reinforced t-slats, flattened expanded metal, woven wire or cast-iron grid | [23] | ||
| Grow-Finish | Walls | Concrete panels or blockwork or pre-stressed concrete panels | [25] |
| Solid floor | Concrete | ||
| Slatted floor | Precast concrete products (BS EN 12737:2004+A1:2007) | [23,24,25] | |
| Door | Polyvinyl chloride (PVC) | [26] | |
| Pen partition | PVC or galvanized steel | ||
| Plastic panel bolted to metal (stainless steel) posts or concrete blocks or concrete panel | [25] | ||
| Steel fence or concrete (cast in place or prefabricated panel) | [23] | ||
| High-density polyethylene (HDPE) | [24] | ||
| Feeder | Black plastic | [25] |
| Group | Material | Ra (µm) | Rq (nm) | Rp (µm) | Rv (µm) | Rz (µm) | Reference |
|---|---|---|---|---|---|---|---|
| Cement | Mortar | 4.32 | - | 10.5 | 10.16 | - | [53] |
| Mortar + hydrophobization | 1.0–2.5 | - | 3.0–5.5 | 4.2–7.9 | - | ||
| Lightweight mortar | 4.29 | - | 10.3 | 10.6 | - | [54] | |
| Plastics | High-density polyethylene (HDPE) | 0.02 | 24.2 | - | - | - | [55] |
| Ethylene vinyl acetate (EVA)/poly-vinylidene dichloride film | - | 13.86 | - | - | - | [56] | |
| Polyvinyl chloride (PVC) | 0.017 | 27 | - | - | - | [57] | |
| HDPE-coated steel (by thermal spraying) | 0.22 ± 0.02 | - | - | - | - | [51] | |
| Metals | 316 Stainless | 0.26 | 8 | - | - | - | [31,58] |
| Stainless steel 304 | - | 19 | - | - | - | [59] | |
| Engineered wood materials | Particleboard | 7.33–9.14 | - | - | - | 51–55 | [60] |
| Medium density fiberboard (MDF) | 2.57–3.81 | - | - | - | 27–34 | [48,60] | |
| Plywood | 4.30–8.59 | - | - | - | - | [61] |
| Group | Material | CA (°) | SFE (mJ·m−2) | Reference |
|---|---|---|---|---|
| Cement base | Mortar | 39.7 a | 59.64 | [53] |
| Mortar + hydrophobization | 103–113 a | 15–21 | ||
| Lightweight mortar | 12.1 a; 29.5 b | 81.1 | [54] | |
| Lightweight mortar + hydrophobization | 38–107 a; 42–98 b | 18–70 | ||
| Plastics | High-density polyethylene (HDPE) | 97 a; 46 b; 60 c | 37 | [63] |
| Ethylene vinyl acetate (EVA)/poly-vinylidene dichloride film | 88 a | - | [56] | |
| Polyvinyl chloride (PVC) | 66 a | - | [57] | |
| Metals | Stainless steel 316 | 86.8 | 26.9 | [31,35] |
| Stainless steel 304 | 80–85 | [59] | ||
| Engineered wood materials | Plywood | 33.8 a | [61] |
| Classification | Bacteria | CA (°) | γBV (mN/m) | Reference |
|---|---|---|---|---|
| Gram-positive aerobic to facultatively anaerobic cocci | Staphylococcus aureus | 25.3 ± 2.9 a | - | [68] |
| 18.5–26.4 b | 69.1 ± 0.6 | [69,70] | ||
| Staphylococcus epidermidis | 18–33 a | - | [71] | |
| 23.4 ± 0.5 b | 67.1 ± 0.3 | [70] | ||
| Enterococcus faecalis ATCC 6055 | 12.7 ± 1.1 b | 73 | [67] | |
| Streptococcus thermophilus ST69 | 28.0 ± 1.5 b | 64 | ||
| Gram-positive aerobic to microaerophilic non-spore-forming bacilli | Listeria monocytogenes | 26.1 ± 1.2 b | 66.3 ± 0.6 | [70] |
| Gram-positive aerobic spore-forming bacilli | Bacillus subtilis | 31.1 ± 9.6 a | - | [35] |
| Bacillus cereus | 8.1 ± 0.8 b | 76 | [67] | |
| Gram-positive anaerobic spore-forming bacilli | Clostridium proteolyticum DSM 3090T | 94.4 ± 0.9 b | 26 | |
| Gram-negative aerobic to facultatively anaerobic bacilli | Escherichia coli | 16.7–22.2 b | 67.9–69.7 | [67,70] |
| Gram-negative | Pseudomonas aeruginosa | 47.9 ± 8.7 a | - | [35] |
| Massilia timonae | 39.6 ± 3.7 a | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanishi, E.Y.; Palacios, J.H.; Godbout, S.; Fournel, S. Interaction between Biofilm Formation, Surface Material and Cleanability Considering Different Materials Used in Pig Facilities—An Overview. Sustainability 2021, 13, 5836. https://doi.org/10.3390/su13115836
Nakanishi EY, Palacios JH, Godbout S, Fournel S. Interaction between Biofilm Formation, Surface Material and Cleanability Considering Different Materials Used in Pig Facilities—An Overview. Sustainability. 2021; 13(11):5836. https://doi.org/10.3390/su13115836
Chicago/Turabian StyleNakanishi, Erika Yukari, Joahnn H. Palacios, Stéphane Godbout, and Sébastien Fournel. 2021. "Interaction between Biofilm Formation, Surface Material and Cleanability Considering Different Materials Used in Pig Facilities—An Overview" Sustainability 13, no. 11: 5836. https://doi.org/10.3390/su13115836
APA StyleNakanishi, E. Y., Palacios, J. H., Godbout, S., & Fournel, S. (2021). Interaction between Biofilm Formation, Surface Material and Cleanability Considering Different Materials Used in Pig Facilities—An Overview. Sustainability, 13(11), 5836. https://doi.org/10.3390/su13115836







