An Overview of the Chemical Characteristics, Bioactivity and Achievements Regarding the Therapeutic Usage of Acetogenins from Annona cherimola Mill.
Abstract
:1. Introduction
2. Classification of ACGs from ACM
2.1. Mono THF Acetogenins
2.2. Adjacent Bis-THF Acetogenins
2.3. Non-Adjacent Bis-THF Acetogenins
3. Methods Applied for the Purification and Isolation of the ACGs Described in ACM
4. Biological Activity and Anticarcinogenic Properties of the ACGs Isolated from ACM
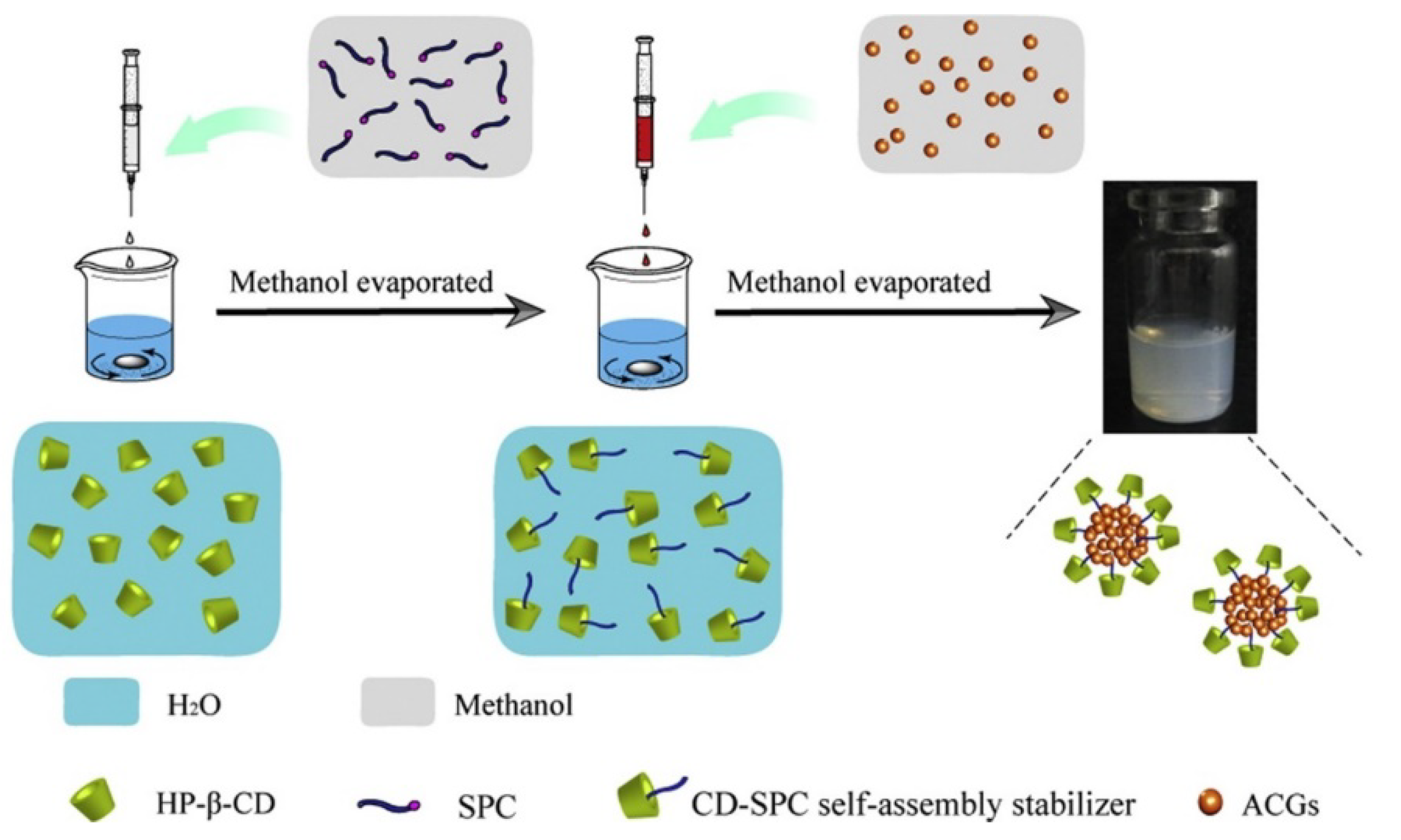
5. How to Apply ACGs from Annona Cherimola Mill.
6. Annona Cherimola Mill. Acetogenin Derivatives
7. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jamkhande, P.G.; Ajgunde, B.R.; Jadge, D.R. Annona cherimola Mill. (Custard apple): A review on its plant profile, nutritional values, traditional claims and ethnomedicinal properties. Orient. Pharm. Exp. Med. 2017, 17, 189–201. [Google Scholar] [CrossRef]
- Quílez, A.M.; Fernández-Arche, M.A.; García-Giménez, M.D.; De la Puerta, R. Potential therapeutic applications of the genus Annona: Local and traditional uses and pharmacology. J. Ethnopharmacol. 2018, 225, 244–270. [Google Scholar] [CrossRef] [PubMed]
- Popenoe, W. The native home of the Cherimoya. J. Hered. 1921, 12, 228–239. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Ramírez-Marez, M.V.; Montalvo-González, E.; Sánchez-Burgos, J.A. Cherimoya (Annona cherimola Mill.). In Fruit and Vegetable Phytochemicals; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 993–1002. [Google Scholar]
- García-Salas, P.; Verardo, V.; Gori, A.; Caboni, M.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of lipid composition of the two principal cherimoya cultivars grown in Andalusian Region. LWT Food Sci. Technol. 2016, 65, 390–397. [Google Scholar] [CrossRef]
- INEbase INe Instituto Nacional de Estadística. Available online: https://www.ine.es/dyngs/INEbase/es/categoria.htm?c=Estadistica_P&cid=1254735570567 (accessed on 24 January 2019).
- Boletín Oficial de la Junta de Andalucía (BOJA); Consejería de Agricultura y Pesca: Sevilla, Spain, 2002; núm. 124, 20553–20562.
- Palma, T.; Aguilera, J.; Stanley, D. A review of postharvest events in cherimoya. Postharvest Biol. Technol. 1993, 2, 187–208. [Google Scholar] [CrossRef]
- Díaz de Cerio, E.; Aguilera Saez, L.M.; Gómez Caravaca, A.M.; Verardo, V.; Fernández Gutiérrez, A.; Fernández, I.; Arráez Román, D. Characterization of bioactive compounds of Annona cherimola L. leaves using a combined approach based on HPLC-ESI-TOF-MS and NMR. Anal. Bioanal. Chem. 2018, 410, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- HORTO INFO. Available online: https://www.hortoinfo.es/index.php/6647-export-chirimoya-240118 (accessed on 24 January 2019).
- Le Ven, J.; Schmitz-Afonso, I.; Lewin, G.; Brunelle, A.; Touboul, D.; Champy, P. Identification of the environmental neurotoxins annonaceous acetogenins in an Annona cherimolia Mill. alcoholic beverage using HPLC-ESI-LTQ-Orbitrap. J. Agric. Food Chem. 2014, 62, 8696–8704. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Laganà, G.; Ficarra, S.; Tellone, E.; Leuzzi, U.; Galtieri, A.; Bellocco, E. Evaluation of the antioxidant and cytoprotective properties of the exotic fruit Annona cherimola Mill. (Annonaceae). Food Res. Int. 2011, 44, 2302–2310. [Google Scholar] [CrossRef]
- Colom, O.A.; Salvatore, A.; Willink, E.; Ordonez, R.; Isla, M.I.; Neske, A.; Bardon, A. Insecticidal, mutagenic and genotoxic evaluation of annonaceous acetogenins. Nat. Prod. Commun. 2010, 5, 391–394. [Google Scholar] [CrossRef] [Green Version]
- Liaw, C.C.; Wu, T.Y.; Chang, F.R.; Wu, Y.C. Historic perspectives on Annonaceous acetogenins from the chemical bench to preclinical trials. Planta Med. 2010, 76, 1390–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neske, A.; Hidalgo, J.R.; Cabedo, N.; Cortes, D. Acetogenins from Annonaceae family. Their potential biological applications. Phytochemistry 2020, 174, 112332. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Hongo, Y.; Tsukagoshi, Y.; Koshino, H. Structural determination of montanacin D by total synthesis. Org. Lett. 2008, 10, 4223–4226. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Polo, M.C.; González, M.C.; Estornell, E.; Sahpaz, S.; Cortes, D. Acetogenins from Annonaceae, inhibitors of mitochondrial complex I. Phytochemistry 1996, 42, 253–271. [Google Scholar] [CrossRef]
- Bermejo, A.; Figadère, B.; Zafra-Polo, M.C.; Barrachina, I.; Estornell, E.; Cortes, D. Acetogenins from Annonaceae: Recent progress in isolation, synthesis and mechanisms of action. Nat. Prod. Rep. 2005, 22, 269–303. [Google Scholar] [CrossRef]
- Liaw, C.-C.; Liou, J.-R.; Wu, T.-Y.; Chang, F.-R.; Wu, Y.-C. Acetogenins from Annonaceae. In Acetogenins from Annonaceae; Kinghorn, A., Falk, H., Gibbons, S.K.J., Eds.; Springer: Cham, Switzerland, 2016; pp. 113–230. [Google Scholar]
- Gutiérrez, M.T.; Durán, A.G.; Mejías, F.J.R.; Molinillo, J.M.G.; Megias, D.; Valdivia, M.M.; Macías, F.A. Bio-guided isolation of acetogenins from Annona cherimola deciduous leaves: Production of nanocarriers to boost the bioavailability properties. Molecules 2020, 25, 4861. [Google Scholar] [CrossRef] [PubMed]
- Qazi, A.K.; Siddiqui, J.A.; Jahan, R.; Chaudhary, S.; Walker, L.A.; Sayed, Z.; Jones, D.T.; Batra, S.K.; Macha, M.A. Emerging therapeutic potential of graviola and its constituents in cancers. Carcinogenesis 2018, 39, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Cao, Y.; Li, Z.; Wu, Z.; Mao, Y.; Chen, H.; Yao, Z.; Wang, L. Annonaceous acetogenin mimic AA005 suppresses human colon cancer cell growth in vivo through downregulation of Mcl-1. Acta Pharmacol. Sin. 2019, 40, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Dal, H.K.; Fang, Z.; Young, E.L.; Mi, H.W. Xylomaticin and gonionenin, cytotoxic annonaceous acetogenins from the seeds of Annona cherimolia. Nat. Prod. Sci. 2007, 13, 355–358. [Google Scholar]
- Kim, D.-H.; Woo, M.-H. Corrosolin and compound-2: Cytotoxic annonaceous acetogenins from the seeds of Annona cherimolia. Yakhak Hoeji 1999, 43, 584–590. [Google Scholar]
- Woo, M.H.; Kim, D.H.; Fotopoulos, S.S.; McLaughlin, J.L. Annocherin and (2,4)-cis- and trans-annocherinones, monotetrahydrofuran annonaceous acetogenins with a C-7 carbonyl group from Annona cherimolia seeds. J. Nat. Prod. 1999, 62, 1250–1255. [Google Scholar] [CrossRef]
- Kim, D.H.; Son, J.K.; Woo, M.H. Annomocherin, annonacin and annomontacin: A novel and two known Bioactive mono-tetrahydrofuran annonaceous acetogenins from Annona cherimolia seeds. Arch. Pharm. Res. 2001, 24, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Sahpaz, S.; González, M.C.; Hocquemiller, R.; Zafra-Polo, M.C.; Cortes, D. Annosenegalin and annogalene: Two cytotoxic mono-tetrahydrofuran acetogenins from Annona senegalensis and Annona cherimolia. Phytochemistry 1996, 42, 103–107. [Google Scholar] [CrossRef]
- Woo, M.-H.; Chung, S.-O.; Kim, D.-H. cis-Annonacin and (2,4)-cis-and trans-isoannonacins: Cytotoxic monotetrahydrofuran annonaceous acetogenins from the seeds of Annona cherimolia. Arch. Pharm. Res. 1999, 22, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Ma, E.S.; Suk, K.D.; Son, J.K.; Lee, J.S.; Woo, M.H. Annomolin and annocherimolin, new cytotoxic annonaceous acetogenins from Annona cherimolia seeds. J. Nat. Prod. 2001, 64, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Son, J.K.; Kim, D.H.; Woo, M.H. Two New Epimeric pairs of acetogenins bearing a carbonyl group from Annona cherimolia Seeds. J. Nat. Prod. 2003, 66, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Cortes, D.; Myint, S.H.; Dupont, B.; Davoust, D. Bioactive acetogenins from seeds of Annona cherimolia. Phytochemistry 1993, 32, 1475–1482. [Google Scholar] [CrossRef]
- Barrachina, I.; Neske, A.; Granell, S.; Bermejo, A.; Chahboune, N.; El Aouad, N.; Alvarez, O.; Bardon, A.; Zafra-Polo, M.C. Tucumanin, a β-Hydroxy-γ-lactone Bistetrahydrofuranic Acetogenin from Annona cherimolia, is a Potent Inhibitor of Mitochondrial Complex I. Planta Med. 2004, 70, 866–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortes, D.; Myint, S.H.; Hocquemiller, R. Molvizarin and motrilin: Two novel cytotoxic bis-tetrahydro-furanic γ-lactone acetogenins from Annona cherimolia. Tetrahedron 1991, 47, 8195–8202. [Google Scholar] [CrossRef]
- Duret, P.; Gromek, D.; Hocquemiller, R.; Cavé, A.; Cortes, D. Isolation and Structure of Three New Bis-Tetrahydrofuran Acetogenins from the Roots of Annona cherimolia. J. Nat. Prod. 1994, 57, 911–916. [Google Scholar] [CrossRef]
- Ríos, J.; Cortes, D.; Valverde, S. Acetogenins, Aporphinoids, and Azaanthraquinone from Annona cherimolia Seeds. Planta Med. 1989, 55, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chang, F.R.; Chiu, H.F.; Wu, M.J.; Wu, Y.C. Aromin-A, an annonaceous acetogenin from Annona cherimola. Phytochemistry 1999, 51, 429–433. [Google Scholar] [CrossRef]
- Alali, F.Q.; Liu, X.-X.; McLaughlin, J.L. Annonaceous acetogenins: Recent Progress. J. Nat. Prod. 1999, 62, 504–540. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Ferrigni, N.; Putnam, J.; Jacobsen, L.; Nichols, D.; McLaughlin, J. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Shah, D.; Seth, N.; Pandey, S.; Yadav, J. Annonaceous acetogenins: The unrevealed area for cytotoxic and pesticidal activities. Syst. Rev. Pharm. 2011, 2, 104. [Google Scholar] [CrossRef] [Green Version]
- Mulia, K.; Krisanti, E.; Maulana, T.; Dianursanti, D. Selective polarity-guided extraction and purification of acetogenins in Annona muricata L. leaves. Int. J. Technol. 2015, 7, 1221–1227. [Google Scholar] [CrossRef] [Green Version]
- Oberlies, N.H.; Jones, J.L.; Corbett, T.H.; Fotopoulos, S.S.; McLaughlin, J.L. Tumor cell growth inhibition by several Annonaceous acetogenins in an in vitro disk diffusion assay. Cancer Lett. 1995, 96, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Jacobo-Herrera, N.; Pérez-Plasencia, C.; Castro-Torres, V.A.; Martínez-Vázquez, M.; González-Esquinca, A.R.; Zentella-Dehesa, A. Selective acetogenins and their potential as anticancer agents. Front. Pharmacol. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.; Xu, S.; Wang, Y.; Li, X.; Cai, B.; Fan, N. Antitumor activity of annonaceous acetogenins in HepS and S180 xenografts bearing mice. Bioorg. Med. Chem. Lett. 2012, 22, 2717–2719. [Google Scholar] [CrossRef]
- Schlie-Guzmán, M.A.; García-Carrancá, A.; González-Esquinca, A.R. In vitro and in vivo antiproliferative activity of laherradurin and cherimolin-2 of annona diversifolia saff. Phyther. Res. 2009, 23, 1128–1133. [Google Scholar] [CrossRef]
- Yuan, S.-S.F.; Chang, H.-L.; Chen, H.-W.; Yeh, Y.-T.; Kao, Y.-H.; Lin, K.-H.; Wu, Y.-C.; Su, J.-H. Annonacin, a mono-tetrahydrofuran acetogenin, arrests cancer cells at the G1 phase and causes cytotoxicity in a Bax- and caspase-3-related pathway. Life Sci. 2003, 72, 2853–2861. [Google Scholar] [CrossRef]
- Roduan, M.R.M.; Hamid, R.A.; Mohtarrudin, N. Modulation of cancer signalling pathway(s) in two -stage mouse skin tumorigenesis by annonacin. BMC Complement. Altern. Med. 2019, 19, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.-Q.; Min, B.-S.; Li, Y.; Nakamura, N.; Qin, G.-W.; Li, C.-J.; Hattori, M. Annonaceous acetogenins from the Leaves of Annona montana. Bioorg. Med. Chem. 2002, 10, 561–565. [Google Scholar] [CrossRef]
- He, K.; Zeng, L.; Ye, Q.; Shi, G.; Oberlies, N.H.; Zhao, G.-X.; Njoku, C.J.; McLaughlin, J.L. Comparative SAR Evaluations of Annonaceous Acetogenins for Pesticidal Activity. Pestic. Sci. 1997, 49, 372–378. [Google Scholar] [CrossRef]
- Lannuzel, A.; Michel, P.P.; Höglinger, G.U.; Champy, P.; Jousset, A.; Medja, F.; Lombès, A.; Darios, F.; Gleye, C.; Laurens, A.; et al. The mitochondrial complex i inhibitor annonacin is toxic to mesencephalic dopaminergic neurons by impairment of energy metabolism. Neuroscience 2003, 121, 287–296. [Google Scholar] [CrossRef]
- Potts, L.F.; Luzzio, F.A.; Smith, S.C.; Hetman, M.; Champy, P.; Litvan, I. Annonacin in Asimina triloba fruit: Implication for neurotoxicity. Neurotoxicology 2012, 33, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Champy, P.; Melot, A.; Guérineau, V.; Gleye, C.; Fall, D.; Höglinger, G.U.; Ruberg, M.; Lannuzel, A.; Laprévote, O.; Laurens, A.; et al. Quantification of acetogenins in Annona muricata linked to atypical Parkinsonism in Guadeloupe. Mov. Disord. 2005, 20, 1629–1633. [Google Scholar] [CrossRef]
- Awodele, O.; Ishola, I.O.; Ikumawoyi, V.O.; Akindele, A.J.; Akintonwa, A. Toxicological evaluation of the lyophilized fruit juice extract of Annona muricata Linn. (Annonaceae) in rodents. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Chang, F.R.; Liaw, C.C.; Wu, Y.C.; Bastow, K.F.; Lee, K.H. Acetogenins as selective inhibitors of the human ovarian 1A9 tumor cell line. J. Med. Chem. 2003, 46, 3185–3188. [Google Scholar] [CrossRef] [PubMed]
- Duval, R.A.; Lewin, G.; Peris, E.; Chahboune, N.; Garofano, A.; Dröse, S.; Cortes, D.; Brandt, U.; Hocquemiller, R. Heterocyclic analogues of squamocin as inhibitors of mitochondrial complex I. On the role of the terminal lactone of annonaceous acetogenins. Biochemistry 2006, 45, 2721–2728. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, D.; Colom, O.Á.; Bardón, A.; Neske, A. Insecticidal effects of acetogenins from rollinia occidentalis seed extract. Nat. Prod. Commun. 2012, 7, 1645–1646. [Google Scholar] [CrossRef] [Green Version]
- Arriaga, Â.M.C.; Feitosa, E.M.A.; Lemos, T.L.G.; Santiago, G.M.P.; Lima, J.Q.; De Oliveira, M.C.F.; e Vasconcelos, J.N.; Rodrigues, F.E.A.; Gomes, T.B.M.; Braz-Filho, R. Chemical constituents and insecticidal activity of Rollinia leptopetala (Annonaceae). Nat. Prod. Commun. 2008, 3, 1934578X0800301021. [Google Scholar] [CrossRef] [Green Version]
- Luna-Cazares, L.M.; Gonzalez-Esquinca, A.R. Susceptibility of complete bacteria and spheroplasts of Escherichia coli, Pseudomonas aeruginosa and Salmonella typhi to rolliniastatin-2. Nat. Prod. Res. 2010, 24, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Hopp, D.C.; Conway, W.D.; McLaughlin, J.L. Using countercurrent chromatography to assist in the purification of new Annonaceous acetogenins from Annona squamosa. Phytochem. Anal. 1999, 10, 339–347. [Google Scholar] [CrossRef]
- Duret, P.; Waechter, A.I.; Margraff, R.; Foucault, A.; Hocquemiller, R.; Cave, A. High-speed countercurrent chromatography: A promising method for the separation of the Annonaceous acetogenins. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 627–635. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Dhakshanamurthy, T.; Kim, K.J.; Hasan, N.; Kwon, S.J.; Chun, S. Sustainable ecofriendly phytoextract mediated one pot green recovery of chitosan. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waechter, A.I.; Yaluff, G.; Inchausti, A.; De Arias, A.R.; Hocquemiller, R.; Cavé, A.; Fournet, A. Leishmanicidal and trypanocidal activities of acetogenins isolated from Annona glauca. Phyther. Res. 1998, 12, 541–544. [Google Scholar] [CrossRef]
- Sahpaz, S.; Laurens, A.; Hocquemiller, R.; Cavé, A.; Cortes, D. Senegalene, une nouvelle acCtogCnine olkfinique mono-tktrahydrofuranique des graines d’Annona senegalensisl. Can. J. Chem. 1994, 72, 3–6. [Google Scholar] [CrossRef]
- Alfonso, D.; Johnson, H.A.; Colman-Saizarbitoria, T.; Presley, C.P.; McCabe, G.P.; McLaughlin, J.L. SARs of annonaceous acetogenins in rat liver mitochondria. Nat. Toxins 1996, 4, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, S.; Gu, Z.M.; Miesbauer, L.R.; Smith, D.L.; Wood, K.V.; Evert, D.R.; McLaughlin, J.L. Parvifloracin and parviflorin: Cytotoxic bistetrahydrofuran acetogenins with 35 carbons from Asimina parviflora (Annonaceae). Can. J. Chem. 1994, 72, 287–293. [Google Scholar] [CrossRef]
- Hidalgo, J.R.; Parellada, E.A.; Blessing, L.D.T.; Bardón, A.; Ameta, K.L.; Vera, N.; Neske, A. Natural and derivatized acetogenins promising for the control of Spodoptera frugiperda smith. J. Agric. Chem. Environ. 2016, 05, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Li, Y.; Li, Y.; Xiao, Y.; Kuang, H.; Wang, X. Annonaceous acetogenins nanosuspensions stabilized by PCL–PEG block polymer: Significantly improved antitumor efficacy. Int. J. Nanomed. 2016, 11, 3239–3253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ao, H.; Gao, Y.; Guo, Y.; Han, M.; Li, H.; Wang, X.; Zhou, X. Nanoparticle with Effect-Enhancing and Toxicity-Reducing Effect on Synergistic and Attenuating Effects on Annonaceous Acetogenins Drugs, and Preparation Method and Application Thereof. CN109223769A, 18 January 2019. [Google Scholar]
- Ao, H.; Fu, J.; Guo, Y.; Han, M.; Li, H.; Wang, X.; Wang, Y. Pharmaceutical Composition for Selective Killing or Efficient killing at nm Level of Drug-Resistant Tumors and Use Thereof. WO2020156329, 6 August 2020. [Google Scholar]
- Hong, J.; Li, X.; Li, Y.; Wang, Y. Nanosuspension of Annonaceous Acetogenin Drugs and Preparation Method of Nanosuspension. CN106420604A, 22 February 2017. [Google Scholar]
- Li, H.; Li, Y.; Ao, H.; Bi, D.; Han, M.; Guo, Y.; Wang, X. Folate-targeting annonaceous acetogenins nanosuspensions: Significantly enhanced antitumor efficacy in HeLa tumor-bearing mice. Drug Deliv. 2018, 25, 880–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mejías, F.J.R.; Gutiérrez, M.T.; Durán, A.G.; Molinillo, J.M.G.; Valdivia, M.M.; Macías, F.A. Provitamin supramolecular polymer micelle with pH responsiveness to control release, bioavailability enhancement and potentiation of cytotoxic efficacy. Colloids Surfaces B Biointerfaces 2019, 173, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, Y.; Cai, S.; Zhuo, R.; Zhang, X.; Liu, L. A facile one-pot construction of supramolecular polymer micelles from α-cyclodextrin and poly (ε-caprolactone ). Angew. Chem. 2008, 47, 5573–5576. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Regulation (EU) 2018/1023 of 23 July 2018; EU: Brussels, Belgium, 2018. [Google Scholar]
- Hong, J.; Sun, Z.; Li, Y.; Guo, Y.; Liao, Y.; Liu, M.; Wang, X. Folate-modified Annonaceous acetogenins nanosuspensions and their improved antitumor efficacy. Int. J. Nanomed. 2017, 12, 5053–5067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Li, Y.; Xiao, Y.; Li, Y.; Guo, Y.; Kuang, H.; Wang, X. Annonaceous acetogenins (ACGs) nanosuspensions based on a self-assembly stabilizer and the significantly improved anti-tumor efficacy. Colloids Surfaces B Biointerfaces 2016, 145, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Liu, Y.; Wang, X. Annonaceous acetogenins Nanoparticles Taking Cyclodextrin and Lecithin as Vectors as well as Preparation Method and Application of Annonaceous acetogenins Nanoparticles. CN Patent CN106389385A, 15 February 2017. [Google Scholar]
- Konno, H.; Hiura, N.; Makabe, H.; Abe, M.; Miyoshi, H. Synthesis and mitochondrial complex I inhibition of dihydroxy-cohibin A, non-THF annonaceous acetogenin analogue. Bioorg. Med. Chem. Lett. 2004, 14, 629–632. [Google Scholar] [CrossRef]
- Fujita, D.; Ichimaru, N.; Abe, M.; Murai, M.; Hamada, T.; Nishioka, T.; Miyoshi, H. Synthesis of non-THF analogs of acetogenin toward simplified mimics. Tetrahedron Lett. 2005, 46, 5775–5779. [Google Scholar] [CrossRef]
- Rodier, S.; Le Huérou, Y.; Renoux, B.; Doyon, J.; Renard, P.; Pierré, A.; Gesson, J.-P.; Grée, R. Synthesis and cytotoxic activity of acetogenin analogues. Bioorg. Med. Chem. Lett. 2000, 10, 1373–1375. [Google Scholar] [CrossRef]
- Yu, B.; Yao, Z.J. A gram-scale laboratory synthesis of Annonaceous acetogenin mimic AA005. Chin. Chem. Lett. 2021, 32, 408–412. [Google Scholar] [CrossRef]
- Huang, G.R.; Jiang, S.; Wu, Y.L.; Jin, Y.; Yao, Z.J.; Wu, J.R. Induction of Cell Death of Gastric Cancer Cells by a Modified Compound of the Annonaceous Acetogenin Family. ChemBioChem 2003, 4, 1216–1221. [Google Scholar] [CrossRef]
- Perciavalle, R.M.; Opferman, J.T. Delving deeper: MCL-1’s contributions to normal and cancer biology. Trends Cell Biol. 2013, 23, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.X.; Huang, G.R.; Zhang, H.M.; Jiang, S.; Wu, J.R.; Yao, Z.J. A structure-activity guided strategy for fluorescent labeling of annonaceous acetogenin mimetics and their application in cell biology. ChemBioChem 2007, 8, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, Y.; Chen, X.G.; Hu, T.S.; Wu, Y.L.; Yao, Z.J. Parallel fragment assembly strategy towards multiple-ether mimicry of anticancer Annonaceous Acetogenins. Angew. Chem. Int. Ed. 2004, 43, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.R.; King, E.F.B.; Menzies, S.K.; Fraser, A.L.; Tulloch, L.B.; Zacharova, M.K.; Smith, T.K.; Florence, G.J. Simplifying nature: Towards the design of broad spectrum kinetoplastid inhibitors, inspired by acetogenins. Bioorganic Med. Chem. 2017, 25, 6126–6136. [Google Scholar] [CrossRef] [Green Version]
- Ichimaru, N.; Murai, M.; Kakutani, N.; Kako, J.; Ishihara, A.; Nakagawa, Y.; Nishioka, T.; Yagi, T.; Miyoshi, H. Synthesis and characterization of new piperazine-type inhibitors for mitochondrial NADH-ubiquinone oxidoreductase (complex I). Biochemistry 2008, 47, 10816–10826. [Google Scholar] [CrossRef] [PubMed]
- Bachan, S.; Tony, K.A.; Kawamura, A.; Montenegro, D.; Joshi, A.; Garg, H.; Mootoo, D.R. Synthesis and anti-tumor activity of carbohydrate analogues of the tetrahydrofuran containing acetogenins. Bioorganic Med. Chem. 2013, 21, 6554–6564. [Google Scholar] [CrossRef] [Green Version]
- Yabunaka, H.; Abe, M.; Kenmochi, A.; Hamada, T.; Nishioka, T.; Miyoshi, H. Synthesis and inhibitory activity of ubiquinone-acetogenin hybrid inhibitor with bovine mitochondrial complex I. Bioorganic Med. Chem. Lett. 2003, 13, 2385–2388. [Google Scholar] [CrossRef]
- Ichimaru, N.; Abe, M.; Kenmochi, A.; Hamada, T.; Nishioka, T.; Miyoshi, H. Synthesis of 13C-Labeled Ubiquinone-Acetogenin Gybrid Inhibitors of Mitochondrial Complex I. J. Pestic. Sci. 2004, 29, 127–129. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.F.; Wu, P.; Jiang, Z.H.; Wei, X.Y. Synthesis and tumor cell growth inhibitory activity of biotinylated annonaceous acetogenins. Eur. J. Med. Chem. 2014, 71, 219–228. [Google Scholar] [CrossRef] [PubMed]





| CAS Number | Name | Other Names | OH Positions | THF System | Relative THF Configuration | Type of γ-Lactone Ring | Molecular Formula | Organ | Number of Biological Studies (Scifinder Database) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 133352-34-8 | Corossolin | Corossoline | 10,15,20 | T-A | th/t/th | L-A | C35H64O6 | Seeds | 15 | [23,24] |
| 246165-35-5 | Annocherin | 4,15,20 | T-A | th/t/th | L-A | C35H62O7 | Seeds | 2 | [25] | |
| 111035-65-5 | Annonacin † | 4,10,15,20 | T-A | th/t/th | L-A | C35H64O7 | Seeds, deciduous leaves | 133 | [20,26] | |
| 130853-76-8 | Annonacin-A † | 4,10,15,20 | T-A | th/t/er | L-A | C35H64O7 | Seeds | 17 | [27] | |
| 137550-92-6 | Annomontacin | 4,10,17,22 | T-A | th/t/th | L-A | C37H68O7 | Seeds | 18 | [26] | |
| 155969-86-1 | Xylomaticin | 4,10,15,20 | T-A | th/t/th | L-A | C37H68O7 | Seeds | 7 | [23] | |
| 155969-65-6 | Gonionenin | (2,4-cis)-Gonioneninone | 4,10,13,18 | T-A | th/t/th | L-A | C37H66O7 | Seeds | 8 | [23] |
| 176200-77-4 | Annosenegalin † | 4,10,15,20 | T-A | th/t/er | L-A | C37H68O7 | Seeds | 1 | [27] | |
| 172586-13-9 | Cis annonacin | 4,10,15,20 | T-A | th/c/th | L-A | C35H64O7 | Seeds | 10 | [28] | |
| 344940-10-9 | Annocherimolin | 4,9,13,18 | T-A | th/t/th | L-A | C37H66O7 | Seeds | 1 | [29] | |
| 373362-55-1 | Annomocherin | 4,10,15,20 | T-A | th/t/th | L-A | C35H62O7 | Seeds | 1 | [26] | |
| 134955-48-9 | Gigantetrocin † | 4,14,17,18 | T-B | t/th-th | L-A | C35H64O7 | Seeds | 13 | [27] | |
| 344940-09-6 | Annomolin | 4,7,8,18 | T-B | th-t/th | L-A | C35H64O7 | Seeds | 1 | [29] | |
| 152784-18-4 and 152784-19-5 | cis/trans isoannonacins | cis-Annonacin-A-one and trans-Annonacin-A-one | 10,15,20 | T-A | th/t/th | L-B1 | C35H64O7 | Seeds | 7 | [28] |
| 246165-37-7 and 246165-38-8 | (2,4)-cis- and trans annocherinones | 15,20 | T-A | th/t/th | L-B1 | C35H62O7 | Seeds | 2 | [25] | |
| 627518-99-4 and 627519-01-1 | Annomolon-A + 34-epi | 15,20,34 | T-A | th/t/th | L-D | C35H62O7 | Seeds | 1 | [30] | |
| 627519-00-0 and 627519-02-2 | Annomolon-B + 34-epi | 4,15,20,34 | T-A | th/t/th | L-D | C35H62O8 | Seeds | 1 | [30] | |
| 139294-55-6 | Jetein † | 10,15,20 | T-A | th/t/er | L-C | C35H66O7 | Seeds | 2 | [31] | |
| 102989-24-2 | Asimicin | Squamocin H | 4,15,24 | T-C | th/t/th/t/th | L-A | C37H66O7 | Seeds | 57 | [32] |
| 123123-32-0 | Bullatacin † | Annonareticin; LI 12105; Rolliniastatin 2; Squamocin G | 4,15,24 | T-C | th/t/th/t/er | L-A | C37H66O7 | Seeds | 229 | [31] |
| 138551-26-5 | Molvizarin † | 4,13,22 | T-C | th/t/th/t/er | L-A | C35H62O7 | Seeds, deciduous leaves | 17 | [20,33] | |
| 194413-43-9 | Annonisin † | 4,8,13,22 | T-C | th/t/th/t/th | L-A | C35H62O8 | Deciduous leaves | 3 | [20] | |
| 120298-30-8 | Squamocin † | Annonin I; Squamocin A | 15,24,28 | T-C | th/t/th/t/er | L-A | C37H66O7 | Seeds, roots | 162 | [31,34] |
| 138551-27-6 | Motrilin † | 15,24,29 | T-C | th/t/th/t/er | L-A | C37H66O7 | Seeds, deciduous leaves | 28 | [20,33] | |
| 159934-23-3 | Squamocin B | 13,22,26 | T-C | th/t/th/t/er | L-A | C35H62O7 | Seeds | 10 | [32] | |
| 123012-00-0 | Bullatacinone † | Isorolliniastatin-2 | 15,24 | T-C | th/t/th/t/er | L-B1 | C37H66O7 | Roots | 69 | [34] |
| 161169-72-8 | Isomolvizarin-1 † | 13,22 | T-C | th/t/th/t/er | L-B1 | C35H62O7 | Roots | 0 | [34] | |
| 158252-75-6 | Isomolvizarin-2 † | 13,22 | T-C | th/t/th/t/th | L-B1 | C35H62O7 | Roots | 1 | [34] | |
| 125276-75-7 | Laherradurin † | 15,24,35 | T-C | th/t/th/t/er | L-C | C37H68O7 | Seeds | 13 | [35] | |
| 139294-54-5 | Itrabin † | 13,22,33 | T-C | th/t/th/t/er | L-C | C35H64O7 | Seeds | 8 | [33] | |
| 832683-48-4 | Tucumanin | 15,24,35 | T-C | th/t/th/t/th | L-C | C37H68O7 | Seeds | 3 | [32] | |
| 92280-15-4 | Otivarin † | 16,19,24,35 | T-D | t/th-th/t/er | L-C | C37H68O8 | Seeds | 6 | [31] | |
| 92280-14-3 | Cherimolin-1 † | Bullatalicin | 4,16,19,24 | T-D | t/th-th/t/er | L-A | C37H66O8 | Seeds, deciduous leaves | 16 | [20,31] |
| 151637-38-6 | Cherimolin-2 † | Bullatanocin | 4,16,19,24 | T-D | t/th-th/t/th | L-A | C37H66O8 | Seeds | 10 | [31] |
| 125620-82-8 | Almunequin † | Squamostatin-A | 16,19,24,28 | T-D | t/th-th/t/er | L-A | C37H66O8 | Seeds, roots | 12 | [31,34] |
| 241822-07-1 | Aromin-A | 15,20 | T-D | t-th/t/er | L-A | C35H60O7 | Stems | 1 | [36] | |
| 157966-80-8 | Isocherimolin-1 † | 16,19,24 | T-D | th-th/t/er | L-B1 | C37H66O8 | Roots | 2 | [34] |
| Method | Eluent System | Isolated ACGs | Organ | Reference |
|---|---|---|---|---|
| Flash chromatography | CH2Cl2-EtOAc-MeOH (4:14:1) | Molvizarin †, motrilin †, bullatacin †, squamocin †, cherimolin-1 †, cherimolin-2 †, almunequin †, itrabin †, laherradurin †, otivarin †, jetein † | Seeds | [31,33] |
| Flash chromatography | CH2Cl2-EtOAc-MeOH (20:19:1) and CH2Cl2-MeOH (24:1) | Annosenegalin †, annonacin-A †, gigantetrocin † | Seeds | [27] |
| Open column chromatography HPLC | MeOH-Hexane (1:1) ACN-H2O (80:20) | Annomolin ‡, Annocherimolin ‡ | Seeds | [29] |
| Open column chromatography HPLC | Hexane-CHCl3 and CHCl3-MeOH gradients ACN-H2O (80:20) | Annomolon A + 34-epi-annomolon A ‡, annomolon B + 34-epi-annomolon B ‡ | Seeds | [30] |
| Open column chromatography HPLC | Hexane-CHCl3-MeOH gradient ACN-H2O (85:15) | Annocherin ‡, (2,4)-cis- and trans-annocherinones ‡ | Seeds | [25] |
| Open column chromatography HPLC | Hexane-EtOAc gradient CH2Cl2-MeOH (95:5) MeOH-H2O-THF (80:20:5) | Molvizarin §, motrilin §, bullatacin §, tucumanin §, squamocin §, squamocin B §, laherradurin §, itrabin §, cherimolin-1 §, almunequin §, asimicin § | Seeds | [32] |
| Open column chromatography HPLC | Hexane-CHCl3-MeOH gradient ACN- H2O (85:15) | cis-Annonacin ‡ and (2,4)-cis- and trans-lsoannonacins ‡ | Seeds | [28] |
| Open column chromatography HPLC | Hexane-CHCl3-MeOH gradient ACN-H2O (85:15) | Annomocherin ‡, annonacin ‡, annomontacin ‡ | Seeds | [26] |
| Open column chromatography HPLC | Hexane-CHCl3-MeOH gradient ACN-H2O (85:15) | Xylomaticin ‡, gonionenin ‡ | Seeds | [23] |
| Open column chromatography PTLC | Hexane-acetone gradient EtOAc-hexane-acetone (10:10:1) EtOAc-acetone (10:1) CHCl3-MeOH (10:1) EtOAc-acetone (15:1) | Aromin-A #, squamocin # | Stems | [36] |
| Open column chromatography HPLC | CH2Cl2-MeOH (97:3) ACN-H2O-THF (70:30:2) | Isocherimolin-1 †, isomolvizarin-1 †, isomolvizarin-2 †, bullatacinone †, squamocin †, almunequin † | Roots | [34] |
| Vacuum column chromatography Open column chromatography HPLC PTLC | H2O-MeOH gradient EtOAc-Hexane gradient Hexane-EtOAc-acetone (7:2:1) CHCl3-MeOH-acetone-H2O (13:7:1:3) | Molvizarin †, cherimolin-1 †, motrilin †, annonacin †, annonisin † | Deciduous leaves | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durán, A.G.; Gutiérrez, M.T.; Mejías, F.J.R.; Molinillo, J.M.G.; Macías, F.A. An Overview of the Chemical Characteristics, Bioactivity and Achievements Regarding the Therapeutic Usage of Acetogenins from Annona cherimola Mill. Molecules 2021, 26, 2926. https://doi.org/10.3390/molecules26102926
Durán AG, Gutiérrez MT, Mejías FJR, Molinillo JMG, Macías FA. An Overview of the Chemical Characteristics, Bioactivity and Achievements Regarding the Therapeutic Usage of Acetogenins from Annona cherimola Mill. Molecules. 2021; 26(10):2926. https://doi.org/10.3390/molecules26102926
Chicago/Turabian StyleDurán, Alexandra G., M. Teresa Gutiérrez, Francisco J. R. Mejías, José M. G. Molinillo, and Francisco A. Macías. 2021. "An Overview of the Chemical Characteristics, Bioactivity and Achievements Regarding the Therapeutic Usage of Acetogenins from Annona cherimola Mill." Molecules 26, no. 10: 2926. https://doi.org/10.3390/molecules26102926
APA StyleDurán, A. G., Gutiérrez, M. T., Mejías, F. J. R., Molinillo, J. M. G., & Macías, F. A. (2021). An Overview of the Chemical Characteristics, Bioactivity and Achievements Regarding the Therapeutic Usage of Acetogenins from Annona cherimola Mill. Molecules, 26(10), 2926. https://doi.org/10.3390/molecules26102926









