The Root Extract of Scutellaria baicalensis Induces Apoptosis in EGFR TKI-Resistant Human Lung Cancer Cells by Inactivation of STAT3
Abstract
1. Introduction
2. Results
2.1. Identification of Baicalin in the Ethanol Extract of SB by HPLC-MS Analysis
2.2. Establishment of EGFR TKI-Resistant PC9 Cell Lines
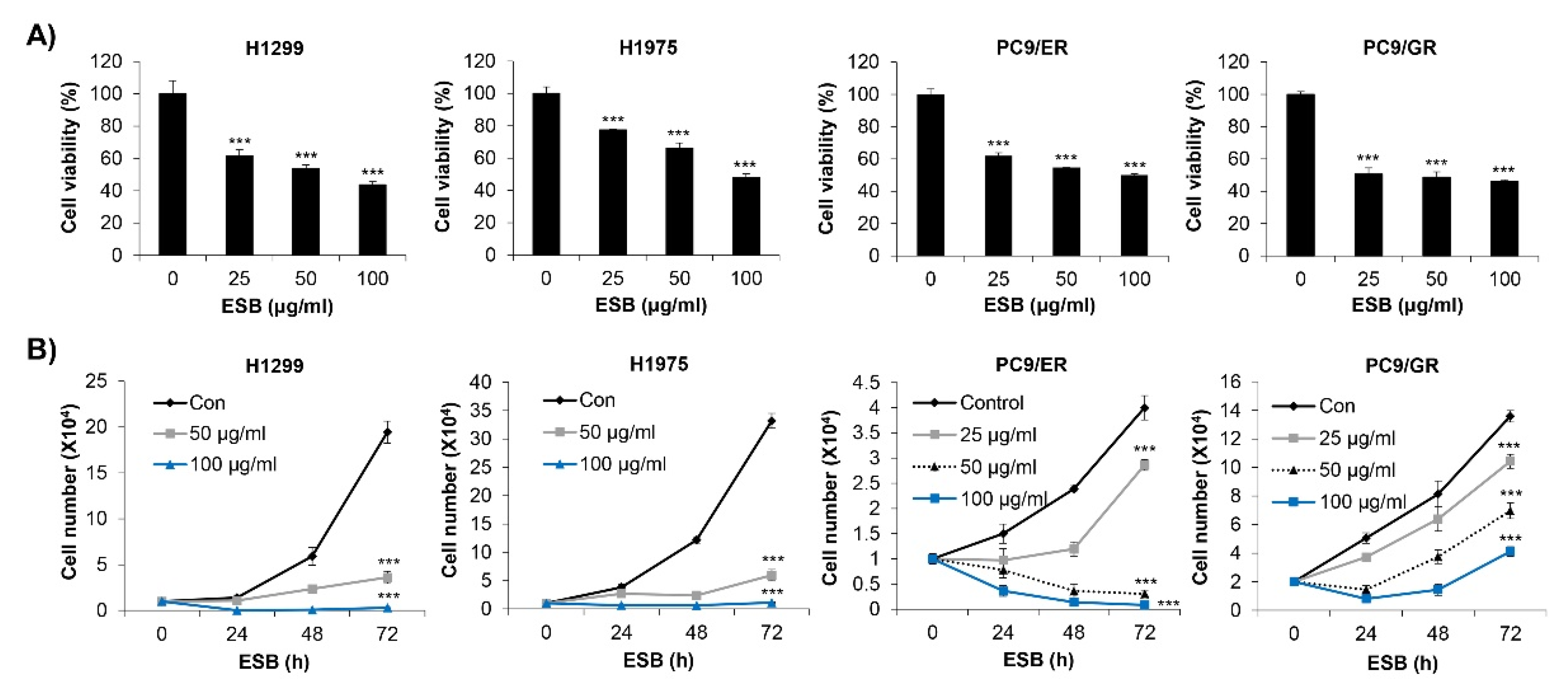
2.3. Inhibition of Cell Growth by ESB in EGFR TKI-Resistant Cell Lines
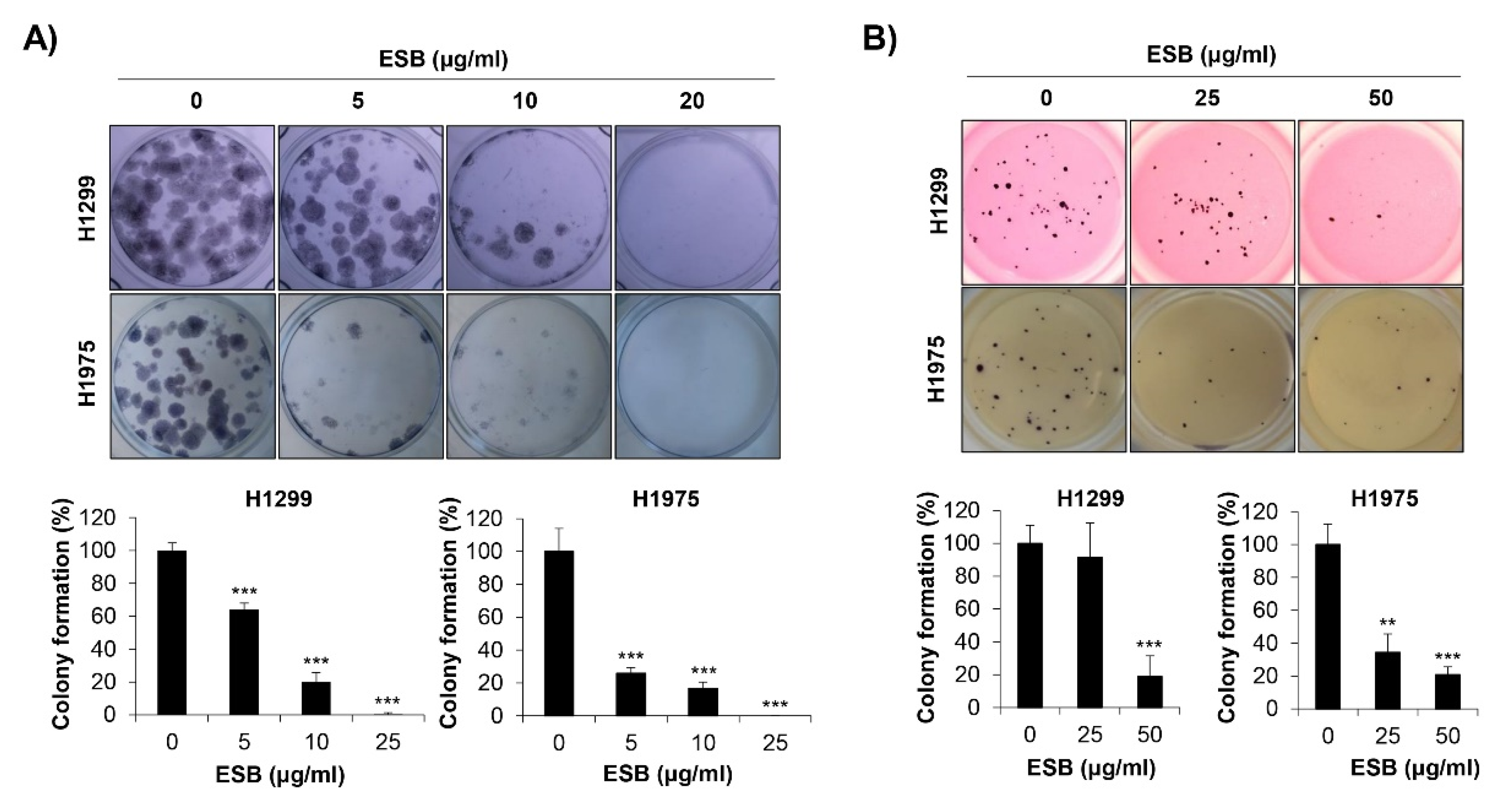
2.4. Inhibition of Colony Formation by ESB in EGFR TKI-Resistant Cell Lines
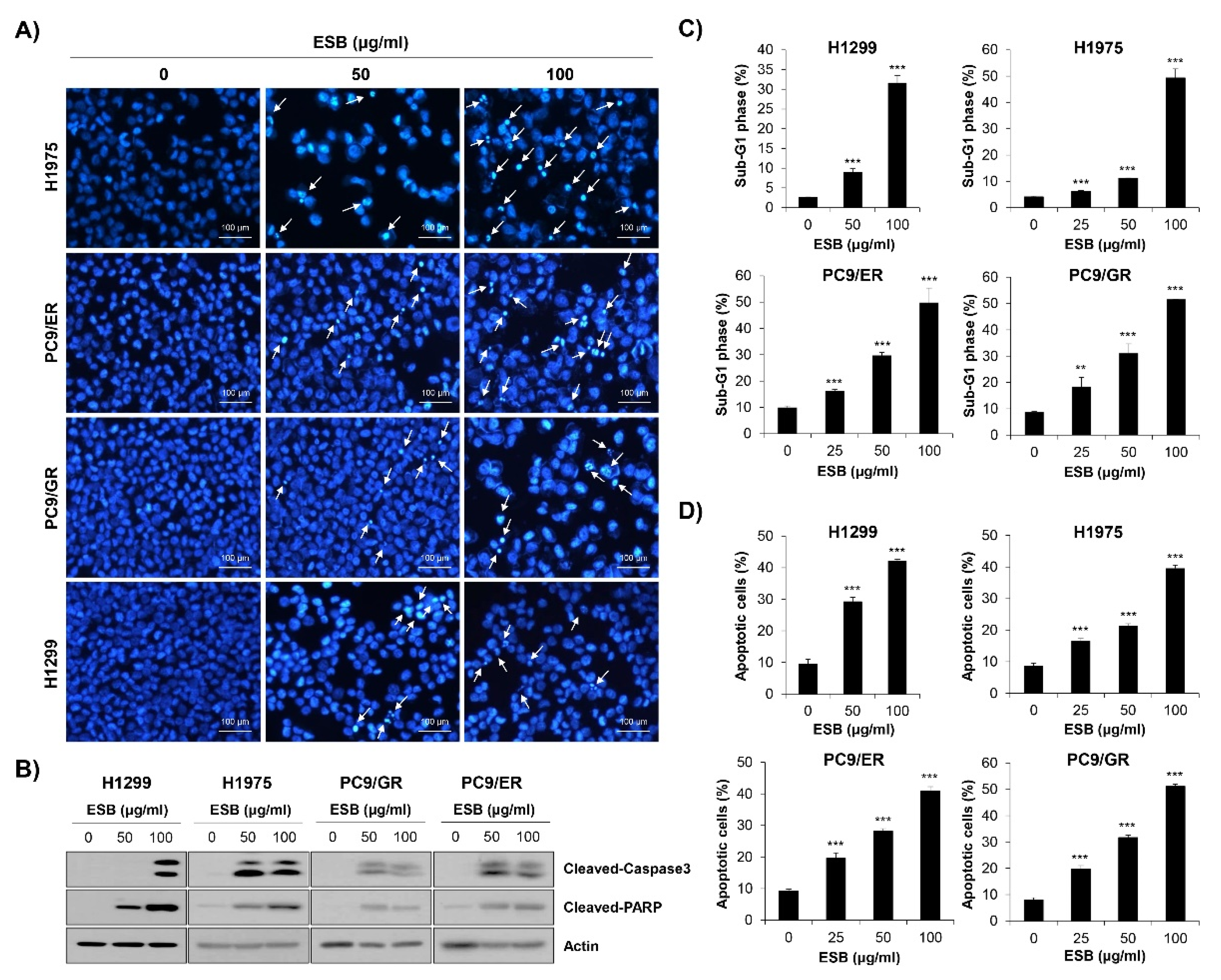
2.5. Induction of Apoptosis by ESB in EGFR TKI-Resistant Cell Lines
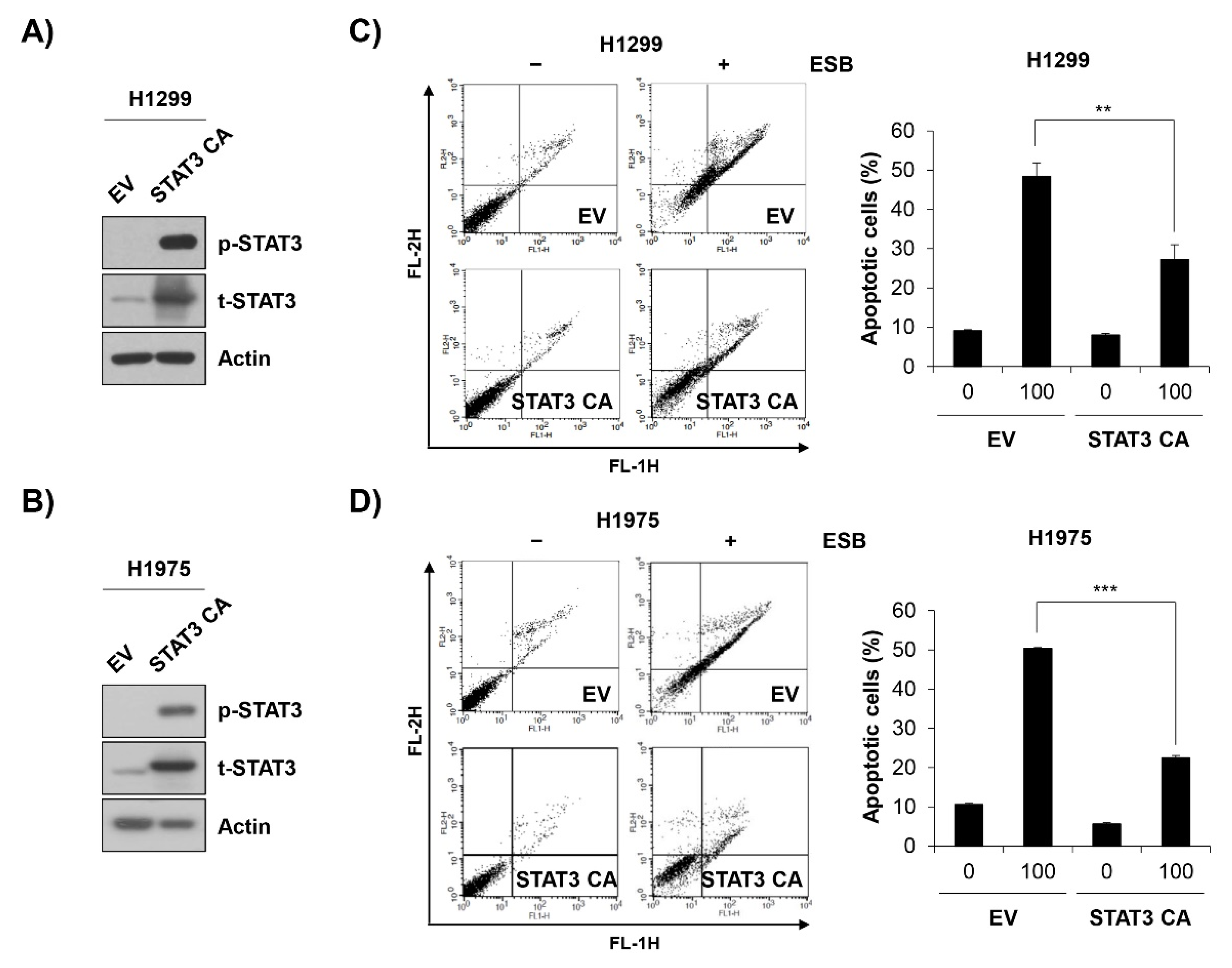
2.6. Inactivation of STAT3 Mediates ESB-Induced Apoptosis in EGFR TKI-Resistant Cell Lines
3. Discussion
4. Materials and Methods
4.1. Preparation of ESB
4.2. HPLC-MS/MS Analysis
4.3. Cell Culture
4.4. MTT Assay
4.5. Trypan Blue Exclusion Assay
4.6. Colony Formation Assay
4.7. DAPI Staining
4.8. Flow Cytometry
4.9. Western Blots
4.10. Transfection
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Burdett, S.; Stewart, L.; Pignon, J.-P. Chemotherapy in non-small cell lung cancer: An update of an individual patient data-based meta-analysis. J. Thorac. Cardiovasc. Surg. 2005, 129, 1205–1206. [Google Scholar] [CrossRef] [PubMed]
- NSCLC Meta-Analyses Collaborative Group. Chemotherapy in Addition to Supportive Care Improves Survival in Advanced Non–Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data from 16 Randomized Controlled Trials. J. Clin. Oncol. 2008, 26, 4617–4625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Yuan, J.-Q.; Wang, K.-F.; Fu, X.-H.; Han, X.-R.; Threapleton, D.; Yang, Z.-Y.; Mao, C.; Tang, J.-L. The prevalence of EGFR mutation in patients with non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget 2016, 7, 78985–78993. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Yatabe, Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010, 277, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Scaltriti, M.; Baselga, J. The Epidermal Growth Factor Receptor Pathway: A Model for Targeted Therapy. Clin. Cancer Res. 2006, 12, 5268–5272. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.L.; Chen, G.; Feng, J.; Liu, X.Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTI-MAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or Chemotherapy for Non–Small-Cell Lung Cancer with Mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar] [CrossRef]
- Cohen, M.H.; Johnson, J.R.; Chen, Y.; Sridhara, R.; Pazdur, R. FDA Drug Approval Summary: Erlotinib (Tarceva ®) Tablets. Oncologist 2005, 10, 461–466. [Google Scholar] [CrossRef]
- Cohen, M.H.; Williams, G.A.; Sridhara, R.; Chen, G.; Pazdur, R. FDA Drug Approval Summary: Gefitinib (ZD1839) (Iressa ®) Tablets. Oncologist 2003, 8, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-G.; Shih, J.-Y. Management of acquired resistance to EGFR TKI–targeted therapy in advanced non-small cell lung cancer. Mol. Cancer 2018, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of Tumor Specimens at the Time of Acquired Resistance to EGFR-TKI Therapy in 155 Patients with EGFR-Mutant Lung Cancers. Clin. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.R.; Jänne, A.P. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat. Med. 2013, 19, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-H.; Lu, J.-J. Osimertinib resistance in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Lett. 2018, 420, 242–246. [Google Scholar] [CrossRef]
- Van Der Wekken, A.; Saber, A.; Hiltermann, T.; Kok, K.; Berg, A.V.D.; Groen, H.J.M. Resistance mechanisms after tyrosine kinase inhibitors afatinib and crizotinib in non-small cell lung cancer, a review of the literature. Crit. Rev. Oncol. 2016, 100, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its tradi-tional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Cheng, C.-S.; Chen, J.; Tan, H.-Y.; Wang, N.; Chen, Z.; Feng, Y. Scutellaria baicalensis and Cancer Treatment: Recent Progress and Perspectives in Biomedical and Clinical Studies. Am. J. Chin. Med. 2018, 46, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Hong, S.H.; Ku, J.M.; Lim, Y.S.; Lee, S.J.; Song, J.; Kim, T.Y.; Cheon, C.; Ko, S.-G. Scutellaria Radix Promotes Apoptosis in Non-Small Cell Lung Cancer Cells via Induction of AMPK-Dependent Autophagy. Am. J. Chin. Med. 2019, 47, 691–705. [Google Scholar] [CrossRef]
- Gao, J.; Morgan, W.A.; Sanchez-Medina, A.; Corcoran, O. The ethanol extract of Scutellaria baicalensis and the active com-pounds induce cell cycle arrest and apoptosis including upregulation of p53 and Bax in human lung cancer cells. Toxicol. Appl. Pharmacol. 2011, 254, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-I.; Park, H.-S.; Kang, S.-R.; Nagappan, A.; Lee, D.-H.; Kim, J.-A.; Han, D.-Y.; Kim, G.-S. Korean Scutellaria baicalensis water extract inhibits cell cycle G1/S transition by suppressing cyclin D1 expression and matrix-metalloproteinase-2 activity in human lung cancer cells. J. Ethnopharmacol. 2011, 133, 634–641. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, H.-J.; Sun, S.-J.; Dai, J.-Y.; Fang, J.-W.; Li, Q.-H.; Yan, C.; Mao, W.-W.; Zhang, Y.-Y. Total flavonoid aglycones extract in Radix scutellariae inhibits lung carcinoma and lung metastasis by affecting cell cycle and DNA synthesis. J. Ethnopharmacol. 2016, 194, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.A.; Grandis, J.R. STAT3 signaling: Anticancer Strategies and Challenges. Mol. Interv. 2011, 11, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kortylewski, M.; Pardoll, D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvi-ronment. Nat. Rev. Immunol. 2007, 7, 41–51. [Google Scholar] [CrossRef]
- Mohrherr, J.; Uras, I.Z.; Moll, H.P.; Casanova, E. STAT3: Versatile Functions in Non-Small Cell Lung Cancer. Cancers 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Dobi, E.; Monnien, F.; Kim, S.; Ivanaj, A.; N’Guyen, T.; Demarchi, M.; Adotevi, O.; Thierry-Vuillemin, A.; Jary, M.; Kantelip, B.; et al. Impact of STAT3 Phosphorylation on the Clinical Effectiveness of Anti-EGFR–Based Therapy in Patients With Metastatic Colorectal Cancer. Clin. Color. Cancer 2013, 12, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Pernas, F.G.; Allen, C.T.; Winters, M.E.; Yan, B.; Friedman, J.; Dabir, B.; Saigal, K.; Mundinger, G.S.; Xu, X.; Morris, J.C.; et al. Proteomic Signatures of Epidermal Growth Factor Receptor and Survival Signal Pathways Correspond to Gefitinib Sensitivity in Head and Neck Cancer. Clin. Cancer Res. 2009, 15, 2361–2372. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.P.; Chang, Q.; Mao, N.; Daly, L.A.; Vogel, R.; Chan, T.; Liu, S.H.; Bournazou, E.; Schori, E.; Zhang, H.; et al. JAK2 inhibition sensitizes resistant EGFR-mutant lung adenocarcinoma to tyrosine kinase inhibitors. Sci. Signal. 2016, 9, ra33. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, A.A.; Tan, F.H.; Putoczki, T.L.; Stylli, S.S.; Luwor, R.B. STAT3 signaling mediates tumour resistance to EGFR targeted therapeutics. Mol. Cell. Endocrinol. 2017, 451, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Tsai, K.W.; Li, Y.Z.; Chang, Y.S.; Lai, Y.C.; Laio, Y.H.; Wu, J.D.; Liu, Y.W. Anti-bladdertumor effect of baicalein from Scutellaria baicalensis Georgi and its application in vivo. Evid. Based Complement. Alternat. Med. 2013, 579751, 2013. [Google Scholar]
- Yang, L.; Zheng, X.L.; Sun, H.; Zhong, Y.J.; Wang, Q.; He, H.N.; Shi, X.W.; Zhou, B.; Li, J.K.; Lin, Y.; et al. Catalase suppres-sion-mediated H2O2 accumulation in cancer cells by wogonin effectively blocks tumor necrosis factor-induced NF-κB activa-tion and sensitizes apoptosis. Cancer Sci. 2011, 102, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Kavandi, L.; Lee, L.R.; Bokhari, A.A.; Pirog, J.E.; Jiang, Y.; Ahmad, K.A.; Syed, V. The Chinese herbs Scutellaria baicalensis and Fritillaria cirrhosa target NFkappaB to inhibit proliferation of ovarian and endometrial cancer cells. Mol. Carcinog. 2015, 54, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ling, Y.; Chen, Y.; Li, C.-L.; Feng, F.; You, Q.-D.; Lu, N.; Guo, Q.-L. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 2010, 297, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-B.; Lu, P.; Guo, Q.-Y.; Zhang, Z.-H.; Meng, X.-Y. Baicalein induces apoptosis in esophageal squamous cell carcinoma cells through modulation of the PI3K/Akt pathway. Oncol. Lett. 2012, 5, 722–728. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, J.; Zheng, J.; Li, J.; Wei, T.; Zheng, Z.; Chen, Y. Down-regulation of the PI3K/Akt signaling pathway and in-duction of apoptosis in CA46 Burkitt lymphoma cells by baicalin. J. Exp. Clin. Cancer Res. 2012, 31, 48. [Google Scholar] [CrossRef]
- Chow, S.; Chen, Y.; Liang, C.; Huang, Y.; Wang, J. Wogonin induces cross-regulation between autophagy and apoptosis via a variety of Akt pathway in human nasopharyngeal carcinoma cells. J. Cell. Biochem. 2012, 113, 3476–3485. [Google Scholar] [CrossRef]
- Zhou, R.-T.; He, M.; Yu, Z.; Liang, Y.; Nie, Y.; Tai, S.; Teng, C.-B. Baicalein inhibits pancreatic cancer cell proliferation and invasion via suppression of NEDD9 expression and its downstream Akt and ERK signaling pathways. Oncotarget 2017, 8, 56351–56363. [Google Scholar] [CrossRef]
- Liu, X.; Tian, S.; Liu, M.; Jian, L.; Zhao, L. Wogonin inhibits the proliferation and invasion, and induces the apoptosis of HepG2 and Bel7402 HCC cells through NF-kappaB/Bcl-2, EGFR and EGFR downstream ERK/AKT signaling. Int. J. Mol. Med. 2016, 38, 1250–1256. [Google Scholar] [CrossRef]
- Wei, L.; Yao, Y.; Zhao, K.; Huang, Y.; Zhou, Y.; Zhao, L.; Guo, Q.; Lu, N. Oroxylin A inhibits invasion and migration through suppressing ERK/GSK-3beta signaling in snail-expressing non-small-cell lung cancer cells. Mol. Carcinog. 2016, 55, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, C.; Chen, H.; Hu, Y.; Tian, L.; Pan, J.; Geng, M. Aβ-induced microglial cell activation is inhibited by baicalin through the JAK2/STAT3 signaling pathway. Int. J. Neurosci. 2014, 124, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhuang, P.; Shen, B.; Zhang, Y.; Shen, J. Baicalin promotes neuronal differentiation of neural stem/progenitor cells through modulating p-stat3 and bHLH family protein expression. Brain Res. 2012, 1429, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Susmitha, G.D.; Miyazato, K.; Ogura, K.; Yokoyama, S.; Hayakawa, Y. Anti-metastatic Effects of Baicalein by Targeting STAT3 Activity in Breast Cancer Cells. Biol. Pharm. Bull. 2020, 43, 1899–1905. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, F.; Wang, Y.; Cai, M.; Wang, Q.; Guo, Q.; Li, Z.; Hu, R. Oroxylin A Inhibits Colitis-associated Carcinogenesis Through Modulating the IL-6/STAT3 Signaling Pathway. Inflamm. Bowel Dis. 2013, 19, 1990–2000. [Google Scholar] [CrossRef]
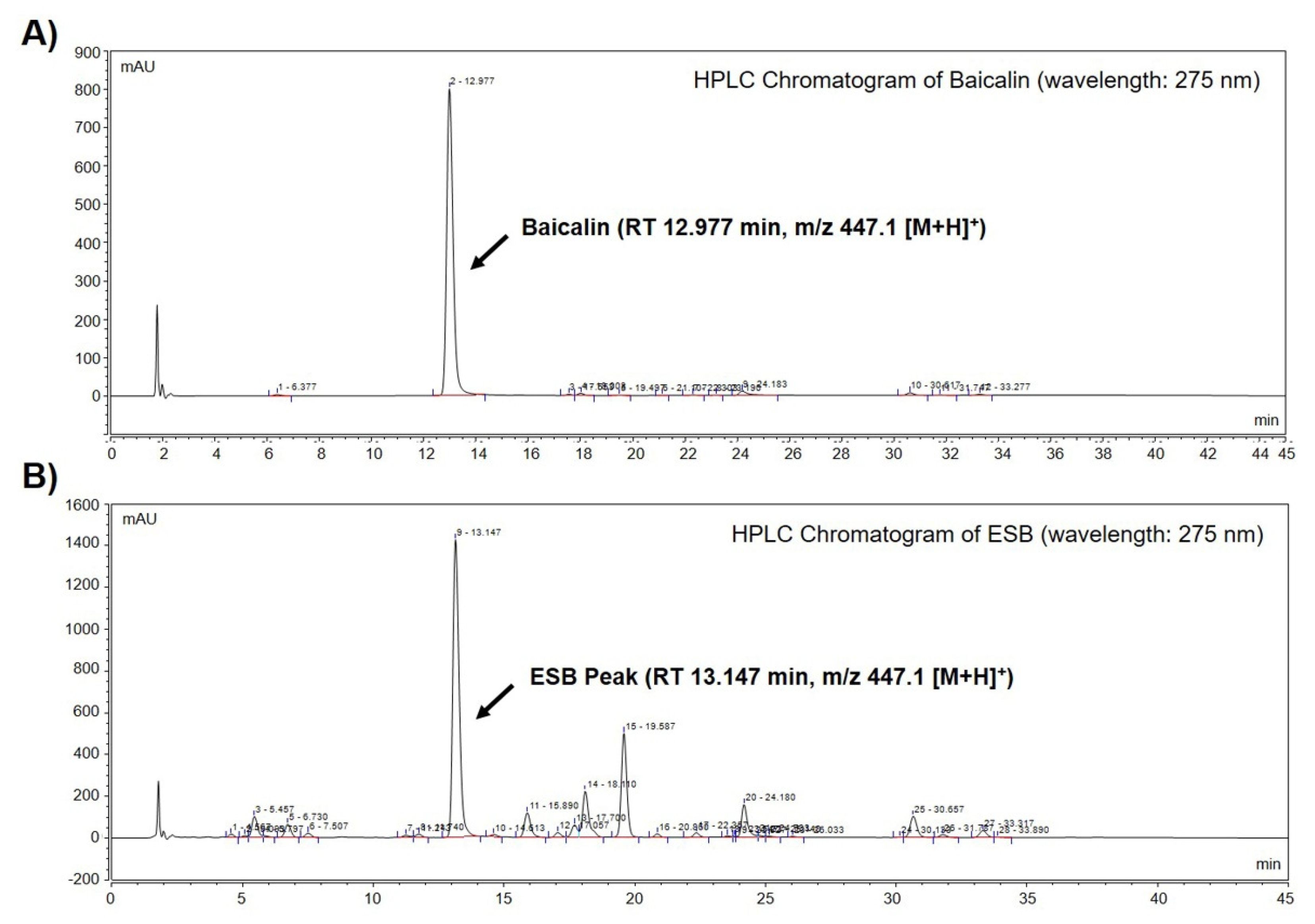


| Name | RT 1 | MS 2 [M+H]+ (m/z) | MS/MS (m/z) | Quantity of Baicalin in ESB ± SD 3 (g/kg) |
|---|---|---|---|---|
| Baicalin | 12.977 | 447.1 447.1 | 271.1 123.0 | 187.76 ± 1.06 |
| ESB peak | 13.147 | 271.1 123.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-J.; Park, S.-H.; Choi, Y.-H.; Chi, G.-Y. The Root Extract of Scutellaria baicalensis Induces Apoptosis in EGFR TKI-Resistant Human Lung Cancer Cells by Inactivation of STAT3. Int. J. Mol. Sci. 2021, 22, 5181. https://doi.org/10.3390/ijms22105181
Park H-J, Park S-H, Choi Y-H, Chi G-Y. The Root Extract of Scutellaria baicalensis Induces Apoptosis in EGFR TKI-Resistant Human Lung Cancer Cells by Inactivation of STAT3. International Journal of Molecular Sciences. 2021; 22(10):5181. https://doi.org/10.3390/ijms22105181
Chicago/Turabian StylePark, Hyun-Ji, Shin-Hyung Park, Yung-Hyun Choi, and Gyoo-Yong Chi. 2021. "The Root Extract of Scutellaria baicalensis Induces Apoptosis in EGFR TKI-Resistant Human Lung Cancer Cells by Inactivation of STAT3" International Journal of Molecular Sciences 22, no. 10: 5181. https://doi.org/10.3390/ijms22105181
APA StylePark, H.-J., Park, S.-H., Choi, Y.-H., & Chi, G.-Y. (2021). The Root Extract of Scutellaria baicalensis Induces Apoptosis in EGFR TKI-Resistant Human Lung Cancer Cells by Inactivation of STAT3. International Journal of Molecular Sciences, 22(10), 5181. https://doi.org/10.3390/ijms22105181







