Polymorphisms in EGFR Gene Predict Clinical Outcome in Unresectable Non-Small Cell Lung Cancer Treated with Radiotherapy and Platinum-Based Chemoradiotherapy
Abstract
1. Introduction
2. Results
2.1. Individual SNPs and Survival
2.2. Survival Analysis According to the Combined SNPs
2.3. Haplotype Analysis
3. Discussion
4. Materials and Methods
4.1. Patients and Treatment
4.2. SNP Identification
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Willers, H.; Azzoli, C.G.; Santivasi, W.L.; Xia, F. Basic mechanisms of therapeutic resistance to radiation and chemotherapy in lung cancer. Cancer J. 2013, 19, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Weihua, Z. Rethink of EGFR in Cancer With Its Kinase Independent Function on Board. Front. Oncol. 2019, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.I.; Gee, J.M.; Harper, M.E. EGFR and cancer prognosis. Eur. J. Cancer 2001, 37 (Suppl. 4), 9–15. [Google Scholar] [CrossRef]
- Scagliotti, G.V.; Selvaggi, G.; Novello, S.; Hirsch, F.R. The biology of epidermal growth factor receptor in lung cancer. Clin. Cancer Res. 2004, 10, 4227s–4232s. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.C.; Jin, X.; Wang, Y.; Wang, K. Role of epidermal growth factor receptor in lung cancer and targeted therapies. Am. J. Cancer Res. 2017, 7, 187–202. [Google Scholar]
- Gonzalez-Conchas, G.A.; Rodriguez-Romo, L.; Hernandez-Barajas, D.; Gonzalez-Guerrero, J.F.; Rodriguez-Fernandez, I.A.; Verdines-Perez, A.; Templeton, A.J.; Ocana, A.; Seruga, B.; Tannock, I.F.; et al. Epidermal growth factor receptor overexpression and outcomes in early breast cancer: A systematic review and a meta-analysis. Cancer Treat. Rev. 2018, 62, 1–8. [Google Scholar] [CrossRef]
- London, M.; Gallo, E. Epidermal growth factor receptor (EGFR) involvement in epithelial-derived cancers and its current antibody-based immunotherapies. Cell Biol. Int. 2020, 44, 1267–1282. [Google Scholar] [CrossRef]
- Akimoto, T.; Hunter, N.R.; Buchmiller, L.; Mason, K.; Ang, K.K.; Milas, L. Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin. Cancer Res. 1999, 5, 2884–2890. [Google Scholar] [PubMed]
- Harari, P.M.; Huang, S.M. Epidermal growth factor receptor modulation of radiation response: Preclinical and clinical development. Semin. Radiat. Oncol. 2002, 12, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Milas, L.; Raju, U.; Liao, Z.; Ajani, J. Targeting molecular determinants of tumor chemo-radioresistance. Semin. Oncol. 2005, 32, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.J.; Nirodi, C.S. The epidermal growth factor receptor: A role in repair of radiation-induced DNA damage. Clin. Cancer Res. 2007, 13, 6555–6560. [Google Scholar] [CrossRef]
- Wang, S.C.; Hung, M.C. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin. Cancer Res. 2009, 15, 6484–6489. [Google Scholar] [CrossRef]
- Dittmann, K.; Mayer, C.; Rodemann, H.P. Inhibition of radiation-induced EGFR nuclear import by C225 (Cetuximab) suppresses DNA-PK activity. Radiother. Oncol. 2005, 76, 157–161. [Google Scholar] [CrossRef]
- Liccardi, G.; Hartley, J.A.; Hochhauser, D. EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treatment. Cancer Res. 2011, 71, 1103–1114. [Google Scholar] [CrossRef]
- Liccardi, G.; Hartley, J.A.; Hochhauser, D. Importance of EGFR/ERCC1 interaction following radiation-induced DNA damage. Clin. Cancer Res. 2014, 20, 3496–3506. [Google Scholar] [CrossRef]
- Huang, S.M.; Harari, P.M. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: Inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin. Cancer Res. 2000, 6, 2166–2174. [Google Scholar]
- Benhar, M.; Engelberg, D.; Levitzki, A. Cisplatin-induced activation of the EGF receptor. Oncogene 2002, 21, 8723–8731. [Google Scholar] [CrossRef]
- Meyn, R.E.; Munshi, A.; Haymach, J.V.; Milas, L.; Ang, K.K. Receptor signaling as a regulatory mechanism of DNA repair. Radiother. Oncol. 2009, 92, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Fatemian, T.; Chowdhury, E.H. Targeting oncogenes and tumor suppressors genes to mitigate chemoresistance. Curr. Cancer Drug Targets 2014, 14, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Cuneo, K.C.; Nyati, M.K.; Ray, D.; Lawrence, T.S. EGFR targeted therapies and radiation: Optimizing efficacy by appropriate drug scheduling and patient selection. Pharmacol. Ther. 2015, 154, 67–77. [Google Scholar] [CrossRef]
- Toulany, M. Targeting DNA Double-Strand Break Repair Pathways to Improve Radiotherapy Response. Genes 2019, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ye, Y.; Rosell, R.; Amos, C.I.; Stewart, D.J.; Hildebrandt, M.A.; Roth, J.A.; Minna, J.D.; Gu, J.; Lin, J.; et al. Genome-wide association study of survival in non-small cell lung cancer patients receiving platinum-based chemotherapy. J. Natl. Cancer Inst. 2011, 103, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Zienolddiny, S.; Skaug, V. Single nucleotide polymorphisms as susceptibility, prognostic, and therapeutic markers of nonsmall cell lung cancer. Lung Cancer Targets Ther. 2011, 3, 1–14. [Google Scholar] [CrossRef][Green Version]
- Butkiewicz, D.; Krześniak, M.; Drosik, A.; Giglok, M.; Gdowicz-Kłosok, A.; Kosarewicz, A.; Rusin, M.; Masłyk, B.; Gawkowska-Suwińska, M.; Suwiński, R. The VEGFR2, COX-2 and MMP-2 polymorphisms are associated with clinical outcome of patients with inoperable non-small cell lung cancer. Int. J. Cancer 2015, 137, 2332–2342. [Google Scholar] [CrossRef]
- Yang, W.C.; Hsu, F.M.; Yang, P.C. Precision radiotherapy for non-small cell lung cancer. J. Biomed. Sci. 2020, 27, 82. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.; Ribeiro, R.; Azevedo, I.; Coelho, A.; Soares, M.; Sousa, B.; Pinto, D.; Lopes, C.; Medeiros, R.; Scagliotti, G.V. Genetic polymorphisms of the epidermal growth factor and related receptor in non-small cell lung cancer--a review of the literature. Oncologist 2007, 12, 201–210. [Google Scholar] [CrossRef]
- Gregorc, V.; Hidalgo, M.; Spreafico, A.; Cusatis, G.; Ludovini, V.; Ingersoll, R.G.; Marsh, S.; Steinberg, S.M.; Viganò, M.G.; Ghio, D.; et al. Germline polymorphisms in EGFR and survival in patients with lung cancer receiving gefitinib. Clin. Pharmacol. Ther. 2008, 83, 477–484. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Tzeng, C.H.; Chen, M.H.; Chen, P.M.; Wang, W.S. Epidermal growth factor receptor R521K polymorphism shows favorable outcomes in KRAS wild-type colorectal cancer patients treated with cetuximab-based chemotherapy. Cancer Sci. 2012, 103, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Resteghini, C.; Paielli, N.; Licitra, L.; Pilotti, S.; Perrone, F. Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget 2016, 7, 74362–74379. [Google Scholar] [CrossRef]
- Jurisic, V.; Vukovic, V.; Obradovic, J.; Gulyaeva, L.F.; Kushlinskii, N.E.; Djordjević, N. EGFR Polymorphism and Survival of NSCLC Patients Treated with TKIs: A Systematic Review and Meta-Analysis. J. Oncol. 2020, 2020, 1973241. [Google Scholar] [CrossRef]
- Ensembl Database 102. Available online: http://www.ensembl.org/ (accessed on 15 December 2020).
- Dong, J.; Dai, J.; Shu, Y.; Pan, S.; Xu, L.; Chen, W.; Wang, Y.; Jin, G.; Ma, H.; Zhang, M.; et al. Polymorphisms in EGFR and VEGF contribute to non-small-cell lung cancer survival in a Chinese population. Carcinogenesis 2010, 31, 1080–1086. [Google Scholar] [CrossRef]
- Guo, H.; Xing, Y.; Mu, A.; Li, X.; Li, T.; Bian, X.; Yang, C.; Zhang, X.; Liu, Y.; Wang, X. Correlations between EGFR gene polymorphisms and pleural metastasis of lung adenocarcinoma. Onco. Targets Ther. 2016, 9, 5257–5270. [Google Scholar] [CrossRef]
- Costa, B.M.; Viana-Pereira, M.; Fernandes, R.; Costa, S.; Linhares, P.; Vaz, R.; Pinheiro, C.; Lima, J.; Soares, P.; Silva, A.; et al. Impact of EGFR genetic variants on glioma risk and patient outcome. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Spindler, K.L.; Nielsen, J.N.; Lindebjerg, J.; Brandslund, I.; Jakobsen, A. Prediction of response to chemoradiation in rectal cancer by a gene polymorphism in the epidermal growth factor receptor promoter region. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Ali Beg, M.M.; Fahdil, S.R.; Yadav, P.; Shukla, K.K.; Mohan, A.; Saxena, A. Association of EGFR 1 Gene Alteration and their Association with Lung Adenocarcinoma Patients. Asian Pac. J. Cancer Prev. 2019, 20, 825–830. [Google Scholar] [CrossRef]
- Liu, W.; Innocenti, F.; Wu, M.H.; Desai, A.A.; Dolan, M.E.; Cook, E.H., Jr.; Ratain, M.J. A functional common polymorphism in a Sp1 recognition site of the epidermal growth factor receptor gene promoter. Cancer Res. 2005, 65, 46–53. [Google Scholar]
- Liu, W.; He, L.; Ramírez, J.; Krishnaswamy, S.; Kanteti, R.; Wang, Y.C.; Salgia, R.; Ratain, M.J. Functional EGFR germline polymorphisms may confer risk for EGFR somatic mutations in non-small cell lung cancer, with a predominant effect on exon 19 microdeletions. Cancer Res. 2011, 71, 2423–2427. [Google Scholar] [CrossRef]
- Moriai, T.; Kobrin, M.S.; Hope, C.; Speck, L.; Korc, M. A variant epidermal growth factor receptor exhibits altered type alpha transforming growth factor binding and transmembrane signaling. Proc. Natl. Acad. Sci. USA 1994, 91, 10217–10221. [Google Scholar] [CrossRef]
- Sasaki, H.; Okuda, K.; Shimizu, S.; Takada, M.; Kawahara, M.; Kitahara, N.; Okumura, M.; Matsumura, A.; Iuchi, K.; Kawaguchi, T.; et al. EGFR R497K polymorphism is a favorable prognostic factor for advanced lung cancer. J. Cancer Res. Clin. Oncol. 2009, 135, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Fan, X.M.; Li, K.X.; Niu, S.H.; Zhang, Q.Y. The Association between Epidermal Growth Factor Receptor Single Nucleotide Polymorphisms and Radiochemotherapy Response in Cervical Cancer. Pathol. Oncol. Res. 2020, 26, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Su, N.W.; Leu, Y.S.; Lee, J.C.; Liu, C.J.; Cheng, C.Y.; Lin, J.S.; Chen, Y.J.; Chen, C.K.; Fang, I.C.; Hsieh, R.K.; et al. EGF and EGFR genetic polymorphisms predict prognosis in locally advanced pharyngolaryngeal squamous cell carcinoma patients receiving postoperative concurrent chemoradiotherapy. Onco Targets Ther. 2014, 7, 2197–2204. [Google Scholar] [CrossRef][Green Version]
- Wang, W.S.; Chen, P.M.; Chiou, T.J.; Liu, J.H.; Lin, J.K.; Lin, T.C.; Wang, H.S.; Su, Y. Epidermal growth factor receptor R497K polymorphism is a favorable prognostic factor for patients with colorectal carcinoma. Clin. Cancer Res. 2007, 13, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.A.; Morlock, E.V.; Karagas, M.R.; Kelsey, K.T.; Marsit, C.J.; Schned, A.R.; Andrew, A.S. EGFR pathway polymorphisms and bladder cancer susceptibility and prognosis. Carcinogenesis 2009, 30, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Press, O.A.; Zhang, W.; Gordon, M.A.; Yang, D.; Lurje, G.; Iqbal, S.; El-Khoueiry, A.; Lenz, H.J. Gender-related survival differences associated with EGFR polymorphisms in metastatic colon cancer. Cancer Res. 2008, 68, 3037–3042. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.B.; Schaffner, S.F.; Nguyen, H.; Moore, J.M.; Roy, J.; Blumenstiel, B.; Higgins, J.; DeFelice, M.; Lochner, A.; Faggart, M.; et al. The structure of haplotype blocks in the human genome. Science 2002, 296, 2225–2229. [Google Scholar] [CrossRef]
- Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, MA, USA. Available online: https://www.broadinstitute.org/haploview/haploview (accessed on 25 November 2020).
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Stephens, M.; Smith, N.J.; Donnelly, P. A new statistical method for haplotype reconstruction from population data. Am. J. Hum. Genet. 2001, 68, 978–989. [Google Scholar] [CrossRef]
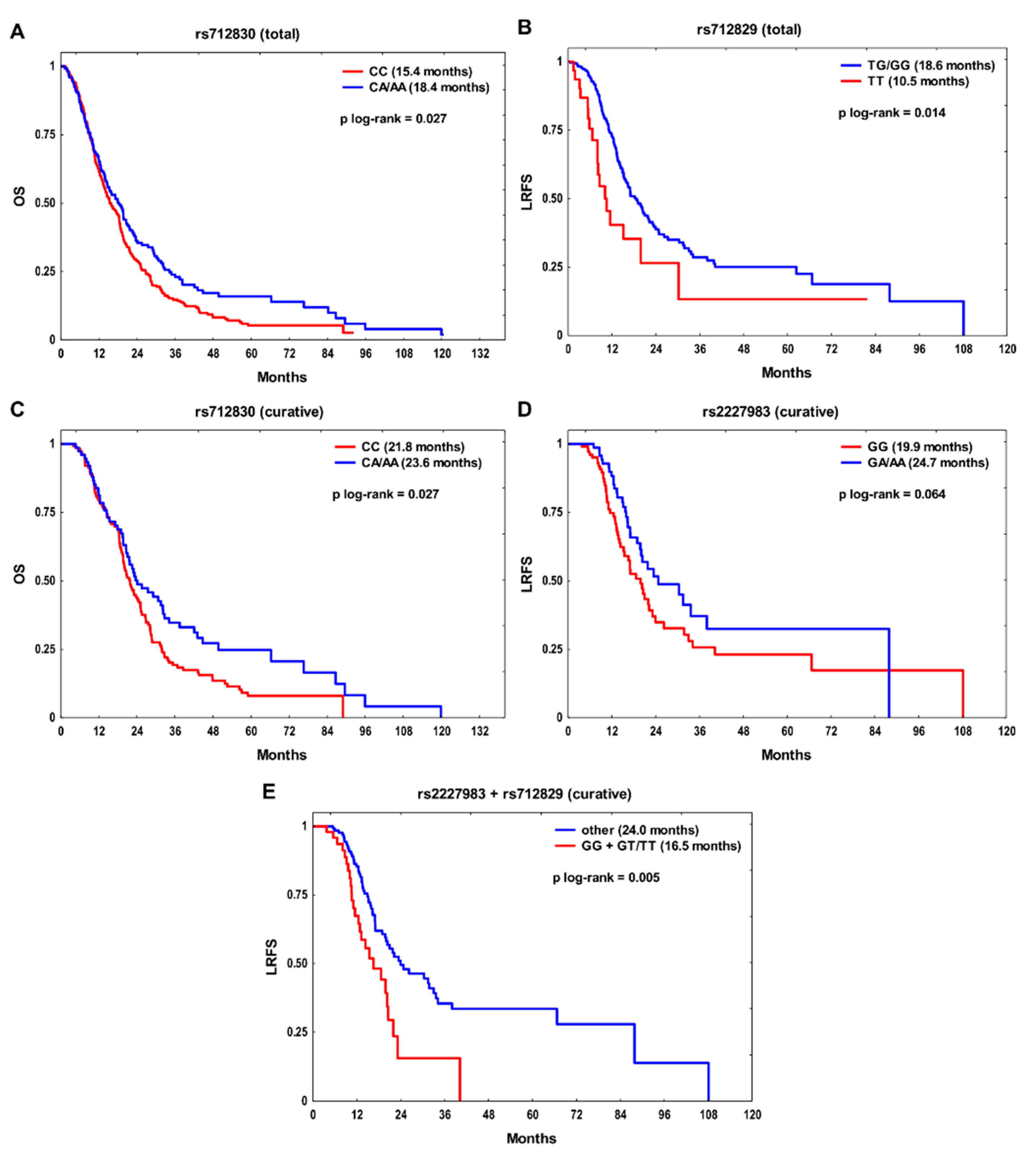
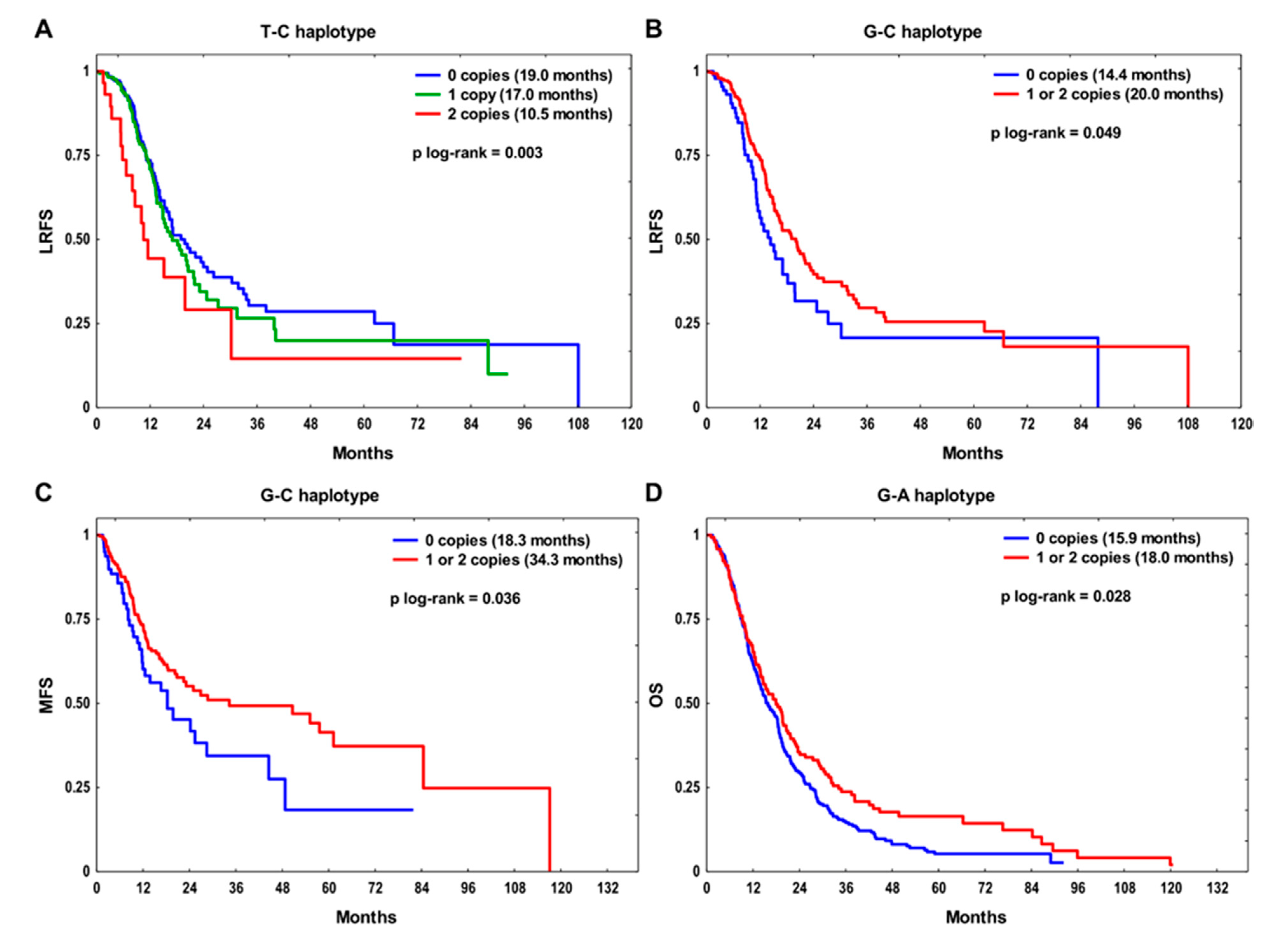
| Feature | OS | LRFS | MFS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Event | mOS | HR (95% CI) | p-Value | Event | mLRFS | HR (95% CI) | p-Value | Event | mMFS | HR (95% CI) | p-Value | |
| n | n | n | |||||||||||
| Sex | |||||||||||||
| Female | 125 (29) | 100 | 18.4 | 1 | 54 | 19.8 | 1 | 45 | 20.8 | 1 | |||
| Male | 311 (71) | 255 | 15.4 | 1.23 (0.97–1.55) | 0.087 | 107 | 17 | 1.04 (0.75–1.45) | 0.796 | 81 | 26.9 | 0.94 (0.65–1.35) | 0.726 |
| p log-rank | 0.077 | 0.786 | 0.713 | ||||||||||
| Age | |||||||||||||
| <63 | 206 (47) | 164 | 19.5 | 1 | 84 | 21 | 1 | 78 | 22.5 | 1 | |||
| ≥63 | 230 (53) | 191 | 14 | 1.38 (1.12–1.71) | 0.002 | 77 | 14.1 | 1.36 (0.99–1.85) | 0.055 | 48 | 44.5 | 0.82 (0.57–1.17) | 0.273 |
| p log-rank | 0.002 | 0.058 | 0.276 | ||||||||||
| Histology | |||||||||||||
| SCC | 266 (61) | 226 | 18.4 | 1 | 105 | 16.3 | 1 | 72 | 25.4 | 1 | |||
| AC | 77 (18) | 62 | 17.3 | 0.85 (0.64–1.13) | 0.258 | 21 | 31.8 | 0.57 (0.36–0.91) | 0.019 | 23 | 26.9 | 1.18 (0.73–1.89) | 0.5 |
| NOS | 93 (21) | 67 | 18 | 0.77 (0.59–1.02) | 0.064 | 35 | 16.5 | 0.95 (0.64–1.39) | 0.777 | 31 | 18.3 | 1.23 (0.80–1.87) | 0.345 |
| p log-rank | 0.433 | 0.318 | 0.478 | ||||||||||
| Clinical stage | |||||||||||||
| I–II | 38 (9) | 31 | 23.6 | 1 | 10 | 24 | 1 | 9 | 50.6 | 1 | |||
| III | 317 (73) | 255 | 18.1 | 1.24 (0.86–1.81) | 0.253 | 127 | 16.9 | 2.15 (1.13–4.11) | 0.02 | 102 | 25.4 | 1.66 (0.84–3.29) | 0.144 |
| IV | 81 (18) | 69 | 10.4 | 2.29 (1.49–3.50) | 1 × 10−4 | 24 | 13.2 | 3.53 (1.68–7.42) | 9 × 10−4 | 15 | 4.2 | 11.85 (5.15–27.30) | <1 × 10−6 |
| p log-rank | <1 × 10−5 | 8 × 10−4 | 3 × 10−4 | ||||||||||
| ECOG/Zubrod PS | |||||||||||||
| 0 | 114 (26) | 93 | 20 | 1 | 49 | 20.4 | 1 | 39 | 44.5 | 1 | |||
| 1 | 290 (67) | 235 | 15.2 | 1.34 (1.05–1.71) | 0.017 | 101 | 16.5 | 1.33 (0.94–1.89) | 0.102 | 82 | 25 | 1.33 (0.91–1.96) | 0.145 |
| 2 | 32 (7) | 27 | 11.2 | 2.29 (1.49–3.53) | 2 × 10−4 | 11 | 7.8 | 3.19 (1.64–6.18) | 6 × 10−4 | 5 | 16.1 | 1.64 (0.64–4.18) | 0.303 |
| p log-rank | 1 × 10−4 | 3 × 10−4 | 0.268 | ||||||||||
| Smoking status | |||||||||||||
| Never | 25 (6) | 17 | 26.4 | 1 | 10 | 33.6 | 1 | 9 | – | 1 | |||
| Ever | 411 (94) | 338 | 15.5 | 1.98 (1.22–3.23) | 0.006 | 151 | 16.5 | 1.82 (0.96–3.47) | 0.068 | 117 | 25.4 | 1.24 (0.63–2.44) | 0.542 |
| p log-rank | 6 × 10−4 | 0.014 | 0.458 | ||||||||||
| Chemotherapy | |||||||||||||
| No | 135 (31) | 111 | 9.8 | 1 | 39 | 13.1 | 1 | 22 | 28.5 | 1 | |||
| Yes | 301 (69) | 244 | 19.4 | 0.50 (0.40–0.63) | <1 × 10−6 | 122 | 19.8 | 0.57 (0.40–0.82) | 0.002 | 104 | 25 | 0.89 (0.56–1.41) | 0.61 |
| p log-rank | <1 × 10−5 | 0.016 | 0.686 | ||||||||||
| Radiation dose | |||||||||||||
| <60 Gy | 231 (53) | 192 | 11.4 | 1 | 71 | 13.1 | 1 | 58 | 12.1 | 1 | |||
| ≥60 Gy | 205 (47) | 163 | 22.5 | 0.45 (0.37–0.56) | <1 × 10−6 | 90 | 21 | 0.47 (0.34–0.64) | 3x10−6 | 68 | 48.7 | 0.40 (0.28–0.57) | <1 × 10−6 |
| p log-rank | <1 × 10−5 | 2 × 10−5 | 1 × 10−5 | ||||||||||
| Endpoint | SNP | Genotype | Event/n | uHR (95% CI) | p-Value | mHR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|
| OS | rs2227983 | GA/AA | 157/196 | 1 | 1 | ||
| GG | 190/231 | 1.02 (0.82–1.26) | 0.878 | 1.04 (0.84–1.29) | 0.708 | ||
| rs712830 | CA/AA | 119/147 | 1 | 1 | |||
| CC | 236/289 | 1.28 (1.02–1.60) | 0.032 | 1.28 (1.01–1.61) | 0.039 | ||
| rs712829 | GG | 177/211 | 1 | 1 | |||
| GT/TT | 178/225 | 1.08 (0.87–1.33) | 0.478 | 0.96 (0.78–1.19) | 0.723 | ||
| LRFS | rs2227983 | GA/AA | 69/196 | 1 | 1 | ||
| GG | 87/231 | 1.13 (0.83–1.56) | 0.44 | 1.25 (0.91–1.73) | 0.172 | ||
| rs712830 | CA/AA | 57/147 | 1 | 1 | |||
| CC | 104/289 | 1.11 (0.80–1.55) | 0.516 | 1.02 (0.73–1.44) | 0.905 | ||
| rs712829 | TG/GG | 143/402 | 1 | 1 | |||
| TT | 18/34 | 1.97 (1.21–3.23) | 0.007 | 2.03 (1.23–3.34) | 0.006 | ||
| MFS | rs2227983 | GA/AA | 58/196 | 1 | 1 | ||
| GG | 66/231 | 0.91 (0.64–1.30) | 0.616 | 0.89 (0.62–1.28) | 0.532 | ||
| rs712830 | CA/AA | 44/147 | 1 | 1 | |||
| CC | 82/289 | 1.24 (0.86–1.80) | 0.252 | 1.31 (0.89–1.94) | 0.171 | ||
| rs712829 | GG | 62/211 | 1 | 1 | |||
| GT/TT | 64/225 | 1.17 (0.82–1.67) | 0.377 | 1.29 (0.88–1.87) | 0.188 |
| Endpoint | SNP | Genotype | Event/n | uHR (95% CI) | p-Value | mHR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|
| OS | rs2227983 | GA/AA | 70/91 | 1 | 1 | ||
| GG | 87/107 | 1.01 (0.73–1.38) | 0.97 | 1.03 (0.74–1.43) | 0.878 | ||
| rs712830 | CA/AA | 57/77 | 1 | 1 | |||
| CC | 106/128 | 1.44 (1.04–2.01) | 0.03 | 1.44 (1.05–2.07) | 0.047 | ||
| rs712829 | GG | 81/102 | 1 | 1 | |||
| GT/TT | 82/103 | 1.28 (0.94–1.75) | 0.12 | 1.20 (0.86–1.67) | 0.273 | ||
| LRFS | rs2227983 | GA/AA | 33/91 | 1 | 1 | ||
| GG | 53/107 | 1.50 (0.97–2.32) | 0.069 | 1.67 (1.06–2.64) | 0.028 | ||
| rs712830 | CA/AA | 36/77 | 1 | 1 | |||
| CC | 54/128 | 1.14 (0.74–1.76) | 0.54 | 1.00 (0.61–1.64) | 0.991 | ||
| rs712829 | GG | 43/102 | 1 | 1 | |||
| GT/TT | 47/103 | 1.43 (0.94–2.17) | 0.099 | 1.32 (0.84–2.07) | 0.23 | ||
| MFS | rs2227983 | GA/AA | 30/91 | 1 | 1 | ||
| GG | 37/107 | 0.91 (0.56–1.49) | 0.722 | 0.99 (0.59–1.64) | 0.954 | ||
| rs712830 | CA/AA | 24/77 | 1 | 1 | |||
| CC | 44/128 | 1.50 (0.90–2.50) | 0.115 | 1.45 (0.85–2.50) | 0.174 | ||
| rs712829 | GG | 34/102 | 1 | 1 | |||
| GT/TT | 34/103 | 1.44 (0.88–2.37) | 0.145 | 1.60 (0.95–2.71) | 0.079 |
| Total | |||
|---|---|---|---|
| Endpoint | Variable | HR (95% CI) | p-Value |
| OS | rs712830 CC | 1.31 (1.04–1.64) | 0.02 |
| Stage III | 1.59 (1.07–2.36) | 0.02 | |
| Stage IV | 1.93 (1.22–3.06) | 0.005 | |
| Ever smoking: yes | 2.02 (1.24–3.30) | 0.005 | |
| Chemotherapy: no | 1.84 (1.43–2.37) | 2 × 10−6 | |
| RT dose < 60 Gy | 1.74 (1.36–2.23) | 1.2 × 10−5 | |
| LRFS | rs712829 TT | 2.15 (1.31–3.52) | 0.003 |
| SCC | 1.40 (1.01–1.96) | 0.046 | |
| Zubrod PS 2 | 2.08 (1.10–3.93) | 0.025 | |
| Stage III | 2.61 (1.32–5.14) | 0.006 | |
| Stage IV | 3.11 (1.39–7.00) | 0.006 | |
| Chemotherapy: no | 1.77 (1.18–2.66) | 0.006 | |
| RT dose < 60 Gy | 1.70 (1.17–2.48) | 0.006 | |
| MFS | Stage IV | 4.75 (2.62–8.59) | <1 × 10−6 |
| RT dose < 60 Gy | 2.01 (1.36–2.97) | 0.0004 | |
| Curative treatment subgroup | |||
| Endpoint | Variable | HR (95% CI) | p-value |
| OS | rs712830 CC | 1.44 (1.04–2.01) | 0.03 |
| LRFS | rs2227983 GG | 1.57 (1.01–2.43) | 0.046 |
| rs712829 GT/TT | 1.66 (1.07–2.57) | 0.023 | |
| Stage III–IV | 2.39 (1.10–5.19) | 0.028 | |
| LRFS | rs2227983 GG + rs712829 GT/TT | 2.00 (1.25–3.22) | 0.004 |
| Stage III–IV | 2.24 (1.03–4.88) | 0.041 | |
| MFS | rs712829 GT/TT | 1.68 (1.01–2.78) | 0.045 |
| Age < 63 years | 1.71 (1.00–2.93) | 0.049 | |
| Non–SCC | 1.73 (1.06–2.82) | 0.028 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butkiewicz, D.; Krześniak, M.; Gdowicz-Kłosok, A.; Giglok, M.; Marszałek-Zeńczak, M.; Suwiński, R. Polymorphisms in EGFR Gene Predict Clinical Outcome in Unresectable Non-Small Cell Lung Cancer Treated with Radiotherapy and Platinum-Based Chemoradiotherapy. Int. J. Mol. Sci. 2021, 22, 5605. https://doi.org/10.3390/ijms22115605
Butkiewicz D, Krześniak M, Gdowicz-Kłosok A, Giglok M, Marszałek-Zeńczak M, Suwiński R. Polymorphisms in EGFR Gene Predict Clinical Outcome in Unresectable Non-Small Cell Lung Cancer Treated with Radiotherapy and Platinum-Based Chemoradiotherapy. International Journal of Molecular Sciences. 2021; 22(11):5605. https://doi.org/10.3390/ijms22115605
Chicago/Turabian StyleButkiewicz, Dorota, Małgorzata Krześniak, Agnieszka Gdowicz-Kłosok, Monika Giglok, Małgorzata Marszałek-Zeńczak, and Rafał Suwiński. 2021. "Polymorphisms in EGFR Gene Predict Clinical Outcome in Unresectable Non-Small Cell Lung Cancer Treated with Radiotherapy and Platinum-Based Chemoradiotherapy" International Journal of Molecular Sciences 22, no. 11: 5605. https://doi.org/10.3390/ijms22115605
APA StyleButkiewicz, D., Krześniak, M., Gdowicz-Kłosok, A., Giglok, M., Marszałek-Zeńczak, M., & Suwiński, R. (2021). Polymorphisms in EGFR Gene Predict Clinical Outcome in Unresectable Non-Small Cell Lung Cancer Treated with Radiotherapy and Platinum-Based Chemoradiotherapy. International Journal of Molecular Sciences, 22(11), 5605. https://doi.org/10.3390/ijms22115605






