Temperature Effects on Properties of Rice Husk Biochar and Calcinated Burkina Phosphate Rock
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock and Biochar Preparation
2.2. Calcination of Burkina Phosphate Rock (BPR)
2.3. Mineralogical Analysis
2.4. Chemical Analysis
3. Results and Discussion
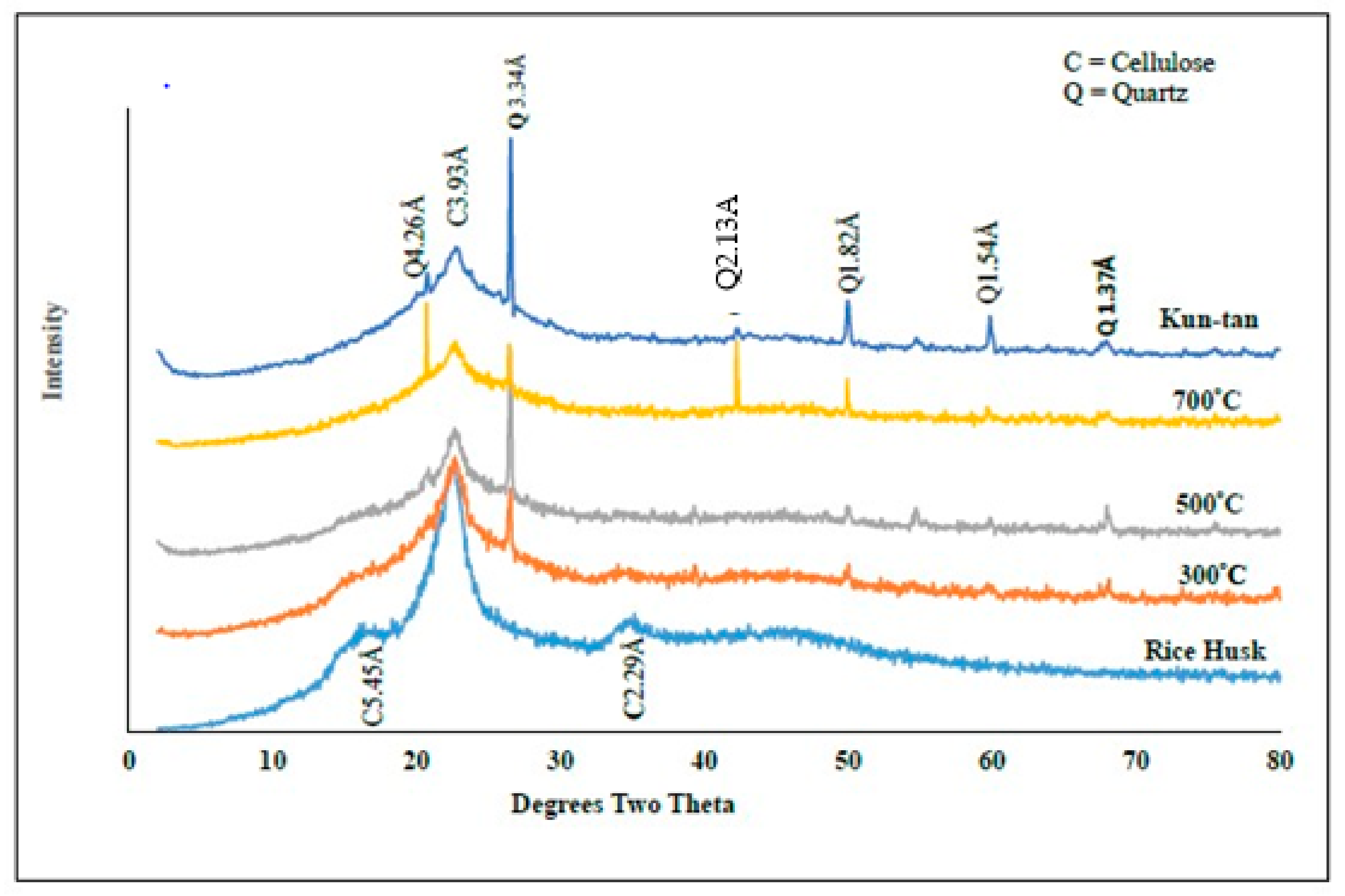
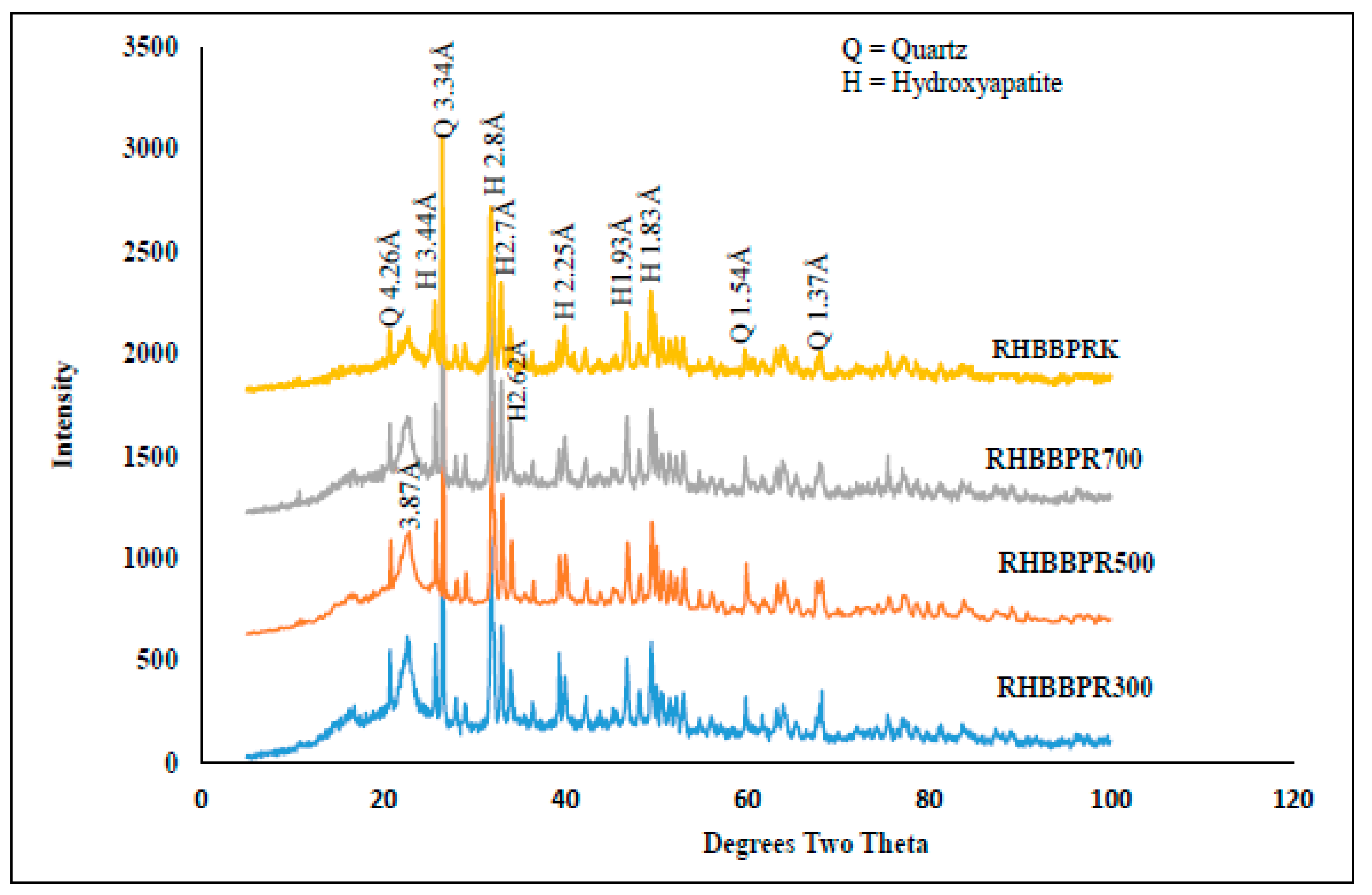
3.1. Mineralogical Properties of RHB, BPR, and RHB-BPR Mixture
3.2. Nitrogen, Hydrogen, and Sulfur Content of RHB, BPR, and RHB-BPR Mixtures
3.3. Carbon and Ash
3.4. Effect of Temperature on pH of RHB, BPR, and RHB-BPR Mixture
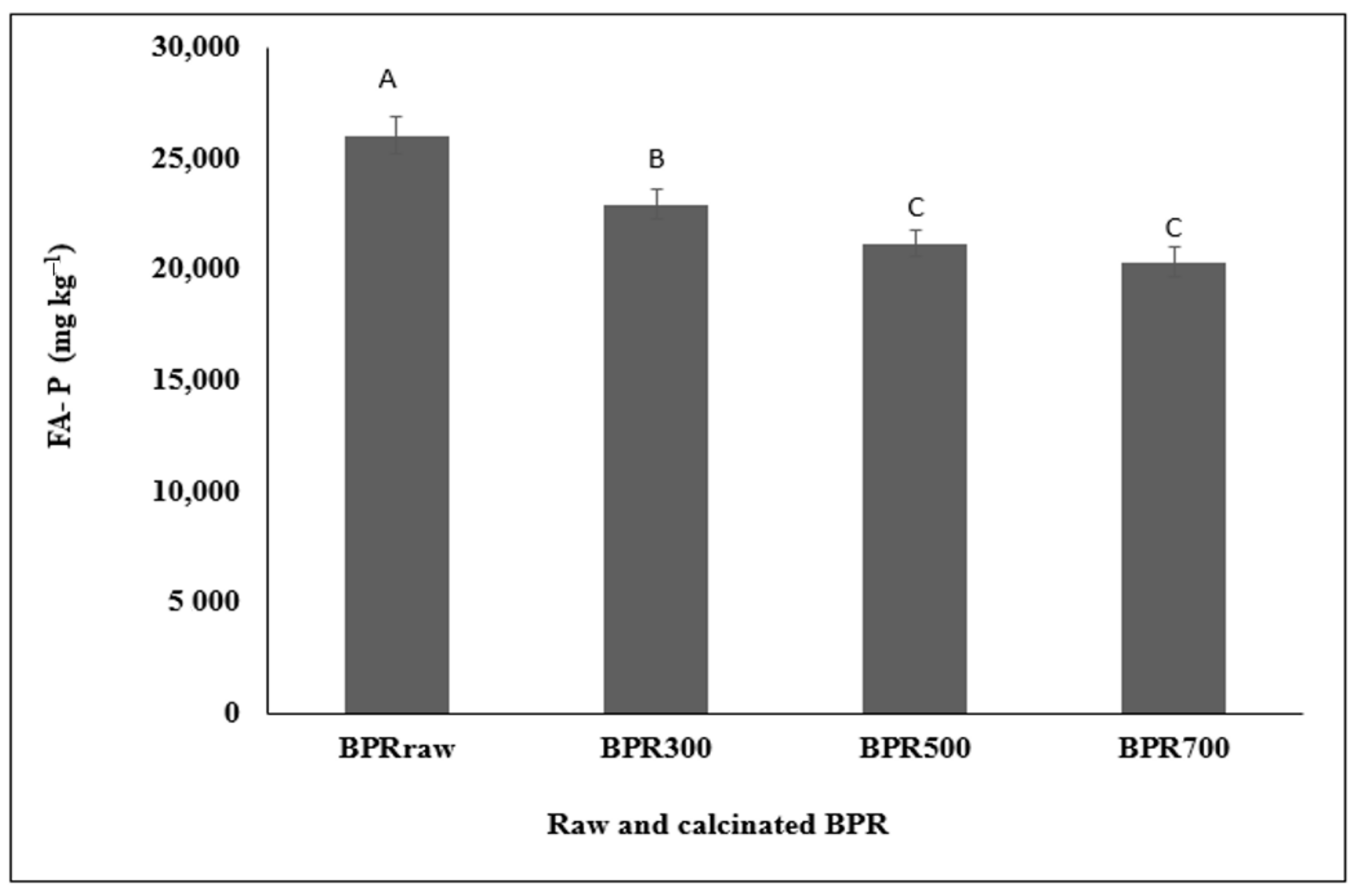
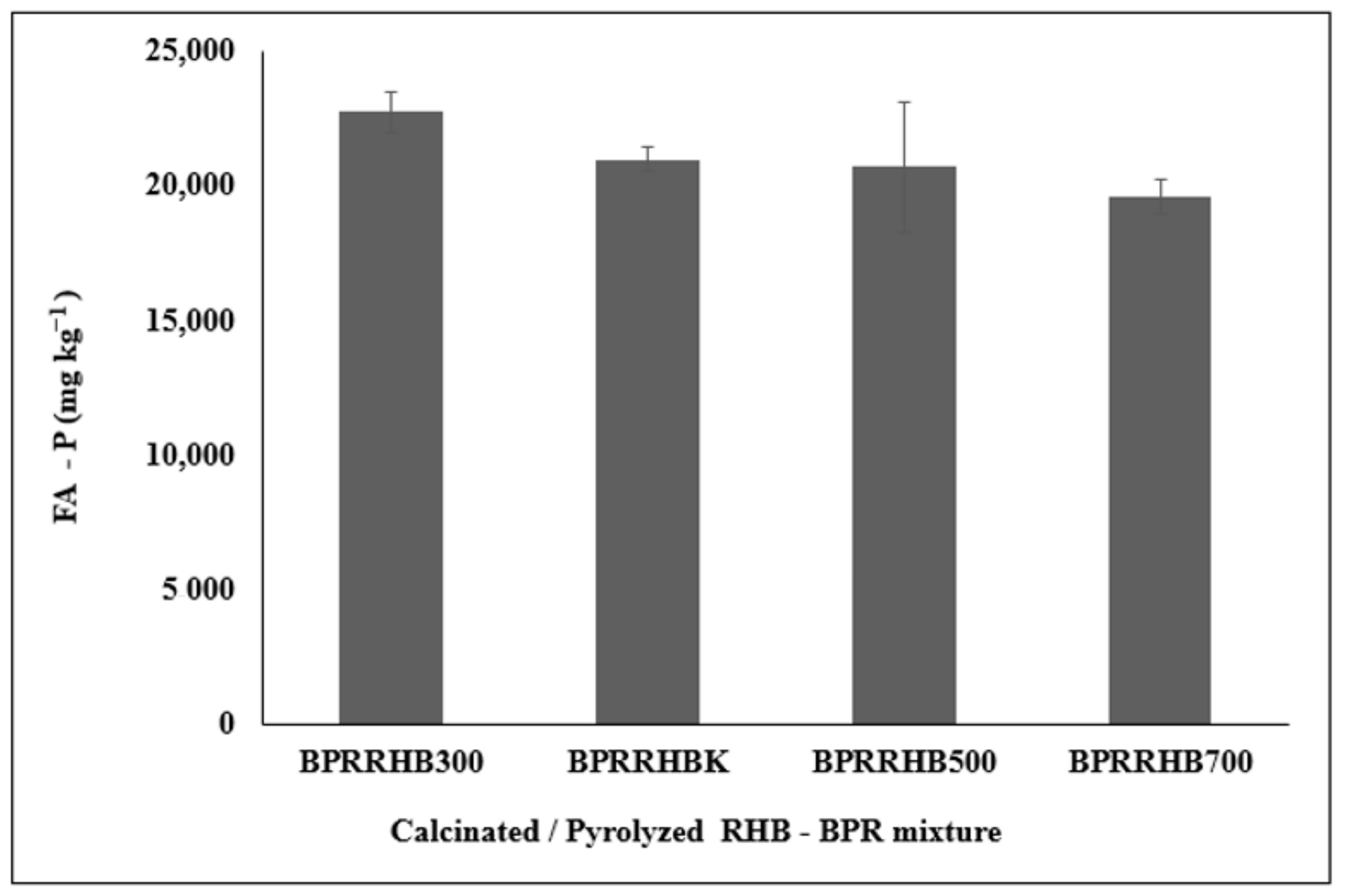
3.5. FA-Extractable P in RHB, BPR, and RHB-BPR Mixture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiessen, H.; Hauffe, H.K.; Mermut, A.R. Deposition of Harmattan dust and its influence on base saturation of soils in northern Ghana. Geoderma 1991, 49, 285–299. [Google Scholar] [CrossRef]
- Amatekpor, J.K. Soils and land-use in the Volta basin: State of the art. In The Sustainable Integrated Development of the Volta Basin in Ghana; Gordon, C., Amatekpor, J.K., Eds.; Volta Basin Research Project, University of Ghana: Legon, Accra, Ghana, 1999; pp. 91–106. [Google Scholar]
- Asiamah, R.D. Soil resources of Ghana. In Synthesis of Soil, Water and Nutrient Management Research in the Volta Basin; Bationo, A., Tabo, R., Waswa, B., Okeyo, J., Kihara, J., Fosu, M., Kabore, S., Eds.; Ecomedia Ltd: Nairobi, Kenya, 2008; p. 331. ISBN 978-92-9059-220-04. [Google Scholar]
- Adu, S.V. Soils of the Nasia Basin, Northern Region, Ghana; Memoir No. 11; CSIR-Soil Research Institute, Advent Press: Kwadaso–Kumasi, Ghana, 1995. [Google Scholar]
- Adu, S.V. Soils of the Bole-Bamboi Area, Northern Region, Ghana; Memoir No. 14; CSIR-Soil Research Institute; Advent Press: Kwadaso–Kumasi, Ghana, 1995. [Google Scholar]
- Haefele, S.M.; Wopereis, M.C.S.; Schloebohm, A.; Wiechmann, H. Long-term fertility experiments for irrigated rice in the West African Sahel: Effect on soil characteristics. Field Crop. Res. 2004, 85, 61–77. [Google Scholar] [CrossRef]
- Mwangi, W.M. Low use of fertilizers and low productivity in sub-Saharan Africa. Nutr. Cycl. Agroecosyst. 1997, 47, 135–147. [Google Scholar] [CrossRef]
- Morris, M.; Kelly, V.A.; Kopicki, R.J.; Byerlee, D. Fertilizer Use in African Agriculture: Lessons Learned and Good Practice Guidelines; World Bank Publications: Washington, DC, USA, 2007; p. 137. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Current World Fertilizer Trends and Outlook to 2015; FAO: Rome, Italy, 2011; p. 40. [Google Scholar]
- Fianko, J.R.; Donkor, A.S.; Lowor, T.; Yeboah, P.O. Agrochemicals and the Ghanaian Environment, a Review. J. Environ. Protect. 2011, 2, 221–230. [Google Scholar] [CrossRef]
- Ragasa, C.; Dankyi, A.; Acheampong, P.; Wiredu, A.N.; Chapoto, A.; Asamoah, M.; Tripp, R. Patterns of Adoption of Improved Rice Technologies in Ghana. IFPRI WORKING PAPER 35. 2013. Available online: http://www.ifpri.org/publication/patterns-adoptionimproved-rice-technologies-ghana (accessed on 20 March 2019).
- SIssaka, R.N.N.; Awuni, J.A.; Dzomeku, I.K.; Buri, M.M.; Avornyo, V.K.; Adjei, E.O.; Fukuda, M.; Awere, D.A.; Tobita, S. Soil Fertility Management for Sustainable Lowland Rice Production in Ghana-Farmer’s Perspectives and Soil Physicochemical Properties. Trop. Agric. Dev. 2016, 60, 119–131. [Google Scholar]
- Fearon, J.; Adraki, P.; Boateng, V. Fertilizer Subsidy Programme in Ghana: Evidence of Performance after Six Years of Implementation. J. Biol. Agric. Healthc. 2015, 5, 100–107. [Google Scholar]
- SIssaka, R.N.N.; Dzomeku, I.K.; Fukuda, M.; Buri, M.M.; Avornyo, V.K.; Owusu Adjei, E.; Awuni, J.A.; Tobita, S. Improvement of Soil Fertility with Use of Indigenous Resources in Lowland Rice Systems. In Soil Fertility Improvement and Integrated Nutrient Management: A Global Perspective; Whalen, J.K., Ed.; InTech: Rijeka, Croatia, 2012; ISBN 978-953-307-945-5. [Google Scholar]
- Issaka, R.N.; Buri, M.M.; Tobita, S.; Nakamura, S.; Owusu-Adjei, E. Indigenous Fertilizing Materials to Enhance Soil Productivity in Ghana. In Soil Fertility Improvement and Integrated Nutrient Management: A Global Perspective; Whalen, J.K., Ed.; InTech: Rijeka, Croatia, 2011; ISBN 978-953-307-945-5. [Google Scholar]
- Japan International Research Center for Agricultural Science. The Study of Improvement of Soil Fertility with Use of Indigenous Resources in Rice Systems of Sub-Sahara Africa; Business Report; Japan International Research Center for Agricultural Science: Tsukuba, Ibaraki, Japan, 2009. [Google Scholar]
- Buri, M.M.; Issaka, R.N.; Wakatsuki, T.; Otoo, E. Soil organic amendments and mineral fertilizers: Options for sustainable lowland rice production in the forest agro-ecology of Ghana. Agric. Food Sci. J. Ghana 2004, 3, 237–324. [Google Scholar]
- Knoblauch, C.; Maarifat, A.A.; Pfeiffer, E.M.; Haefele, S.M. Degradability of black carbon and its impact on trace gas fluxes and carbon turnover in paddy soils. Soil Biol. Biochem. 2011, 43, 1768–1778. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Brewer, C.E.; Hu, Y.Y.; Schmidt-Rohr, K.; Loynachan, T.E.; Laird, D.A.; Brown, R.C. Extent of pyrolysis impacts on fast pyrolysis biochar properties. J. Environ. Qual. 2012, 41, 1115–1122. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef]
- Spokas, K.A.; Reicosky, D.C. Impacts of sixteen different biochars on soil greenhouse gas production. Ann. Environ. Sci. 2009, 3, 179–193. [Google Scholar]
- Lindsay, W.L.; Vlek, P.L.G. Phosphate minerals. In Minerals in the Soil Environment; Dixon, J.B., Weed, S.B., Eds.; Springer Science & Business Media: Madison, WI, USA, 1977; pp. 539–672. [Google Scholar]
- Bolan, N.S.; Hedley, M.J.; Loganathan, P. Preparation, forms and properties of controlled-release phosphate fertilizers. Fertil. Res. 1993, 35, 13–24. [Google Scholar]
- Ando, J. Thermal phosphate. In Manual of Fertilizer Processing; Nielsson, F.T., Ed.; Marcel Dekker Inc: New York, NY, USA, 1987; pp. 93–124. [Google Scholar]
- Nakamura, S. Technology for the solubilization of phosphate rock and its advantages―Phosphate rock solubilization by low-temperature calcination. In Soil Fertility Improvement with Indigenous Resources in Lowland Rice Ecologies in Ghana; Satoshi, T.S.N., Ed.; JIRCAS Working Report No.86; Japan International Research Center for Agricultural Science: Tsukuba, Ibaraki, Japan, 2018; pp. 71–76. [Google Scholar]
- Asuming-Brempong, S.; Anipa, B. The use of rock phosphate and phosphate solubilizing fungi (Aspergillus niger) to improve the growth and the yield of upland rice on Typic Kandiudalf. West Afr. J. Appl. Ecol. 2013, 22, 27–39. [Google Scholar]
- Kpomblekou, K.A.; Tabatabai, M.A. Effect of organic acids on the release of phosphorous from phosphate rock. Soil Sci. 1994, 158, 442–453. [Google Scholar] [CrossRef]
- Owusu-Bennoah, E.; Acquaye, D.K. Greenhouse evaluation of agronomic potential of different sources of phosphate fertilizer in a typical concretionary soil of Northern Ghana. Fert. Res. 1996, 44, 1091–1106. [Google Scholar] [CrossRef]
- Abekoe, M.; Tiessen, H. Fertilizer P transformations and P availability in hillslope soils of northern Ghana. Nutr. Cycl Agroecosys. 1998, 52, 45–54. [Google Scholar] [CrossRef]
- Hammond, L.L.; Chien, S.H.; Roy, A.H.; Mokwunye, A.U. Solubility and agronomic effectiveness of partially acidulated phosphate rocks as influenced by their iron and aluminium oxide content. Fertil. Res. 1989, 19, 93–98. [Google Scholar] [CrossRef]
- Gilkes, R.J.; Palmer, B. Calcined Christmas Island C-Grade Rock Phosphate fertilizers: Mineralogical properties, reversion and assessment by chemical extraction. Aust. J. Soil Res. 1979, 17, 467–481. [Google Scholar] [CrossRef]
- Wang, T.; Camps-Arbestain, M.; Hedley, M.; Bishop, P. Predicting phosphorus bioavailability from high-ash biochars. Plant Soil. 2012, 357, 173–187. [Google Scholar] [CrossRef]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous cellulose structure and characterization. Cell. Chem. Technol. 2011, 45, 13–21. [Google Scholar]
- Ju, X.; Bowden, M.; Browna, E.E.; Zhang, X. An improved X-ray diffraction method for cellulose crystallinity measurement. Carbohyd. Polym. 2015, 123, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y. Rice husk silica derived nanomaterials for sustainable applications. Renew. Sustain. Energy Rev. 2017, 80, 453–466. [Google Scholar] [CrossRef]
- Asuncion, M.Z.; Hasegawa, I.; Kampf, J.W.; Laine, R.M. The selective dissolution of rice hull ash to form [OSiO1.5]8[R4N]8 (R = Me, CH2CH2OH) octasilicates. Basic nano building blocks and possible models of intermediates formed during biosilicification processes. J. Mater. Chem. 2005, 15, 2114–2121. [Google Scholar] [CrossRef]
- Ding, T.P.; Ma, G.R.; Shui, M.X.; Wan, D.F.; Li, R.H. Silicon isotope study on rice plants from the Zhejiang province, China. Chem. Geol. 2005, 218, 41–50. [Google Scholar] [CrossRef]
- Yalçin, N.; Sevinç, V. Studies on silica obtained from rice husk. Ceram. Int. 2001, 27, 219–224. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Lyons, G.; English, P.; Guppy, C.N. Silicon nutrition of rice is affected by soil pH, weathering, and silicon fertilization. J. Plant Nutr. Soil Sc. 2011, 174, 437–446. [Google Scholar] [CrossRef]
- Savant, N.K.; Synder, G.H.; Datnoff, L.E. Silicon management and sustainable rice production. Adv. Agron. 1997, 58, 151–199. [Google Scholar]
- Kamruzzaman, M.; Sadrul Islam, A.K.M. Physical and thermochemical properties of rice husk in Bangladesh. Int. J. Biol. Res. 2011, 11, 45–49. [Google Scholar]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef]
- Aria, M.M.; Lakzian, A.; Haghnia, G.H.; Berenji, A.R.; Besharati, H.; Fotova, A. Effect of Thiobacillus, sulfur, and vermicompost on the water-soluble phosphorus of hard rock phosphate. Bioresour. Technol. 2010, 101, 551–554. [Google Scholar]
- Bangar, K.C.; Shankar, S.K.; Kapoor, K.; Kukreja, K.; Mishra, M.M. Preparation of nitrogen and phosphorous-enriched paddy straw compost and its effect on the yield and nutrient uptake by wheat (Triticum aestivum L.). Biol. Fert. Soils 1989, 8, 339–342. [Google Scholar] [CrossRef]
- Nakamura, S.; Imai, T.; Nagumo, F.; Toriyama, K.; Tobita, S.; Matsunaga, R. Solubilization of Burkina Faso phosphate rock through calcination method. Jpn. J. Soil Sci. Plant Nutr. 2015, 86, 534–538. [Google Scholar]
- Chien, S.H.; Menon, R.G. Agronomic evaluation of modified phosphate rock products IFDS’s experience. Fertil. Res. 1995, 41, 197–209. [Google Scholar] [CrossRef]


| Materials | N % | H % | S % | pHw | Ash % | C % |
|---|---|---|---|---|---|---|
| RH feedstock | 0.39d | 4.97a | 0.22a | 6.20 | 19.59 | 35.07c |
| RHB 300 °C | 0.48b | 2.71a | 0.26ab | 6.38 | 39.79 | 40.36a |
| RHB 500 °C | 0.54a | 2.06c | 0.08bc | 6.64 | 46.55 | 34.62c |
| RHB 700 °C | 0.43cd | 1.82d | 0.06bc | 7.69 | 47.82 | 40.81a |
| RHBK | 0.43c | 2.05c | 0.04c | 6.53 | 43.89 | 37.99b |
| BPR RAW | BDL | 0.34a | 0.17a | 6.40 | NA | BDL |
| BPR 300 °C | BDL | 0.23ab | 0.12b | 7.03 | NA | BDL |
| BPR 500 °C | BDL | 0.20b | 0.08bc | 6.48 | NA | BDL |
| BPR 700 °C | BDL | 0.20b | 0.05c | 6.37 | NA | BDL |
| RHB-BPR 300 °C | 0.01b | 1.20a | 0.05b | 6.76 | NA | 16.17a |
| RHB-BPR 500 °C | 0.02b | 0.99ab | 0.04b | 7.07 | NA | 17.12a |
| RHB-BPR 700 °C | 0.02b | 0.85b | 0.03b | 7.26 | NA | 16.13a |
| RHB-BPRK | 0.20a | 0.99ab | 0.73a | 6.50 | NA | 15.44a |
| SiO2 | Al2O3 | Fe2O3 | SO3 | CaO | MgO | K2O | Na2O | P2O5 | TiO2 | BaO | SrO | Mn2O3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | Basis |
| 20 | 3.56 | 2.13 | 0.19 | 40.1 | 0.32 | 0.28 | 0.15 | 29.8 | 0.22 | 0.07 | 0.17 | 0.05 | LOI-free |
| 19.4 | 3.46 | 2.06 | 0.18 | 38.9 | 0.31 | 0.27 | 0.15 | 28.9 | 0.21 | 0.07 | 0.16 | 0.05 | Oven-dry |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avornyo, V.K.; Manu, A.; Laird, D.A.; Thompson, M.L. Temperature Effects on Properties of Rice Husk Biochar and Calcinated Burkina Phosphate Rock. Agriculture 2021, 11, 432. https://doi.org/10.3390/agriculture11050432
Avornyo VK, Manu A, Laird DA, Thompson ML. Temperature Effects on Properties of Rice Husk Biochar and Calcinated Burkina Phosphate Rock. Agriculture. 2021; 11(5):432. https://doi.org/10.3390/agriculture11050432
Chicago/Turabian StyleAvornyo, Vincent K., Andrew Manu, David A. Laird, and Michael L. Thompson. 2021. "Temperature Effects on Properties of Rice Husk Biochar and Calcinated Burkina Phosphate Rock" Agriculture 11, no. 5: 432. https://doi.org/10.3390/agriculture11050432
APA StyleAvornyo, V. K., Manu, A., Laird, D. A., & Thompson, M. L. (2021). Temperature Effects on Properties of Rice Husk Biochar and Calcinated Burkina Phosphate Rock. Agriculture, 11(5), 432. https://doi.org/10.3390/agriculture11050432





