Angiogenic CD34 Stem Cell Therapy in Coronary Microvascular Repair—A Systematic Review
Abstract
:1. Introduction
2. Discovery of CD34+ Cells as Endothelial Progenitor
3. Preclinical Studies on CD34 Therapy for Ischemic Disease
4. Clinical Studies on CD34 Therapy for Ischemic Disease
4.1. CD34 Therapy for Peripheral Ischemia
4.2. CD34 Therapy for Nonischemic Cardiomyopathy
4.3. CD34 Therapy for Myocardial Infarction
4.4. CD34 Therapy for Ischemic Stroke
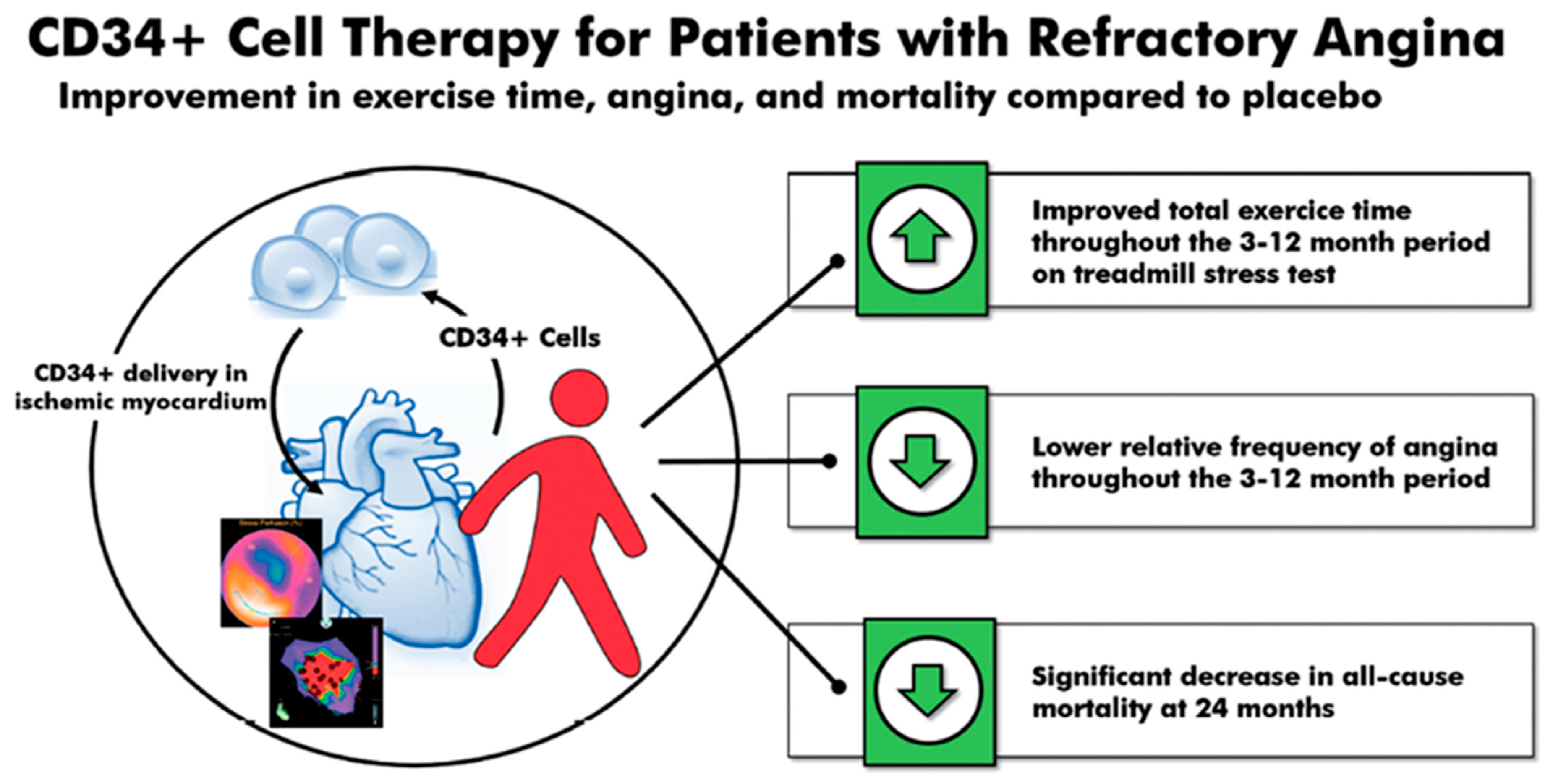
4.5. CD34 Therapy for Refractory Angina
5. Ischemia with Nonobstructive Coronary Artery (INOCA) Disease and Coronary Microvascular Dysfunction (CMD)
5.1. Risk Factors in INOCA and CMD
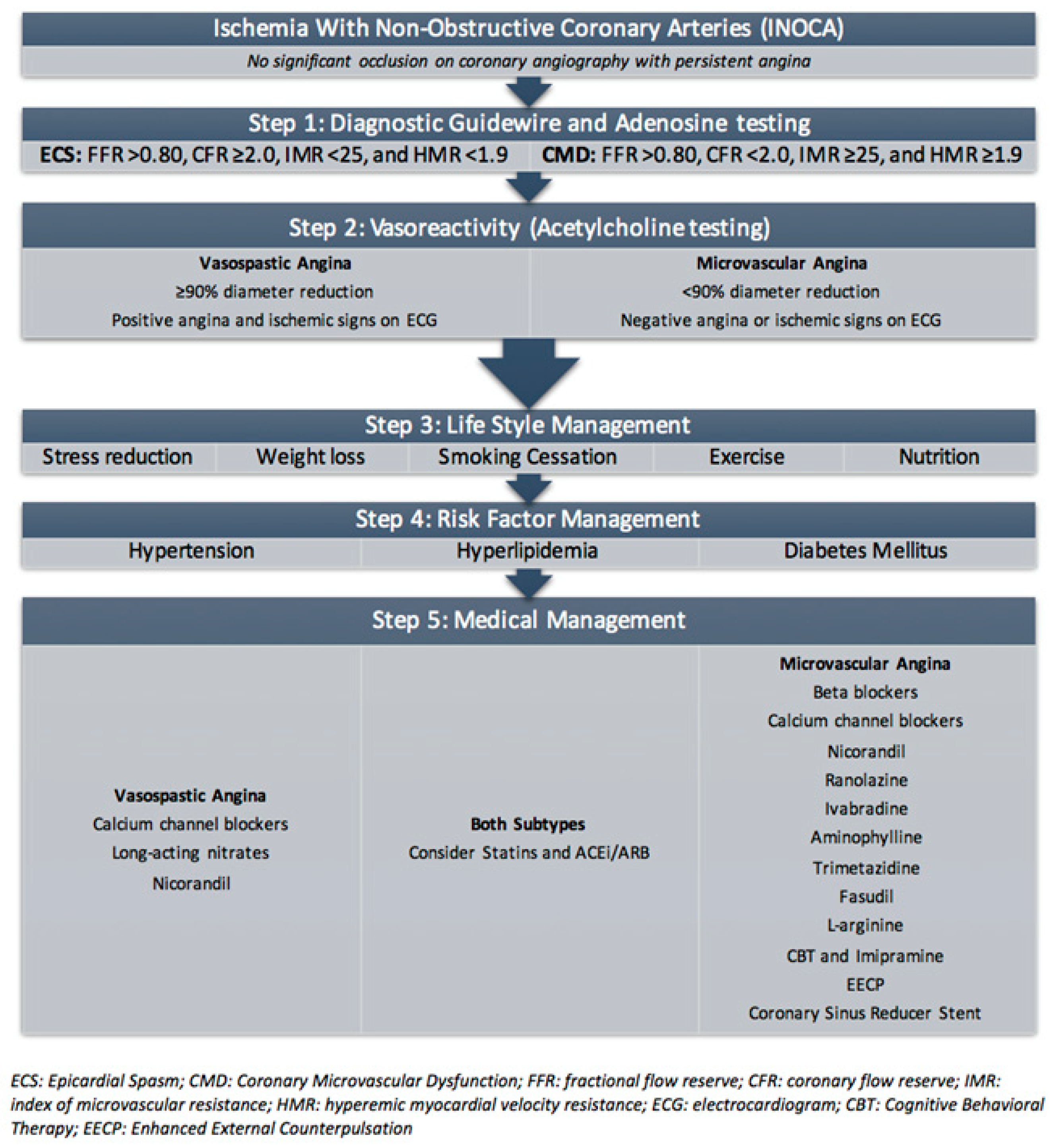
5.2. Diagnosis of INOCA and CMD
6. Current Treatment of Coronary Microvascular Dysfunction
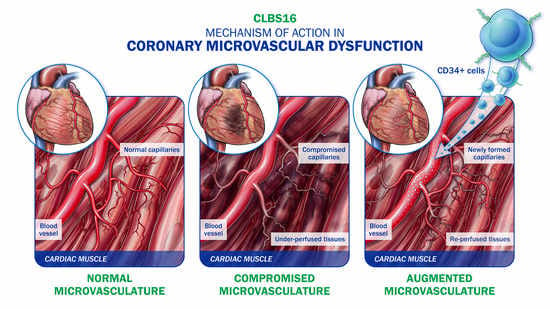
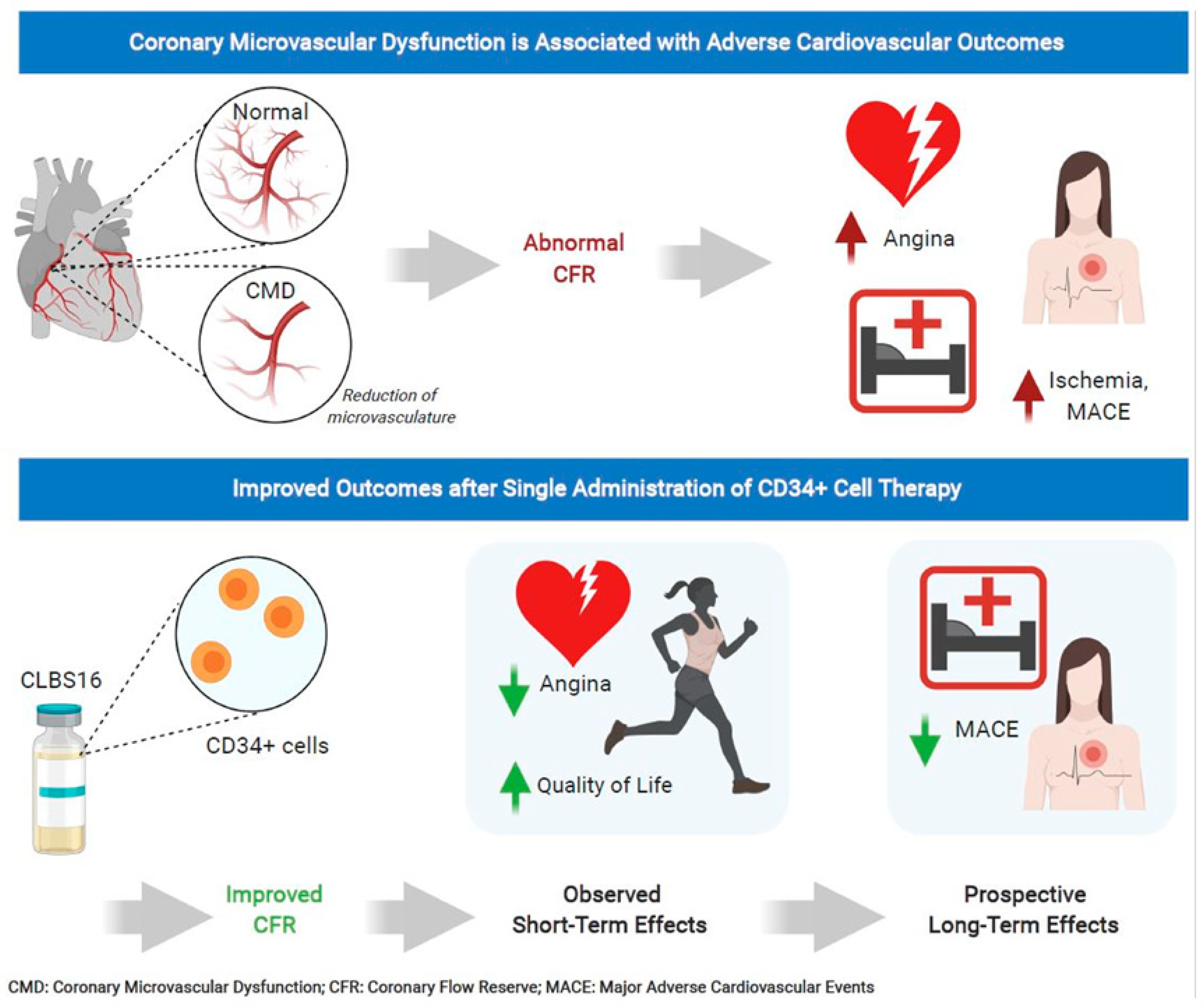
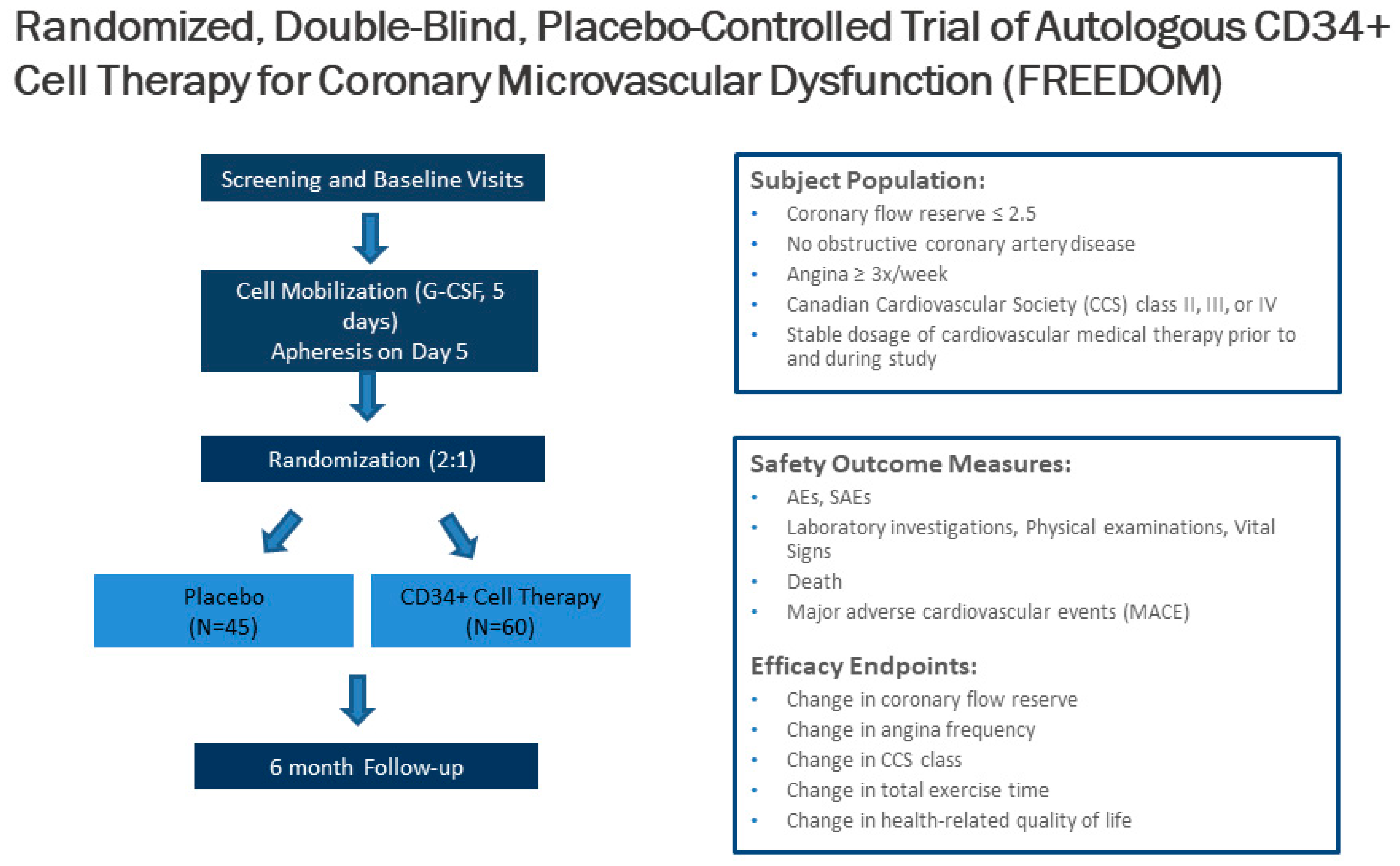
7. CD34 Therapy as a Novel Treatment for Coronary Microvascular Dysfunction
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bairey Merz, C.N.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation 2017, 135, 1075–1092. [Google Scholar] [CrossRef]
- Widmer, R.J.; Samuels, B.; Samady, H.; Price, M.J.; Jeremias, A.; Anderson, R.D.; Jaffer, F.A.; Escaned, J.; Davies, J.; Prasad, M.; et al. The functional assessment of patients with non-obstructive coronary artery disease: Expert review from an international microcirculation working group. EuroIntervention 2019, 14, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention 2021, 16, 1049–1069. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, L.; Hvelplund, A.; Abildstrøm, S.Z.; Pedersen, F.; Galatius, S.; Madsen, J.K.; Jørgensen, E.; Kelbæk, H.; Prescott, E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012, 33, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.; Widmer, R.J.; Matsuzawa, Y.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. Jacc. Cardiovasc. Interv. 2015, 8, 1445–1453. [Google Scholar] [CrossRef]
- Recio-Mayoral, A.; Mason, J.C.; Kaski, J.C.; Rubens, M.B.; Harari, O.A.; Camici, P.G. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur. Heart J. 2009, 30, 1837–1843. [Google Scholar] [CrossRef] [Green Version]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study Group. International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20. [Google Scholar] [CrossRef]
- Shaw, L.J.; Shaw, R.E.; Merz, C.N.; Brindis, R.G.; Klein, L.W.; Nallamothu, B.; Douglas, P.S.; Krone, R.J.; McKay, C.R.; Block, P.C.; et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation 2008, 117, 1787–1801. [Google Scholar] [CrossRef] [Green Version]
- Reis, S.E.; Holubkov, R.; Lee, J.S.; Sharaf, B.; Reichek, N.; Rogers, W.J.; Walsh, E.G.; Fuisz, A.R.; Kerensky, R.; Detre, K.M.; et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women’s Ischemia Syndrome Evaluation (WISE) study. J. Am. Coll. Cardiol. 1999, 33, 1469–1475. [Google Scholar] [CrossRef] [Green Version]
- Reis, S.E.; Holubkov, R.; Conrad Smith, A.J.; Kelsey, S.F.; Sharaf, B.L.; Reichek, N.; Rogers, W.J.; Merz, C.N.; Sopko, G.; Pepine, C.J.; et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: Results from the NHLBI WISE study. Am. Heart J. 2001, 141, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Pepine, C.J.; Anderson, R.D.; Sharaf, B.L.; Reis, S.E.; Smith, K.M.; Handberg, E.M.; Johnson, B.D.; Sopko, G.; Bairey Merz, C.N. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J. Am. Coll. Cardiol. 2010, 55, 2825–2832. [Google Scholar] [CrossRef] [Green Version]
- Sietsema, W.K.; Kawamoto, A.; Takagi, H.; Losordo, D.W. Autologous CD34+ Cell Therapy for Ischemic Tissue Repair. Circ. J. 2019, 83, 1422–1430. [Google Scholar] [CrossRef] [Green Version]
- Prasad, M.; Corban, M.T.; Henry, T.D.; Dietz, A.B.; Lerman, L.O.; Lerman, A. Promise of autologous CD34+ stem/progenitor cell therapy for treatment of cardiovascular disease. Cardiovasc. Res. 2020, 116, 1424–1433. [Google Scholar] [CrossRef]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef]
- Losordo, D.W.; Schatz, R.A.; White, C.J.; Udelson, J.E.; Veereshwarayya, V.; Durgin, M.; Poh, K.K.; Weinstein, R.; Kearney, M.; Chaudhry, M.; et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: A phase I/IIa double-blind, randomized controlled trial. Circulation 2007, 115, 3165–3172. [Google Scholar] [CrossRef] [Green Version]
- Losordo, D.W.; Henry, T.D.; Davidson, C.; Sup Lee, J.; Costa, M.A.; Bass, T.; Mendelsohn, F.; Fortuin, F.D.; Pepine, C.J.; Traverse, J.H.; et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ. Res. 2011, 109, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Henry, T.D.; Schaer, G.L.; Traverse, J.H.; Povsic, T.J.; Davidson, C.; Lee, J.S.; Costa, M.A.; Bass, T.; Mendelsohn, F.; Fortuin, F.D.; et al. Autologous CD34(+) Cell Therapy for Refractory Angina: 2-Year Outcomes From the ACT34-CMI Study. Cell Transpl. 2016, 25, 1701–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Povsic, T.J.; Henry, T.D.; Traverse, J.H.; Fortuin, F.D.; Schaer, G.L.; Kereiakes, D.J.; Schatz, R.A.; Zeiher, A.M.; White, C.J.; Stewart, D.J.; et al. The RENEW Trial: Efficacy and Safety of Intramyocardial Autologous CD34(+) Cell Administration in Patients With Refractory Angina. Jacc. Cardiovasc. Interv. 2016, 9, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.D.; Losordo, D.W.; Traverse, J.H.; Schatz, R.A.; Jolicoeur, E.M.; Schaer, G.L.; Clare, R.; Chiswell, K.; White, C.J.; Fortuin, F.D.; et al. Autologous CD34+ cell therapy improves exercise capacity, angina frequency and reduces mortality in no-option refractory angina: A patient-level pooled analysis of randomized double-blinded trials. Eur. Heart J. 2018, 39, 2208–2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quyyumi, A.A.; Vasquez, A.; Kereiakes, D.J.; Klapholz, M.; Schaer, G.L.; Abdel-Latif, A.; Frohwein, S.; Henry, T.D.; Schatz, R.A.; Dib, N.; et al. PreSERVE-AMI: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Intracoronary Administration of Autologous CD34+ Cells in Patients With Left Ventricular Dysfunction Post STEMI. Circ. Res. 2017, 120, 324–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, T.D.; Noel Bairey Merz, C.; Wei, J.; Corban, M.T.; Quesada, O.; Joung, S.; Kotynski, C.L.; Wang, J.; Lewis, M.; Schumacher, A.M.; et al. CD34+ stem cell therapy increases coronary flow reserve and reduces angina in patients with coronary microvascular dysfunction. Circ. Cardiovasc. Interv. 2021. under review. [Google Scholar]
- Roscoe, R.A.; Rybka, W.B.; Winkelstein, A.; Houston, A.M.; Kiss, J.E. Enumeration of CD34+ hematopoietic stem cells for reconstitution following myeloablative therapy. Cytometry 1994, 16, 74–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshioka, T.; Ageyama, N.; Shibata, H.; Yasu, T.; Misawa, Y.; Takeuchi, K.; Matsui, K.; Yamamoto, K.; Terao, K.; Shimada, K.; et al. Repair of infarcted myocardium mediated by transplanted bone marrow-derived CD34+ stem cells in a nonhuman primate model. Stem Cells 2005, 23, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Mackie, A.R.; Losordo, D.W. CD34-positive stem cells: In the treatment of heart and vascular disease in human beings. Tex. Heart Inst. J. 2011, 38, 474–485. [Google Scholar]
- Schatteman, G.C.; Hanlon, H.D.; Jiao, C.; Dodds, S.G.; Christy, B.A. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J. Clin. Investig. 2000, 106, 571–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhou, B.; Han, Z.C. Therapeutic neovascularization by transplantation of mobilized peripheral blood mononuclear cells for limb ischemia. A comparison between CD34+ and CD34- mononuclear cells. Thromb. Haemost. 2006, 95, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, P.; Emanueli, C.; Pelosi, E.; Salis, M.B.; Cerio, A.M.; Bonanno, G.; Patti, M.; Stassi, G.; Condorelli, G.; Peschle, C. Transplantation of low dose CD34+KDR+ cells promotes vascular and muscular regeneration in ischemic limbs. FASEB J. 2004, 18, 1737–1739. [Google Scholar] [CrossRef]
- Kocher, A.A.; Schuster, M.D.; Szabolcs, M.J.; Takuma, S.; Burkhoff, D.; Wang, J.; Homma, S.; Edwards, N.M.; Itescu, S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat. Med. 2001, 7, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Murohara, T.; Ikeda, H.; Duan, J.; Shintani, S.; Sasaki, K.; Eguchi, H.; Onitsuka, I.; Matsui, K.; Imaizumi, T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J. Clin. Investig. 2000, 105, 1527–1536. [Google Scholar] [CrossRef] [Green Version]
- Majka, M.; Janowska-Wieczorek, A.; Ratajczak, J.; Ehrenman, K.; Pietrzkowski, Z.; Kowalska, M.A.; Gewirtz, A.M.; Emerson, S.G.; Ratajczak, M.Z. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood 2001, 97, 3075–3085. [Google Scholar] [CrossRef] [Green Version]
- Ohtake, T.; Mochida, Y.; Ishioka, K.; Oka, M.; Maesato, K.; Moriya, H.; Hidaka, S.; Higashide, S.; Ioji, T.; Fujita, Y.; et al. Autologous Granulocyte Colony-Stimulating Factor-Mobilized Peripheral Blood CD34 Positive Cell Transplantation for Hemodialysis Patients with Critical Limb Ischemia: A Prospective Phase II Clinical Trial. Stem Cells Transl. Med. 2018, 7, 774–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamoto, A.; Fujita, Y.; Sietsema, W.K.; Wang, J.; Takagi, H.; Losordo, D.W. Design of a potentially registrational study of sakigake-designated GCSF-mobilized autologous CD34 cell (CLBS12) therapy of no-option critical limb ischemia including arteriosclerosis obliterans and buerger’s disease. Cytotherapy 2020, 22, S61. [Google Scholar] [CrossRef]
- Vrtovec, B.; Poglajen, G.; Lezaic, L.; Sever, M.; Domanovic, D.; Cernelc, P.; Socan, A.; Schrepfer, S.; Torre-Amione, G.; Haddad, F.; et al. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ. Res. 2013, 112, 165–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Bentley, P.; Hamady, M.; Marley, S.; Davis, J.; Shlebak, A.; Nicholls, J.; Williamson, D.A.; Jensen, S.L.; Gordon, M.; et al. Intra-Arterial Immunoselected CD34+ Stem Cells for Acute Ischemic Stroke. Stem Cells Transl. Med. 2014, 3, 1322–1330. [Google Scholar] [CrossRef]
- Sargento-Freitas, J.; Pereira, A.; Gomes, A.; Amorim, P.; Matos, T.; Cardoso, C.M.P.; Silva, F.; Santo, G.C.; Nunes, C.; Galego, O.; et al. STROKE34 Study Protocol: A Randomized Controlled Phase IIa Trial of Intra-Arterial CD34+ Cells in Acute Ischemic Stroke. Front. Neurol. 2018, 9, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, G.L.; Henry, T.D.; Povsic, T.J.; Losordo, D.W.; Garberich, R.F.; Stanberry, L.I.; Strauss, C.E.; Traverse, J.H. CD34(+) cell therapy significantly reduces adverse cardiac events, health care expenditures, and mortality in patients with refractory angina. Stem Cells Transl. Med. 2020, 9, 1147–1152. [Google Scholar] [CrossRef]
- Wang, S.; Cui, J.; Peng, W.; Lu, M. Intracoronary autologous CD34+ stem cell therapy for intractable angina. Cardiology 2010, 117, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Sorop, O.; Merkus, D.; de Beer, V.J.; Houweling, B.; Pistea, A.; McFalls, E.O.; Boomsma, F.; van Beusekom, H.M.; van der Giessen, W.J.; VanBavel, E.; et al. Functional and structural adaptations of coronary microvessels distal to a chronic coronary artery stenosis. Circ. Res. 2008, 102, 795–803. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef]
- Taqueti, V.R.; Hachamovitch, R.; Murthy, V.L.; Naya, M.; Foster, C.R.; Hainer, J.; Dorbala, S.; Blankstein, R.; Di Carli, M.F. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 2015, 131, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Khuddus, M.A.; Pepine, C.J.; Handberg, E.M.; Bairey Merz, C.N.; Sopko, G.; Bavry, A.A.; Denardo, S.J.; McGorray, S.P.; Smith, K.M.; Sharaf, B.L.; et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: A substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). J. Interv. Cardiol. 2010, 23, 511–519. [Google Scholar] [CrossRef]
- Gulati, M.; Cooper-DeHoff, R.M.; McClure, C.; Johnson, B.D.; Shaw, L.J.; Handberg, E.M.; Zineh, I.; Kelsey, S.F.; Arnsdorf, M.F.; Black, H.R.; et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: A report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch. Intern. Med. 2009, 169, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taqueti, V.R.; Shaw, L.J.; Cook, N.R.; Murthy, V.L.; Shah, N.R.; Foster, C.R.; Hainer, J.; Blankstein, R.; Dorbala, S.; Di Carli, M.F. Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation 2017, 135, 566–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.M.; Huo, T.; Delia Johnson, B.; Bittner, V.; Kelsey, S.F.; Vido Thompson, D.; Noel Bairey Merz, C.; Pepine, C.J.; Cooper-Dehoff, R.M. Cardiovascular and mortality risk of apparent resistant hypertension in women with suspected myocardial ischemia: A report from the NHLBI-sponsored WISE Study. J. Am. Heart Assoc. 2014, 3, e000660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Carli, M.F.; Janisse, J.; Grunberger, G.; Ager, J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J. Am. Coll. Cardiol. 2003, 41, 1387–1393. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Nelson, M.D.; Szczepaniak, E.W.; Smith, L.; Mehta, P.K.; Thomson, L.E.; Berman, D.S.; Li, D.; Bairey Merz, C.N.; Szczepaniak, L.S. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H14–H19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, P.A.; Gnecchi-Ruscone, T.; Schäfers, K.P.; Lüscher, T.F.; Camici, P.G. Low density lipoprotein cholesterol and coronary microvascular dysfunction in hypercholesterolemia. J. Am. Coll. Cardiol. 2000, 36, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Vitiello, L.; Spoletini, I.; Gorini, S.; Pontecorvo, L.; Ferrari, D.; Ferraro, E.; Stabile, E.; Caprio, M.; la Sala, A. Microvascular inflammation in atherosclerosis. Int. J. Cardiol. Metab. Endocr. 2014, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Taqueti, V.R.; Everett, B.M.; Murthy, V.L.; Gaber, M.; Foster, C.R.; Hainer, J.; Blankstein, R.; Dorbala, S.; Di Carli, M.F. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation 2015, 131, 528–535. [Google Scholar] [CrossRef] [Green Version]
- Samim, A.; Nugent, L.; Mehta, P.K.; Shufelt, C.; Bairey Merz, C.N. Treatment of angina and microvascular coronary dysfunction. Curr. Treat. Options. Cardiovasc. Med. 2010, 12, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Harris, K.F.; Matthews, K.A. Interactions between autonomic nervous system activity and endothelial function: A model for the development of cardiovascular disease. Psychosom. Med. 2004, 66, 153–164. [Google Scholar] [CrossRef]
- Mygind, N.D.; Michelsen, M.M.; Pena, A.; Frestad, D.; Dose, N.; Aziz, A.; Faber, R.; Host, N.; Gustafsson, I.; Hansen, P.R.; et al. Coronary Microvascular Function and Cardiovascular Risk Factors in Women with Angina Pectoris and No Obstructive Coronary Artery Disease: The iPOWER Study. J. Am. Heart Assoc. 2016, 5, e003064. [Google Scholar] [CrossRef] [Green Version]
- Taqueti, V.R.; Di Carli, M.F. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2625–2641. [Google Scholar] [CrossRef]
- Feher, A.; Sinusas, A.J. Quantitative Assessment of Coronary Microvascular Function: Dynamic Single-Photon Emission Computed Tomography, Positron Emission Tomography, Ultrasound, Computed Tomography, and Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2017, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murthy, V.L.; Naya, M.; Taqueti, V.R.; Foster, C.R.; Gaber, M.; Hainer, J.; Dorbala, S.; Blankstein, R.; Rimoldi, O.; Camici, P.G.; et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014, 129, 2518–2527. [Google Scholar] [CrossRef] [Green Version]
- Thomson, L.E.; Wei, J.; Agarwal, M.; Haft-Baradaran, A.; Shufelt, C.; Mehta, P.K.; Gill, E.B.; Johnson, B.D.; Kenkre, T.; Handberg, E.M.; et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ. Cardiovasc. Imaging 2015, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, M.; Weinberg, N.; Pohost, G.M.; Bairey Merz, C.N.; Shaw, L.J.; Sopko, G.; Fuisz, A.; Rogers, W.J.; Walsh, E.G.; Johnson, B.D.; et al. Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: Results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. Jacc. Cardiovasc. Imaging 2010, 3, 1030–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K.; et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J. Am. Coll. Cardiol. 2018, 72, 2841–2855. [Google Scholar] [CrossRef]
- Ludmer, P.L.; Selwyn, A.P.; Shook, T.L.; Wayne, R.R.; Mudge, G.H.; Alexander, R.W.; Ganz, P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N. Engl. J. Med. 1986, 315, 1046–1051. [Google Scholar] [CrossRef]
- Fearon, W.F.; Kobayashi, Y. Invasive Assessment of the Coronary Microvasculature: The Index of Microcirculatory Resistance. Circ. Cardiovasc. Interv. 2017, 10, e005361. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.R.; Zeitz, C.J.; Rajendran, S.; Di Fiore, D.P.; Tavella, R.; Beltrame, J.F. Clinical and coronary haemodynamic determinants of recurrent chest pain in patients without obstructive coronary artery diseas-A pilot study. Int. J. Cardiol. 2018, 267, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study Group. International standardization of diagnostic criteria for vasospastic angina. Eur. Heart J. 2017, 38, 2565–2568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, A.; Takahashi, J.; Hao, K.; Kikuchi, Y.; Shindo, T.; Ikeda, S.; Sato, K.; Sugisawa, J.; Matsumoto, Y.; Miyata, S.; et al. Coronary Functional Abnormalities in Patients with Angina and Nonobstructive Coronary Artery Disease. J. Am. Coll. Cardiol. 2019, 74, 2350–2360. [Google Scholar] [CrossRef]
- Jespersen, L.; Abildstrom, S.Z.; Hvelplund, A.; Prescott, E. Persistent angina: Highly prevalent and associated with long-term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin. Res. Cardiol. 2013, 102, 571–581. [Google Scholar] [CrossRef]
- Brainin, P.; Frestad, D.; Prescott, E. The prognostic value of coronary endothelial and microvascular dysfunction in subjects with normal or non-obstructive coronary artery disease: A systematic review and meta-analysis. Int. J. Cardiol. 2018, 254, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Suhrs, H.E.; Michelsen, M.M.; Prescott, E. Treatment strategies in coronary microvascular dysfunction: A systematic review of interventional studies. Microcirculation 2019, 26, e12430. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. [2018 ESC/ESH Guidelines for the management of arterial hypertension]. Kardiol. Pol. 2019, 77, 71–159. [Google Scholar] [CrossRef] [Green Version]
- Pauly, D.F.; Johnson, B.D.; Anderson, R.D.; Handberg, E.M.; Smith, K.M.; Cooper-DeHoff, R.M.; Sopko, G.; Sharaf, B.M.; Kelsey, S.F.; Merz, C.N.; et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am. Heart J. 2011, 162, 678–684. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, Q.; Zhao, J.; Li, X.; Sun, X.; Yang, H.; Wu, Z.; Yang, J. Effects of combination of statin and calcium channel blocker in patients with cardiac syndrome X. Coron. Artery. Dis. 2014, 25, 40–44. [Google Scholar] [CrossRef]
- Task Force, M.; Montalescot, G.; Sechtem, U.; Achenbach, S.; Andreotti, F.; Arden, C.; Budaj, A.; Bugiardini, R.; Crea, F.; Cuisset, T.; et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 2013, 34, 2949–3003. [Google Scholar] [CrossRef]
- Wei, J.; Shufelt, C.; Bairey Merz, C.N. Women’s health: Making cardiovascular disease real. Curr. Opin. Cardiol. 2018, 33, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Berry, C. How to Diagnose and Manage Angina Without Obstructive Coronary Artery Disease: Lessons from the British Heart Foundation CorMicA Trial. Interv. Cardiol. 2019, 14, 76–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, G.; Di Franco, A.; Lamendola, P.; Tarzia, P.; Nerla, R.; Stazi, A.; Villano, A.; Sestito, A.; Lanza, G.A.; Crea, F. Lack of effect of nitrates on exercise stress test results in patients with microvascular angina. Cardiovasc. Drugs 2013, 27, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Bairey Merz, C.N.; Handberg, E.M.; Shufelt, C.L.; Mehta, P.K.; Minissian, M.B.; Wei, J.; Thomson, L.E.; Berman, D.S.; Shaw, L.J.; Petersen, J.W.; et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): Impact on angina and myocardial perfusion reserve. Eur. Heart J. 2016, 37, 1504–1513. [Google Scholar] [CrossRef]
- Guarini, G.; Huqi, A.; Morrone, D.; Capozza, P.; Todiere, G.; Marzilli, M. Pharmacological approaches to coronary microvascular dysfunction. Pharm. Ther. 2014, 144, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Villano, A.; Di Franco, A.; Nerla, R.; Sestito, A.; Tarzia, P.; Lamendola, P.; Di Monaco, A.; Sarullo, F.M.; Lanza, G.A.; Crea, F. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am. J. Cardiol. 2013, 112, 8–13. [Google Scholar] [CrossRef]
- Yesildag, O.; Yazici, M.; Yilmaz, O.; Ucar, R.; Sagkan, O. The effect of aminophylline infusion on the exercise capacity in patients with syndrome X. Acta Cardiol. 1999, 54, 335–337. [Google Scholar]
- Shi, J.; Wei, L. Rho kinases in cardiovascular physiology and pathophysiology: The effect of fasudil. J. Cardiovasc. Pharm. 2013, 62, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Palmer, R.M.; Ashton, D.S.; Moncada, S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 1988, 333, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Asbury, E.A.; Kanji, N.; Ernst, E.; Barbir, M.; Collins, P. Autogenic training to manage symptomology in women with chest pain and normal coronary arteries. Menopause 2009, 16, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.O., 3rd; Quyyumi, A.A.; Mincemoyer, R.; Stine, A.M.; Gracely, R.H.; Smith, W.B.; Geraci, M.F.; Black, B.C.; Uhde, T.W.; Waclawiw, M.A.; et al. Imipramine in patients with chest pain despite normal coronary angiograms. N. Engl. J. Med. 1994, 330, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, B.; Shukla, J.; Henry, T.D.; Quesada, O. Angiogenic CD34 Stem Cell Therapy in Coronary Microvascular Repair—A Systematic Review. Cells 2021, 10, 1137. https://doi.org/10.3390/cells10051137
Rai B, Shukla J, Henry TD, Quesada O. Angiogenic CD34 Stem Cell Therapy in Coronary Microvascular Repair—A Systematic Review. Cells. 2021; 10(5):1137. https://doi.org/10.3390/cells10051137
Chicago/Turabian StyleRai, Balaj, Janki Shukla, Timothy D. Henry, and Odayme Quesada. 2021. "Angiogenic CD34 Stem Cell Therapy in Coronary Microvascular Repair—A Systematic Review" Cells 10, no. 5: 1137. https://doi.org/10.3390/cells10051137