Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables
Abstract
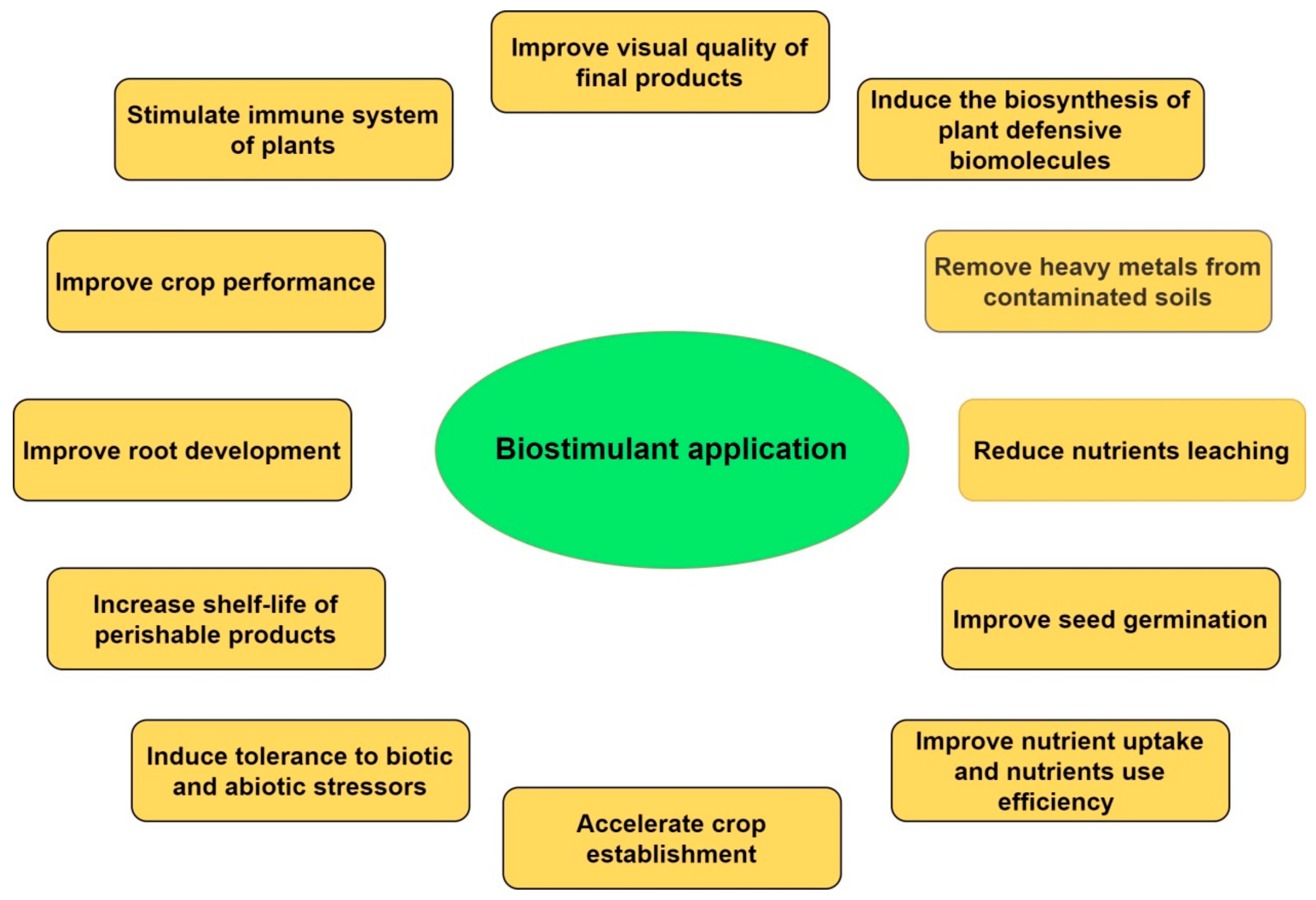
:1. Introduction
2. Biostimulant Categories
3. Practical Applications of Biostimulants and Biostimulatory Products on Horticultural Crops
4. Future Remarks and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russo, R.O.; Berlyn, G.P. The Use of Organic Biostimulants to Help Low Input Sustainable Agriculture. J. Sustain. Agric. 1991, 1, 19–42. [Google Scholar] [CrossRef]
- Szparaga, A.; Kuboń, M.; Kocira, S.; Czerwińska, E.; Pawłowska, A.; Hara, P.; Kobus, Z.; Kwaśniewski, D. Towards Sustainable Agriculture—Agronomic and Economic Effects of Biostimulant Use in Common Bean Cultivation. Sustainability 2019, 11, 4575. [Google Scholar] [CrossRef] [Green Version]
- Postel, S.L. Entering an era of water scarcity: The challenges ahead. Ecol. Appl. 2000, 10, 941–948. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total. Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2014, 31, 1–17. [Google Scholar] [CrossRef]
- Caradonia, F.; Battaglia, V.; Righi, L.; Pascali, G.; La Torre, A. Plant Biostimulant Regulatory Framework: Prospects in Europe and Current Situation at International Level. J. Plant Growth Regul. 2019, 38, 438–448. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2018, 82, 277–285. [Google Scholar] [CrossRef]
- Askari-Khorasgani, O.; Hatterman-Valenti, H.; Pardo, F.B.F.; Pessarakli, M. Plant and symbiont metabolic regulation and biostimulants application improve symbiotic performance and cold acclimation. J. Plant Nutr. 2019, 42, 2151–2163. [Google Scholar] [CrossRef]
- Massa, D.; Lenzi, A.; Montoneri, E.; Ginepro, M.; Prisa, D.; Burchi, G. Plant response to biowaste soluble hydrolysates in hibiscus grown under limiting nutrient availability. J. Plant Nutr. 2017, 41, 396–409. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The Use of Biostimulants for Enhancing Nutrient Uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Taofiq, O.; Fernandes, Â.; Tzortzakis, N.; Ciric, A.; Sokovic, M.; Barros, L.; Ferreira, I.C. Bioactive properties of greenhouse-cultivated green beans (Phaseolus vulgaris L.) under biostimulants and water-stress effect. J. Sci. Food Agric. 2019, 99, 6049–6059. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R. Biostimulants Application Alleviates Water Stress Effects on Yield and Chemical Composition of Greenhouse Green Bean (Phaseolus vulgaris L.). Agronomy 2020, 10, 181. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Dias, M.I.; Petropoulos, S.A.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Calhelha, R.C.; Ivanov, M.; Stojković, D.; Soković, M.; et al. The Effects of Biostimulants, Biofertilizers and Water-Stress on Nutritional Value and Chemical Composition of Two Spinach Genotypes (Spinacia oleracea L.). Molecules 2019, 24, 4494. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, C.; Gonzalez-Coloma, A.; Prigent-Combaret, C. Plant metabolomics to the benefit of crop protection and growth stimulation. Adv. Bot. Res. 2021, 98, 107–132. [Google Scholar] [CrossRef]
- Colla, G.; Svecová, E.; Cardarelli, M.; Rouphael, Y.; Reynaud, H.; Canaguier, R.; Planques, B. Effectiveness of A Plant-Derived Protein Hydrolysate to Improve Crop Performances under Different Growing Conditions. Acta Hortic. 2013, 175–179. [Google Scholar] [CrossRef]
- Cerdán, M.; Sánchez-Sánchez, A.; Jordá, J.D.; Juárez, M.; Sánchez-Andreu, J. Effect of commercial amino acids on iron nutrition of tomato plants grown under lime-induced iron deficiency. J. Plant Nutr. Soil Sci. 2013, 176, 859–866. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Tejada, M.; Benítez, C.; Gómez, I.; Parrado, J. Use of biostimulants on soil restoration: Effects on soil biochemical properties and microbial community. Appl. Soil Ecol. 2011, 49, 11–17. [Google Scholar] [CrossRef]
- Karapouloutidou, S.; Gasparatos, D. Effects of Biostimulant and Organic Amendment on Soil Properties and Nutrient Status of Lactuca sativa in a Calcareous Saline-Sodic Soil. Agriculture 2019, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiee, H.; Badi, H.N.; Mehrafarin, A.; Qaderi, A.; Zarinpanjeh, N.; Sekara, A.; Zand, E. Application of Plant Biostimulants as New Approach to Improve the Biological Responses of Medicinal Plants- A Critical Review. J. Med. Plants 2016, 15, 1–39. [Google Scholar]
- Vasconsuelo, A.; Boland, R. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 2007, 172, 861–875. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a Sustainable Agriculture Through Plant Biostimulants: From Experimental Data to Practical Applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.; del Cerro, P.; Espuny, M.; Jiménez-Guerrero, I.; López-Baena, F.; Ollero, F.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef] [Green Version]
- De Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Geelen, D.; Xu, L. The Chemical Biology of Plant Biostimulants; Wiley: Ghent, Belgium, 2020. [Google Scholar]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; et al. Morphological and Physiological Responses Induced by Protein Hydrolysate-Based Biostimulant and Nitrogen Rates in Greenhouse Spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Gupta, V.; Kumar, M.; Brahmbhatt, H.; Reddy, C.; Seth, A.; Jha, B. Simultaneous determination of different endogenetic plant growth regulators in common green seaweeds using dispersive liquid–liquid microextraction method. Plant Physiol. Biochem. 2011, 49, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Sivasankari, S.; Venkatesalu, V.; Anantharaj, M.; Chandrasekaran, M. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour. Technol. 2006, 97, 1745–1751. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Kulkarni, M.G.; Pendota, S.C.; Van Staden, J. Enhancing growth, phytochemical constituents and aphid resistance capacity in cabbage with foliar application of eckol—A biologically active phenolic molecule from brown seaweed. New Biotechnol. 2016, 33, 273–279. [Google Scholar] [CrossRef]
- Ghosh, A.; Anand, K.V.; Seth, A. Life cycle impact assessment of seaweed based biostimulant production from onshore cultivated Kappaphycus alvarezii (Doty) Doty ex Silva—Is it environmentally sustainable? Algal Res. 2015, 12, 513–521. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-Based Biostimulants Modulate Rhizosphere Microbial Populations and Improve N Uptake Efficiency, Yield, and Nutritional Quality of Leafy Vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Marra, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Olivares, F.L.; Busato, J.G.; De Paula, A.M.; Lima, L.D.S.; Aguiar, N.O.; Canellas, L.P. Plant growth promoting bacteria and humic substances: Crop promotion and mechanisms of action. Chem. Biol. Technol. Agric. 2017, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced Systemic Resistance and Promotion of Plant Growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef] [Green Version]
- Glick, R.B. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kloepper, J.W.; Ryu, C.-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef]
- Wong, W.S.; Zhong, H.T.; Cross, A.T.; Wan, J.; Yong, H. Plant Biostimulants in Vermicomposts: Characteristics and Plausible Mechanisms. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Jackson, M.B. Are Plant Hormones Involved in Root to Shoot Communication? In Advances in Botanical Research; Callow, J.A., Ed.; Academic Press: Cambridge, MA, USA, 1993; Volume 19, pp. 103–187. ISBN 0065-2296. [Google Scholar]
- Lu, Y.; Wang, E.; Tang, Z.; Rui, J.; Li, Y.; Tang, Z.; Dong, W.; Liu, X.; George, T.S.; Song, A.; et al. Roots and microbiome jointly drive the distributions of 17 phytohormones in the plant soil continuum in a phytohormone-specific manner. Plant Soil 2021, 1–13. [Google Scholar] [CrossRef]
- Rosier, A.; Medeiros, F.H.V.; Bais, H.P. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil 2018, 428, 35–55. [Google Scholar] [CrossRef] [Green Version]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular Mycorrhizal Fungi and Associated Microbiota as Plant Biostimulants: Research Strategies for the Selection of the Best Performing Inocula. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef] [Green Version]
- Puglisi, I.; Barone, V.; Sidella, S.; Coppa, M.; Broccanello, C.; Gennari, M.; Baglieri, A. Biostimulant activity of humic-like substances from agro-industrial waste on Chlorella vulgaris and Scenedesmus quadricauda. Eur. J. Phycol. 2018, 53, 433–442. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Francioso, O.; Ertani, A.; Muscolo, A.; Nardi, S. Isopentenyladenosine and cytokinin-like activity of different humic substances. J. Geochem. Explor. 2013, 129, 70–75. [Google Scholar] [CrossRef]
- Vujinović, T.; Zanin, L.; Venuti, S.; Contin, M.; Ceccon, P.; Tomasi, N.; Pinton, R.; Cesco, S.; De Nobili, M. Biostimulant Action of Dissolved Humic Substances from a Conventionally and an Organically Managed Soil on Nitrate Acquisition in Maize Plants. Front. Plant Sci. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- De Hita Mejía, D.; Fuentes, M.; García, A.; Olaetxea, M.; Baigorri, R.; Zamarreño, A.M.; Berbara, R.; Garcia-Mina, J.M. Humic substances: A valuable agronomic tool for improving crop adaptation to saline water irrigation. Water Sci. Technol. Water Supply 2019, 19, 1735–1740. [Google Scholar] [CrossRef]
- Olaetxea, M.; De Hita, D.; Garcia, C.A.; Fuentes, M.; Baigorri, R.; Mora, V.; Garnica, M.; Urrutia, O.; Erro, J.; Zamarreño, A.M.; et al. Hypothetical framework integrating the main mechanisms involved in the promoting action of rhizospheric humic substances on plant root- and shoot- growth. Appl. Soil Ecol. 2018, 123, 521–537. [Google Scholar] [CrossRef]
- Jindo, K.; Martim, S.A.; Navarro, E.C.; Pérez-Alfocea, F.; Hernandez, T.; Garcia, C.; Aguiar, N.O.; Canellas, L.P. Root growth promotion by humic acids from composted and non-composted urban organic wastes. Plant Soil 2012, 353, 209–220. [Google Scholar] [CrossRef]
- Garcia, A.C.; Olaetxea, M.; Santos, L.A.; Mora, V.; Baigorri, R.; Fuentes, M.; Zamarreño, A.M.; Berbara, R.L.; Garcia-Mina, J.M. Involvement of hormone- and ROS- signaling pathways in the beneficial action of humic substances on plants growing under normal and stressing conditions. BioMed. Res. Int. 2016, 2016, 3747501. [Google Scholar] [CrossRef]
- Conselvan, G.B.; Fuentes, D.; Merchant, A.; Peggion, C.; Francioso, O.; Carletti, P. Effects of humic substances and indole-3-acetic acid on Arabidopsis sugar and amino acid metabolic profile. Plant Soil 2018, 426, 17–32. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Scaglia, B.; Pognani, M.; Adani, F. The anaerobic digestion process capability to produce biostimulant: The case study of the dissolved organic matter (DOM) vs. auxin-like property. Sci. Total. Environ. 2017, 589, 36–45. [Google Scholar] [CrossRef]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. Biostimulant activity of phosphite in horticulture. Sci. Hortic. 2015, 196, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Rickard, D.A. Review of phosphorus acid and its salts as fertilizer materials. J. Plant Nutr. 2000, 23, 161–180. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Production of chitosan oligosaccharides for inclusion in a plant biostimulant. Pure Appl. Chem. 2016, 88, 881–889. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Chitosan as Soil Amendment Affects Lettuce Growth, Photochemical Efficiency, and Gas Exchange. HortTechnology 2018, 28, 476–480. [Google Scholar] [CrossRef] [Green Version]
- Singh, S. Enhancing phytochemical levels, enzymatic and antioxidant activity of spinach leaves by chitosan treatment and an insight into the metabolic pathway using DART-MS technique. Food Chem. 2016, 199, 176–184. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Recent Advances of Chitosan Applications in Plants. Polymers 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ugolini, L.; Cinti, S.; Righetti, L.; Stefan, A.; Matteo, R.; D’Avino, L.; Lazzeri, L. Production of an enzymatic protein hydrolyzate from defatted sunflower seed meal for potential application as a plant biostimulant. Ind. Crop. Prod. 2015, 75, 15–23. [Google Scholar] [CrossRef]
- Pane, C.; Palese, A.M.; Spaccini, R.; Piccolo, A.; Celano, G.; Zaccardelli, M. Enhancing sustainability of a processing tomato cultivation system by using bioactive compost teas. Sci. Hortic. 2016, 202, 117–124. [Google Scholar] [CrossRef]
- Messias, R.D.S.; Silveira, C.A.P.; Galli, V.; Pillon, C.N.; Rombaldi, C.V. Multimineral and Organic Composition of a Liquid By-Product from the Pyrobituminous Shale Pyrolysis Process and its Potential Use in Agriculture. J. Plant Nutr. 2015, 38, 959–972. [Google Scholar] [CrossRef]
- Debnath, B.; Sikdar, A.; Islam, S.; Hasan, K.; Li, M.; Qiu, D. Physiological and Molecular Responses to Acid Rain Stress in Plants and the Impact of Melatonin, Glutathione and Silicon in the Amendment of Plant Acid Rain Stress. Molecules 2021, 26, 862. [Google Scholar] [CrossRef]
- Hanson, A.D.; Beaudoin, G.A.; Mccarty, D.R.; Gregory, J.F. Does Abiotic Stress Cause Functional B Vitamin Deficiency in Plants? Plant Physiol. 2016, 172, 2082–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teklić, T.; Parađiković, N.; Špoljarević, M.; Zeljković, S.; Lončarić, Z.; Lisjak, M. Linking abiotic stress, plant metabolites, biostimulants and functional food. Ann. Appl. Biol. 2021, 178, 169–191. [Google Scholar] [CrossRef]
- Vílchez, J.I.; Navas, A.; González-López, J.; Arcos, S.C.; Manzanera, M. Biosafety test for plant growth-promoting bacteria: Proposed Environmental and Human Safety Index (EHSI) Protocol. Front. Microbiol. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Barros-Rodríguez, A.; Rangseekaew, P.; Lasudee, K.; Pathom-Aree, W.; Manzanera, M. Regulatory risks associated with bacteria as biostimulants and biofertilizers in the frame of the European Regulation (EU) 2019/1009. Sci. Total Environ. 2020, 740, 140239. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.F.; Rosen, C.J.; Venterea, R.T. Contrasting effects of inhibitors and biostimulants on agronomic performance and reactive nitrogen losses during irrigated potato production. Field Crop. Res. 2019, 240, 143–153. [Google Scholar] [CrossRef]
- Toscano, S.; Romano, D.; Massa, D.; Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulant applications in low input horticultural cultivation systems. Italus Hortus 2018, 27–36. [Google Scholar] [CrossRef]
- Ahmad, R.; Lim, C.J.; Kwon, S.-Y. Glycine betaine: A versatile compound with great potential for gene pyramiding to improve crop plant performance against environmental stresses. Plant Biotechnol. Rep. 2013, 7, 49–57. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, D. Developing Biostimulants from Agro-Food and Industrial By-Products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [Green Version]
- Vioque, J.; Sánchez-Vioque, R.; Clemente, A.; Pedroche, J.; Millán, F. Partially hydrolyzed rapeseed protein isolates with improved functional properties. J. Am. Oil Chem. Soc. 2000, 77, 447–450. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agricola 2016, 73, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. Environ. Boil. Fishes 2011, 23, 371–393. [Google Scholar] [CrossRef]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed Oligosaccharides Stimulate Plant Growth by Enhancing Carbon and Nitrogen Assimilation, Basal Metabolism, and Cell Division. J. Plant Growth Regul. 2012, 32, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi Pirbalouti, A.; Malekpoor, F.; Salimi, A.; Golparvar, A. Exogenous application of chitosan on biochemical and phys-iological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Sci. Hortic. 2017, 217, 114–122. [Google Scholar] [CrossRef]
- Varadarajan, D.K.; Karthikeyan, A.S.; Matilda, P.D.; Raghothama, K.G. Phosphite, an Analog of Phosphate, Suppresses the Coordinated Expression of Genes under Phosphate Starvation. Plant Physiol. 2002, 129, 1232–1240. [Google Scholar] [CrossRef] [Green Version]
- Tambascio, C.; Covacevich, F.; Lobato, M.C.; de Lasa, C.; Caldiz, D.; Dosio, G.; Andreu, A. The Application of K Phosphites to Seed Tubers Enhanced Emergence, Early Growth and Mycorrhizal Colonization in Potato (Solanum tuberosum). Am. J. Plant Sci. 2014, 5, 132–137. [Google Scholar] [CrossRef]
- Olivieri, F.; Feldman, M.; Machinandiarena, M.; Lobato, M.; Caldiz, D.; Daleo, G.; Andreu, A. Phosphite applications induce molecular modifications in potato tuber periderm and cortex that enhance resistance to pathogens. Crop. Prot. 2012, 32, 1–6. [Google Scholar] [CrossRef]
- Lobato, M.C.; Machinandiarena, M.F.; Tambascio, C.; Dosio, G.A.A.; Caldiz, D.O.; Daleo, G.R.; Andreu, A.B.; Olivieri, F.P. Effect of foliar applications of phosphite on post-harvest potato tubers. Eur. J. Plant Pathol. 2011, 130, 155–163. [Google Scholar] [CrossRef]
- Oyarburo, N.S.; Machinandiarena, M.F.; Feldman, M.L.; Daleo, G.R.; Andreu, A.B.; Olivieri, F.P. Potassium phosphite increases tolerance to UV-B in potato. Plant Physiol. Biochem. 2015, 88, 1–8. [Google Scholar] [CrossRef]
- Moor, U.; Põldma, P.; Tõnutare, T.; Karp, K.; Starast, M.; Vool, E. Effect of phosphite fertilization on growth, yield and fruit composition of strawberries. Sci. Hortic. 2009, 119, 264–269. [Google Scholar] [CrossRef]
- Estrada-Ortiz, E.; Trejo-Téllez, L.I.; Gómez-Merino, F.C.; Núñez-Escobar, R.; Sandoval-Villa, M. The effects of phosphite on strawberry yield and fruit quality. J. Soil Sci. Plant Nutr. 2013, 13, 612–620. [Google Scholar] [CrossRef] [Green Version]
- Lovatt, C.J. Properly Timing Foliar-applied Fertilizers Increases Efficacy: A Review and Update on Timing Foliar Nutrient Applications to Citrus and Avocado. HortTechnology 2013, 23, 536–541. [Google Scholar] [CrossRef] [Green Version]
- Aremu, A.O.; Stirk, W.A.; Kulkarni, M.G.; Tarkowská, D.; Turečková, V.; Gruz, J.; Šubrtová, M.; Pěnčík, A.; Novák, O.; Dolezal, K.; et al. Evidence of phytohormones and phenolic acids variability in garden-waste-derived vermicompost leachate, a well-known plant growth stimulant. Plant Growth Regul. 2014, 75, 483–492. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, S.N.; Teo, C.H.; Yew, Y.R.; Ge, L.; Chen, X.; Yong, J.W.H. Analysis of phytohormones in vermicompost using a novel combinative sample preparation strategy of ultrasound-assisted extraction and solid-phase extraction coupled with liquid chromatography–tandem mass spectrometry. Talanta 2015, 139, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Hodges, D.M.; Critchley, A.T.; Prithiviraj, B. A Commercial Extract of Brown Macroalga (Ascophyllum nodosum) Affects Yield and the Nutritional Quality of Spinach In Vitro. Commun. Soil Sci. Plant Anal. 2013, 44, 1873–1884. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Zhang, J.; Kirby, C.W.; Ji, X.; Locke, S.J.; Critchley, A.T.; Prithiviraj, B. Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem. 2011, 124, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant- and Seaweed-Based Extracts Increase Yield but Differentially Modulate Nutritional Quality of Greenhouse Spinach through Biostimulant Action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Chrysargyris, A.; Xylia, P.; Anastasiou, M.; Pantelides, I.; Tzortzakis, N. Effects of Ascophyllum nodosum seaweed extracts on lettuce growth, physiology and fresh-cut salad storage under potassium deficiency. J. Sci. Food Agric. 2018, 98, 5861–5872. [Google Scholar] [CrossRef] [PubMed]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Ascophyllum extract application can promote plant growth and root yield in carrot associated with increased root-zone soil microbial activity. Can. J. Plant Sci. 2014, 94, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Effect of Ascophyllum extract application on plant growth, fruit yield and soil microbial communities of strawberry. Can. J. Plant Sci. 2013, 93, 23–36. [Google Scholar] [CrossRef]
- Weber, N.; Schmitzer, V.; Jakopic, J.; Stampar, F. First fruit in season: Seaweed extract and silicon advance organic strawberry (Fragaria×ananassa Duch.) fruit formation and yield. Sci. Hortic. 2018, 242, 103–109. [Google Scholar] [CrossRef]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. A novel type of seaweed extract as a natural alternative to the use of iron chelates in strawberry production. Sci. Hortic. 2010, 125, 263–269. [Google Scholar] [CrossRef]
- Galvão, Í.M.; dos Santos, O.F.; de Souza, M.L.C.; de Jesus Guimarães, J.; Kühn, I.E.; Broetto, F. Biostimulants action in common bean crop submitted to water deficit. In Toward a Sustainable Agriculture Through Plant Biostimulants: From Data to Practical Applications; Rouphael, Y., Colla, G., Eds.; MDPI: Basel, Switzerland, 2019; Volume 225, p. 105762. [Google Scholar]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. Environ. Boil. Fishes 2019, 32, 573–597. [Google Scholar] [CrossRef] [Green Version]
- Rayorath, P.; Jithesh, M.N.; Farid, A.; Khan, W.; Palanisamy, R.; Hankins, S.D.; Critchley, A.T.; Prithiviraj, B. Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. Environ. Boil. Fishes 2007, 20, 423–429. [Google Scholar] [CrossRef]
- Dookie, M.; Ali, O.; Ramsubhag, A.; Jayaraman, J. Flowering gene regulation in tomato plants treated with brown seaweed extracts. Sci. Hortic. 2021, 276, 109715. [Google Scholar] [CrossRef]
- Shukla, P.S.; Prithiviraj, B. Ascophyllum nodosum Biostimulant Improves the Growth of Zea mays Grown Under Phosphorus Impoverished Conditions. Front. Plant Sci. 2021, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; El Nakhel, C.; Leone, V.; Mori, M. Effect of seaweed (Ecklonia maxima) extract and legume-derived protein hydrolysate biostimulants on baby leaf lettuce grown on optimal doses of nitrogen under greenhouse conditions. Aust. J. Crop. Sci. 2020, 14, 1456–1464. [Google Scholar] [CrossRef]
- Herrera, D.D.F.; Muñoz-Ochoa, M.; Hernández-Herrera, R.M.; Hernández-Carmona, G. Biostimulant activity of individual and blended seaweed extracts on the germination and growth of the mung bean. Environ. Boil. Fishes 2018, 31, 2025–2037. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Rengasamy, K.R.; Pendota, S.C.; Gruz, J.; Plačková, L.; Novák, O.; Doležal, K.; Van Staden, J. Bioactive molecules derived from smoke and seaweed Ecklonia maxima showing phytohormone-like activity in Spinacia oleracea L. New Biotechnol. 2019, 48, 83–89. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M.; Sikorska, A.; Grzywacz, K.; Niewęgłowski, M. Marketable yield of potato and its quantitative parameters after application of herbicides and biostimulants. Agriculture 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Wadas, W.; Dziugieł, T. Changes in assimilation area and chlorophyll content of very early potato (Solanum tuberosum L.) cultivars as influenced by biostimulants. Agronomy 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Wadas, W.; Dziugieł, T. Quality of new potatoes (Solanum tuberosum L.) in response to plant biostimulants application. Agriculture 2020, 10, 265. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M.; Mystkowska, I.; Sikorska, A. Total and True Protein Content in Potato Tubers Depending on Herbicides and Biostimulants. Agronomy 2020, 10, 1106. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Kulkarni, M.G.; Papenfus, H.B.; Van Staden, J. Quantification of plant growth biostimulants, phloroglucinol and eckol, in four commercial seaweed liquid fertilizers and some by-products. Algal Res. 2016, 20, 57–60. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Kulkarni, M.G.; Stirk, W.A.; Van Staden, J. Eckol—A new plant growth stimulant from the brown seaweed Ecklonia maxima. Environ. Boil. Fishes 2015, 27, 581–587. [Google Scholar] [CrossRef]
- Stirk, W.A.; Tarkowská, D.; Turečová, V.; Strnad, M.; Van Staden, J. Abscisic acid, gibberellins and brassinosteroids in Kelpak®, a commercial seaweed extract made from Ecklonia maxima. Environ. Boil. Fishes 2013, 26, 561–567. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Consentino, B.B.; Virga, G.; La Placa, G.G.; Sabatino, L.; Rouphael, Y.; Ntatsi, G.; Iapichino, G.; La Bella, S.; Mauro, R.P.; D’Anna, F.; et al. Celery (Apium graveolens L.) Performances as Subjected to Different Sources of Protein Hydrolysates. Plants 2020, 9, 1633. [Google Scholar] [CrossRef]
- García-Santiago, J.C.; Cavazos, C.J.L.; González-Fuentes, J.A.; Zermeño-González, A.; Alvarado, E.R.; Duarte, A.R.; Preciado-Rangel, P.; Troyo-Diéguez, E.; Ramos, F.M.P.; Valdez-Aguilar, L.A.; et al. Effects of fish-derived protein hydrolysate, animal-based organic fertilisers and irrigation method on the growth and quality of grape tomatoes. Biol. Agric. Hortic. 2021, 37, 107–124. [Google Scholar] [CrossRef]
- Madende, M.; Hayes, M. Fish By-Product Use as Biostimulants: An Overview of the Current State of the Art, Including Relevant Legislation and Regulations within the EU and USA. Molecules 2020, 25, 1122. [Google Scholar] [CrossRef] [Green Version]
- Baglieri, A.; Cadili, V.; Monterumici, C.M.; Gennari, M.; Tabasso, S.; Montoneri, E.; Nardi, S.; Negre, M. Fertilization of bean plants with tomato plants hydrolysates. Effect on biomass production, chlorophyll content and N assimilation. Sci. Hortic. 2014, 176, 194–199. [Google Scholar] [CrossRef]
- Chehade, L.A.; Al Chami, Z.; De Pascali, S.A.; Cavoski, I.; Fanizzi, F.P. Biostimulants from food processing by-products: Agronomic, quality and metabolic impacts on organic tomato (Solanum lycopersicum L.). J. Sci. Food Agric. 2018, 98, 1426–1436. [Google Scholar] [CrossRef]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Cuciniello, A.; Cenvinzo, V.; Bonini, P.; Colla, G.; Rouphael, Y. Yield and Nutritional Quality of Vesuvian Piennolo Tomato PDO as Affected by Farming System and Biostimulant Application. Agronomy 2019, 9, 505. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Desoky, E.-S.M.; Osman, A.; Rady, M.M. Pumpkin seed protein hydrolysate treatment alleviates salt stress effects on Phaseolus vulgaris by elevating antioxidant capacity and recovering ion homeostasis. Sci. Hortic. 2020, 271, 109495. [Google Scholar] [CrossRef]
- Koleška, I.; Hasanagić, D.; Todorović, V.; Murtić, S.; Klokić, I.; Parađiković, N.; Kukavica, B. Biostimulant prevents yield loss and reduces oxidative damage in tomato plants grown on reduced NPK nutrition. J. Plant Interact. 2017, 12, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Casadesús, A.; Polo, J.; Munné-Bosch, S. Hormonal Effects of an Enzymatically Hydrolyzed Animal Protein-Based Biostimulant (Pepton) in Water-Stressed Tomato Plants. Front. Plant Sci. 2019, 10, 758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-Wide Identification of Differentially Expressed Genes in Solanum lycopersicon L. in Response to an Alfalfa-Protein Hydrolysate Using Microarrays. Front. Plant Sci. 2017, 8, 1159. [Google Scholar] [CrossRef] [Green Version]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein Hydrolysate Stimulates Growth in Tomato Coupled With N-Dependent Gene Expression Involved in N Assimilation. Front. Plant Sci. 2018, 9, 1233. [Google Scholar] [CrossRef] [Green Version]
- Ceccarelli, A.; Miras-Moreno, B.; Buffagni, V.; Senizza, B.; Pii, Y.; Cardarelli, M.; Rouphael, Y.; Colla, G.; Lucini, L. Foliar Application of Different Vegetal-Derived Protein Hydrolysates Distinctively Modulates Tomato Root Development and Metabolism. Plants 2021, 10, 326. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Reynaud, H.; Canaguier, R.; Trtílek, M.; Panzarová, K.; et al. Understanding the Biostimulant Action of Vegetal-Derived Protein Hydrolysates by High-Throughput Plant Phenotyping and Metabolomics: A Case Study on Tomato. Front. Plant Sci. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A Combined Phenotypic and Metabolomic Approach for Elucidating the Biostimulant Action of a Plant-Derived Protein Hydrolysate on Tomato Grown Under Limited Water Availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef]
- Lucini, L.; Miras-Moreno, B.; Rouphael, Y.; Cardarelli, M.; Colla, G. Combining Molecular Weight Fractionation and Metabolomics to Elucidate the Bioactivity of Vegetal Protein Hydrolysates in Tomato Plants. Front. Plant Sci. 2020, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Hamedani, S.R.; Rouphael, Y.; Colla, G.; Colantoni, A.; Cardarelli, M. Biostimulants as a Tool for Improving Environmental Sustainability of Greenhouse Vegetable Crops. Sustainability 2020, 12, 5101. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Caruso, G.; Cozzolino, E.; De Pascale, S.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Stand-Alone and Combinatorial Effects of Plant-based Biostimulants on the Production and Leaf Quality of Perennial Wall Rocket. Plants 2020, 9, 922. [Google Scholar] [CrossRef]
- Wilson, H.T.; Amirkhani, M.; Taylor, A.G. Evaluation of Gelatin as a Biostimulant Seed Treatment to Improve Plant Performance. Front. Plant Sci. 2018, 9, 1006. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous Application of Amino Acids Improves the Growth and Yield of Lettuce by Enhancing Photosynthetic Assimilation and Nutrient Availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef] [Green Version]
- Tsouvaltzis, P.; Kasampalis, D.S.; Aktsoglou, D.-C.; Barbayiannis, N.; Siomos, A.S. Effect of Reduced Nitrogen and Supplemented Amino Acids Nutrient Solution on the Nutritional Quality of Baby Green and Red Lettuce Grown in a Floating System. Agronomy 2020, 10, 922. [Google Scholar] [CrossRef]
- Abdelhamid, M.T.; Sadak, M.S.; Schmidhalter, U. Effect of Foliar Application of Aminoacids on Plant Yield and Physiological Parameters in Bean Plants Irrigated with Seawater. Acta Biológica Colombiana 2014, 20, 140–152. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Hara, P.; Treder, K.; Findura, P.; Bartoš, P.; Filip, M. Biochemical and economical effect of application biostimulants containing seaweed extracts and amino acids as an element of agroecological management of bean cultivation. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Mostafa, G.G. Improving the Growth of Fennel Plant Grown under Salinity Stress using some Biostimulants. Am. J. Plant Physiol. 2015, 10, 77–83. [Google Scholar] [CrossRef]
- Kałużewicz, A.; Krzesiński, W.; Spiżewski, T.; Zaworska, A. Effect of Biostimulants on Several Physiological Characteristics and Chlorophyll Content in Broccoli under Drought Stress and Re-watering. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Klokić, I.; Koleška, I.; Hasanagić, D.; Murtić, S.; Bosančić, B.; Todorović, V. Biostimulants’ influence on tomato fruit characteristics at conventional and low-input NPK regime. Acta Agric. Scand. Sect. B Plant Soil Sci. 2020, 70, 233–240. [Google Scholar] [CrossRef]
- Azcona, I.; Pascual, I.; Aguirreolea, J.; Fuentes, M.; García-Mina, J.M.; Sánchez-Díaz, M. Growth and development of pepper are affected by humic substances derived from composted sludge. J. Plant Nutr. Soil Sci. 2011, 174, 916–924. [Google Scholar] [CrossRef]
- Sánchez, A.S.; Juárez, M.; Sánchez-Andreu, J.; Jordá, J.; Bermúdez, D. Use of Humic Substances and Amino Acids to Enhance Iron Availability for Tomato Plants from Applications of the Chelate FeEDDHA. J. Plant Nutr. 2005, 28, 1877–1886. [Google Scholar] [CrossRef]
- Dziugieł, T.; Wadas, W. Possibility of increasing early crop potato yield with foliar application of seaweed extracts and humic acids. J. Central Eur. Agric. 2020, 21, 300–310. [Google Scholar] [CrossRef]
- Türkmen, Ö.; Dursun, A.; Turan, M.; Erdinç, Ç. Calcium and humic acid affect seed germination, growth, and nutrient content of tomato (Lycopersicon esculentum L.) seedlings under saline soil conditions. Acta Agric. Scand. Sect. B Plant Soil Sci. 2004, 54, 168–174. [Google Scholar] [CrossRef]
- Paksoy, M.; Türkmen, Ö.; Dursun, A. Effects of potassium and humic acid on emergence, growth and nutrient contents of okra (Abelmoschus esculentus L.) seedling under saline soil conditions. Afr. J. Biotechnol. 2010, 9, 5343–5346. [Google Scholar]
- Pizzeghello, D.; Schiavon, M.; Francioso, O.; Vecchia, F.D.; Ertani, A.; Nardi, S. Bioactivity of Size-Fractionated and Unfractionated Humic Substances from Two Forest Soils and Comparative Effects on N and S Metabolism, Nutrition, and Root Anatomy of Allium sativum L. Front. Plant Sci. 2020, 11, 1203. [Google Scholar] [CrossRef]
- Balmori, D.M.; Domínguez, C.Y.A.; Carreras, C.R.; Rebatos, S.M.; Farías, L.B.P.; Izquierdo, F.G.; Berbara, R.L.L.; García, A.C. Foliar application of humic liquid extract from vermicompost improves garlic (Allium sativum L.) production and fruit quality. Int. J. Recycl. Org. Waste Agric. 2019, 8, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Gemin, L.G.; Mógor, Á.F.; De Oliveira Amatussi, J.; Mógor, G. Microalgae associated to humic acid as a novel biostimulant improving onion growth and yield. Sci. Hortic. 2019, 256, 108560. [Google Scholar] [CrossRef]
- Selim, E.; Mosa, A.; El-Ghamry, A. Evaluation of humic substances fertigation through surface and subsurface drip irrigation systems on potato grown under Egyptian sandy soil conditions. Agric. Water Manag. 2009, 96, 1218–1222. [Google Scholar] [CrossRef]
- Selladurai, R.; Purakayastha, T.J. Effect of Humic Acid Multinutrient Fertilizers on Yield and Nutrient Use Efficiency of Potato. J. Plant Nutr. 2015, 39, 949–956. [Google Scholar] [CrossRef]
- Shalaby, O.A.E.-S.; El-Messairy, M.M. Humic acid and boron treatment to mitigate salt stress on the melon plant. Acta Agric. Slov. 2018, 111, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, E.A.; Ramadan, W.A. Effect of zinc foliar spray alone and combined with humic acid or/and chitosan on growth, nutrient elements content and yield of dry bean (Phaseolus vulgaris L.) plants sown at different dates. Sci. Hortic. 2015, 184, 101–105. [Google Scholar] [CrossRef]
- Hartz, T.K.; Bottoms, T.G. Humic Substances Generally Ineffective in Improving Vegetable Crop Nutrient Uptake or Productivity. HortScience 2010, 45, 906–910. [Google Scholar] [CrossRef] [Green Version]
- Bettoni, M.M.; Mogor, Á.F.; Pauletti, V.; Goicoechea, N.; Aranjuelo, I.; Garmendia, I. Nutritional quality and yield of onion as affected by different application methods and doses of humic substances. J. Food Compos. Anal. 2016, 51, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Waqas, M.; Ahmad, B.; Arif, M.; Munsif, F.; Khan, A.L.; Amin, M.; Kang, S.-M.; Kim, Y.-H.; Lee, I.-J. Evaluation of Humic Acid Application Methods for Yield and Yield Components of Mungbean. Am. J. Plant Sci. 2014, 5, 2269–2276. [Google Scholar] [CrossRef] [Green Version]
- Sani, M.N.H.; Islam, M.N.; Uddain, J.; Chowdhury, M.S.N.; Subramaniam, S. Synergistic effect of microbial and nonmicrobial biostimulants on growth, yield, and nutritional quality of organic tomato. Crop Sci. 2020, 60, 2102–2114. [Google Scholar] [CrossRef]
- Bonini, P.; Rouphael, Y.; Miras-Moreno, B.; Lee, B.; Cardarelli, M.; Erice, G.; Cirino, V.; Lucini, L.; Colla, G. A Microbial-Based Biostimulant Enhances Sweet Pepper Performance by Metabolic Reprogramming of Phytohormone Profile and Secondary Metabolism. Front. Plant Sci. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Carillo, P.; Colla, G.; Fiorentino, N.; Sabatino, L.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cirillo, V.; Shabani, E.; et al. Appraisal of Combined Applications of Trichoderma virens and a Biopolymer-Based Biostimulant on Lettuce Agronomical, Physiological, and Qualitative Properties under Variable N Regimes. Agronomy 2020, 10, 196. [Google Scholar] [CrossRef] [Green Version]
- Patkowska, E.; Mielniczuk, E.; Jamiołkowska, A.; Skwaryło-Bednarz, B.; BłaŻewicz-Woźniak, M. The Influence of Trichoderma harzianum Rifai T-22 and Other Biostimulants on Rhizosphere Beneficial Microorganisms of Carrot. Agronomy 2020, 10, 1637. [Google Scholar] [CrossRef]
- Pereira, T.D.S.; Macêdo, A.G.; Da Silva, J.; Pinheiro, J.B.; De Paula, A.M.; Biscaia, D.; Busato, J.G. Water-extractable fraction of vermicomposts enriched with Trichoderma enhances the growth of bell pepper and tomato as well as their tolerance against Meloidogyne incognita. Sci. Hortic. 2020, 272, 109536. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Chaoxing, H. Does Inoculation with Glomus mosseae Improve Salt Tolerance in Pepper Plants? J. Plant Growth Regul. 2014, 33, 644–653. [Google Scholar] [CrossRef]
- Petropoulos, S.A. Practical Applications of Plant Biostimulants in Greenhouse Vegetable Crop Production. Agronomy 2020, 10, 1569. [Google Scholar] [CrossRef]
- Vasseur-Coronado, M.; du Boulois, H.D.; Pertot, I.; Puopolo, G. Selection of plant growth promoting rhizobacteria sharing suitable features to be commercially developed as biostimulant products. Microbiol. Res. 2021, 245, 126672. [Google Scholar] [CrossRef]
- Sarma, R.K.; Saikia, R. Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil 2014, 377, 111–126. [Google Scholar] [CrossRef]
- Korir, H.; Mungai, N.W.; Thuita, M.; Hamba, Y.; Masso, C.; Tola, Y.H. Co-inoculation Effect of Rhizobia and Plant Growth Promoting Rhizobacteria on Common Bean Growth in a Low Phosphorus Soil. Front. Plant Sci. 2017, 8, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Yong, J.W.H.; Letham, D.S.; Wong, S.C.; Farquhar, G.D. Rhizobium-induced elevation in xylem cytokinin delivery in pigeonpea induces changes in shoot development and leaf physiology. Funct. Plant Biol. 2014, 41, 1323–1335. [Google Scholar] [CrossRef]
- Thao, H.T.B.; Yamakawa, T. Phosphite (phosphorous acid): Fungicide, fertilizer or bio-stimulator? Soil Sci. Plant Nutr. 2009, 55, 228–234. [Google Scholar] [CrossRef]
- Reyes-Pérez, J.J.; Enríquez-Acosta, E.A.; Ramírez-Arrebato, M.Á.; Rodríguez-Pedroso, A.T.; Falcón-Rodríguez, A. Effect of humic acids, mycorrhiza, and chitosan on growth indicators of two tomato cultivars (Solanum lycopersicum L.). Terra Latinoam. 2020, 38, 653–666. [Google Scholar] [CrossRef]
- Asgari-Targhi, G.; Iranbakhsh, A.; Ardebili, Z.O. Potential benefits and phytotoxicity of bulk and nano-chitosan on the growth, morphogenesis, physiology, and micropropagation of Capsicum annuum. Plant Physiol. Biochem. 2018, 127, 393–402. [Google Scholar] [CrossRef]
- Iglesias, M.J.; Colman, S.L.; Terrile, M.C.; París, R.; Martín-Saldaña, S.; Chevalier, A.A.; Álvarez, V.A.; Casalongué, C.A. Enhanced Properties of Chitosan Microparticles over Bulk Chitosan on the Modulation of the Auxin Signaling Pathway with Beneficial Impacts on Root Architecture in Plants. J. Agric. Food Chem. 2019, 67, 6911–6920. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Laane, H.-M. The Effects of Foliar Sprays with Different Silicon Compounds. Plants 2018, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Zhang, Y.; Yao, H.; Wu, J.; Sun, H.; Gong, H. Silicon improves seed germination and alleviates oxidative stress of bud seedlings in tomato under water deficit stress. Plant Physiol. Biochem. 2014, 78, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aranda, M.R.; Jurado, O.; Cuartero, J. Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J. Plant Physiol. 2006, 163, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, Y.; Han, W.; Feng, R.; Hu, Y.; Guo, J.; Gong, H. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant Sci. 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Xu, X.-B.; Hu, Y.-H.; Han, W.-H.; Yin, J.-L.; Li, H.-L.; Gong, H.-J. Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep. 2015, 34, 1629–1646. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, A.S.; Salama, Y.; El-Nemr, M.A.; Abdel-Mawgoud, A. Nano silicon application improves salinity tolerance of sweet pepper plants. Int. J. ChemTech Res. 2015, 8, 11–17. [Google Scholar]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Faisal, M.; Al Sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 33, 2429–2437. [Google Scholar] [CrossRef]
- Przybysz, A.; Gawrońska, H.; Gajc-Wolska, J. Biological mode of action of a nitrophenolates-based biostimulant: Case study. Front. Plant Sci. 2014, 5, 1–15. [Google Scholar] [CrossRef]
- Elmarie, V.D.W.; Johan, C.P.; Van Der Watt, E.; Pretorius, J.C. A triglyceride from Lupinus albus L. seed with bio-stimulatory properties. Afr. J. Biotechnol. 2013, 12, 5431–5443. [Google Scholar] [CrossRef] [Green Version]
- Wadas, W.; Kalinowski, K. Effect of Tytanit on the dry matter and macroelement contents in potato tuber. J. Central Eur. Agric. 2018, 19, 557–570. [Google Scholar] [CrossRef] [Green Version]
- Rady, M.M.; Varma, C.B.; Howladar, S.M. Common bean (Phaseolus vulgaris L.) seedlings overcome NaCl stress as a result of presoaking in Moringa oleifera leaf extract. Sci. Hortic. 2013, 162, 63–70. [Google Scholar] [CrossRef]
- Galambos, N.; Compant, S.; Moretto, M.; Sicher, C.; Puopolo, G.; Wäckers, F.; Sessitsch, A.; Pertot, I.; Perazzolli, M. Humic Acid Enhances the Growth of Tomato Promoted by Endophytic Bacterial Strains Through the Activation of Hormone-, Growth-, and Transcription-Related Processes. Front. Plant Sci. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Olivares, F.L.; Aguiar, N.O.; Rosa, R.C.C.; Canellas, L.P. Substrate biofortification in combination with foliar sprays of plant growth promoting bacteria and humic substances boosts production of organic tomatoes. Sci. Hortic. 2015, 183, 100–108. [Google Scholar] [CrossRef]
- Ekin, Z. Integrated Use of Humic Acid and Plant Growth Promoting Rhizobacteria to Ensure Higher Potato Productivity in Sustainable Agriculture. Sustainability 2019, 11, 3417. [Google Scholar] [CrossRef] [Green Version]
- Sandepogu, M.; Shukla, P.S.; Asiedu, S.; Yurgel, S.; Prithiviraj, B. Combination of Ascophyllum nodosum Extract and Humic Acid Improve Early Growth and Reduces Post-Harvest Loss of Lettuce and Spinach. Agriculture 2019, 9, 240. [Google Scholar] [CrossRef] [Green Version]
- Ngoroyemoto, N.; Kulkarni, M.G.; Stirk, W.A.; Gupta, S.; Finnie, J.F.; van Staden, J. Interactions Between Microorganisms and a Seaweed-Derived Biostimulant on the Growth and Biochemical Composition of Amaranthus hybridus L. Nat. Prod. Commun. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic Biostimulatory Action: Designing the Next Generation of Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [Green Version]
- Shehata, S.A.; AbdelGawad, K.F.; Elmogy, M. Quality and Shelf-life of Onion Bulbs Influenced by Bio-stimulants. Int. J. Veg. Sci. 2017, 23, 362–371. [Google Scholar] [CrossRef]
- Semida, W.M.; El-Mageed, T.A.A.; Hemida, K.; Rady, M.M. Natural bee-honey based biostimulants confer salt tolerance in onion via modulation of the antioxidant defence system. J. Hortic. Sci. Biotechnol. 2019, 94, 632–642. [Google Scholar] [CrossRef]
- Anbarasi, D.; Haripriya, K. Response of aggregatum onion (Allium cepa L. var. aggregatum Don.) to organic inputs, bio fertilizers and biostimulants. Plant Arch. 2020, 20, 759–762. [Google Scholar]
- Agung, I.G.A.M.S.; Diara, I.W. Biostimulants Enhanced Seedling Root Growth and Bulb Yields of True Seed Shallots (Allium cepa var aggregatum L.). Int. J. Environ. Agric. Biotechnol. 2019, 4, 598–601. [Google Scholar] [CrossRef]
- Ngoroyemoto, N.; Gupta, S.; Kulkarni, M.; Finnie, J.; Van Staden, J. Effect of organic biostimulants on the growth and biochemical composition of Amaranthus hybridus L. S. Afr. J. Bot. 2019, 124, 87–93. [Google Scholar] [CrossRef]
- Arthur, G.D.; Aremu, A.O.; Kulkarni, M.G.; Okem, A.; Stirk, W.A.; Davies, T.C.; Van Staden, J. Can the use of natural biostimulants be a potential means of phytoremediating contaminated soils from goldmines in South Africa? Int. J. Phytoremediation 2015, 18, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Kałużewicz, A.; Gąsecka, M.; Spiżewski, T. Influence of biostimulants on phenolic content in broccoli heads directly after harvest and after storage. Folia Hortic. 2017, 29, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Navarro-León, E.; López-Moreno, F.J.; Rios, J.J.; Blasco, B.; Ruiz, J.M. Assaying the use of sodium thiosulphate as a biostimulant and its effect on cadmium accumulation and tolerance in Brassica oleracea plants. Ecotoxicol. Environ. Saf. 2020, 200, 110760. [Google Scholar] [CrossRef]
- Sadeghi, H.; Taban, A. Crushed maize seeds enhance soil biological activity and salt tolerance in caper (Capparis spinosa L.). Ind. Crop. Prod. 2021, 160, 113103. [Google Scholar] [CrossRef]
- Barrajón-Catalán, E.; Álvarez-Martínez, F.J.; Borrás, F.; Pérez, D.; Herrero, N.; Ruiz, J.J.; Micol, V. Metabolomic analysis of the effects of a commercial complex biostimulant on pepper crops. Food Chem. 2020, 310, 125818. [Google Scholar] [CrossRef]
- Dzung, P.D.; Van Phu, D.; Du, B.D.; Ngoc, L.S.; Duy, N.N.; Hiet, H.D.; Nghia, D.H.; Thang, N.T.; Van Le, B.; Hien, N.Q. Effect of foliar application of oligochitosan with different molecular weight on growth promotion and fruit yield enhancement of chili plant. Plant Prod. Sci. 2017, 20, 389–395. [Google Scholar] [CrossRef]
- Shirkhodaei, M.; Darzi, M.T.; Haj, M.; Hadi, M.H.S. Influence of Vermicompost and Biostimulant on the growth and biomass of coriander (Coriandrum sativum L.). Int. J. Adv. Biol. Biomed. Res. 2014, 2, 706–714. [Google Scholar]
- Pokluda, R.; Sękara, A.; Jezdinský, A.; Kalisz, A.; Neugebauerová, J.; Grabowska, A. The physiological status and stress biomarker concentration of Coriandrum sativum L. plants subjected to chilling are modified by biostimulant application. Biol. Agric. Hortic. 2016, 32, 258–268. [Google Scholar] [CrossRef]
- Negro, D.; Montesano, V.; Sonnante, G.; Rubino, P.; De Lisi, A.; Sarli, G. Fertilization strategies on cultivars of globe artichoke: Effects on yield and quality performance. J. Plant Nutr. 2015, 39, 279–287. [Google Scholar] [CrossRef]
- Wszelaczyńska, E.; Szczepanek, M.; Pobereżny, J.; Kazula, M.J. Effect of biostimulant application and long-term storage on the nutritional value of carrot. Hortic. Bras. 2019, 37, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Hernandez, O.L.; Calderín, A.; Huelva, R.; Martínez-Balmori, D.; Guridi, F.; Aguiar, N.O.; Olivares, F.L.; Canellas, L.P. Humic substances from vermicompost enhance urban lettuce production. Agron. Sustain. Dev. 2015, 35, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Torres, P.; Novaes, P.; Ferreira, L.G.; Santos, J.P.; Mazepa, E.; Duarte, M.E.R.; Noseda, M.D.; Chow, F.; Dos Santos, D.Y. Effects of extracts and isolated molecules of two species of Gracilaria (Gracilariales, Rhodophyta) on early growth of lettuce. Algal Res. 2018, 32, 142–149. [Google Scholar] [CrossRef]
- Vetrano, F.; Miceli, C.; Angileri, V.; Frangipane, B.; Moncada, A.; Miceli, A. Effect of Bacterial Inoculum and Fertigation Management on Nursery and Field Production of Lettuce Plants. Agronomy 2020, 10, 1477. [Google Scholar] [CrossRef]
- Alkuwayti, M.; El-Sherif, F.; Yap, Y.-K.; Khattab, S. Foliar application of Moringa oleifera leaves extract altered stress-responsive gene expression and enhanced bioactive compounds composition in Ocimum basilicum. S. Afr. J. Bot. 2020, 129, 291–298. [Google Scholar] [CrossRef]
- Taha, R.; Alharby, H.; Bamagoos, A.; Medani, R.; Rady, M. Elevating tolerance of drought stress in Ocimum basilicum using pollen grains extract; a natural biostimulant by regulation of plant performance and antioxidant defense system. S. Afr. J. Bot. 2020, 128, 42–53. [Google Scholar] [CrossRef]
- Rady, M.; Desoky, E.-S.; Elrys, A.; Boghdady, M. Can licorice root extract be used as an effective natural biostimulant for salt-stressed common bean plants? S. Afr. J. Bot. 2019, 121, 294–305. [Google Scholar] [CrossRef]
- Rady, M.M.; Mohamed, G.F. Modulation of salt stress effects on the growth, physio-chemical attributes and yields of Phaseolus vulgaris L. plants by the combined application of salicylic acid and Moringa oleifera leaf extract. Sci. Hortic. 2015, 193, 105–113. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Ahmed, M.E.; Maswada, H.F.; Xuan, T.D. Enhancing growth, yield, biochemical, and hormonal contents of snap bean (Phaseolus vulgaris L.) sprayed with moringa leaf extract. Arch. Agron. Soil Sci. 2016, 63, 687–699. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Ahmed, M.E.; Maswada, H.F.; Al-Araby, A.A.; Xuan, T.D. Growth traits, physiological parameters and hormonal status of snap bean (Phaseolus vulgaris L.) sprayed with garlic cloves extract. Arch. Agron. Soil Sci. 2018, 64, 1068–1082. [Google Scholar] [CrossRef]
- Merwad, A.-R.M.A. Using Moringa oleifera extract as biostimulant enhancing the growth, yield and nutrients accumulation of pea plants. J. Plant Nutr. 2017, 41, 425–431. [Google Scholar] [CrossRef]
- Desoky, E.-S.M.; ElSayed, A.I.; Merwad, A.-R.M.; Rady, M.M. Stimulating antioxidant defenses, antioxidant gene expression, and salt tolerance in Pisum sativum seedling by pretreatment using licorice root extract (LRE) as an organic biostimulant. Plant Physiol. Biochem. 2019, 142, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Rehman, H. Supplementing organic biostimulants into growing media enhances growth and nutrient uptake of tomato transplants. Sci. Hortic. 2016, 203, 192–198. [Google Scholar] [CrossRef]
- Chanthini, K.M.-P.; Senthil-Nathan, S.; Stanley-Raja, V.; Thanigaivel, A.; Karthi, S.; Sivanesh, H.; Sundar, N.S.; Palanikani, R.; Soranam, R. Chaetomorpha antennina (Bory) Kützing derived seaweed liquid fertilizers as prospective bio-stimulant for Lycopersicon esculentum (Mill). Biocatal. Agric. Biotechnol. 2019, 20, 101190. [Google Scholar] [CrossRef]
- Colman, S.L.; Salcedo, M.F.; Mansilla, A.Y.; Iglesias, M.J.; Fiol, D.F.; Martín-Saldaña, S.; Alvarez, V.A.; Chevalier, A.A.; Casalongué, C.A. Chitosan microparticles improve tomato seedling biomass and modulate hormonal, redox and defense pathways. Plant Physiol. Biochem. 2019, 143, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Maach, M.; Boudouasar, K.; Akodad, M.; Skalli, A.; Moumen, A.; Baghour, M. Application of biostimulants improves yield and fruit quality in tomato. Int. J. Veg. Sci. 2020, 1–6. [Google Scholar] [CrossRef]
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Efficacy of microalgal extracts as biostimulants through seed treatment and foliar spray for tomato cultivation. Ind. Crops Prod. 2020, 151, 112453. [Google Scholar] [CrossRef]
- Rahou, Y.A.; Ait-El-Mokhtar, M.; Anli, M.; Boutasknit, A.; Ben-Laouane, R.; Douira, A.; Benkirane, R.; El Modafar, C.; Meddich, A. Use of mycorrhizal fungi and compost for improving the growth and yield of tomato and its resistance to Verticillium dahliae. Arch. Phytopathol. Plant Prot. 2020, 1–26. [Google Scholar] [CrossRef]
- Dong, C.; Wang, G.; Du, M.; Niu, C.; Zhang, P.; Zhang, X.; Ma, D.; Ma, F.; Bao, Z. Biostimulants promote plant vigor of tomato and strawberry after transplanting. Sci. Hortic. 2020, 267, 109355. [Google Scholar] [CrossRef]
- Rachidi, F.; Benhima, R.; Sbabou, L.; El Arroussi, H. Microalgae polysaccharides bio-stimulating effect on tomato plants: Growth and metabolic distribution. Biotechnol. Rep. 2020, 25, e00426. [Google Scholar] [CrossRef] [PubMed]
- Pohl, A.; Grabowska, A.; Kalisz, A.; Sękara, A. Biostimulant Application Enhances Fruit Setting in Eggplant—An Insight into the Biology of Flowering. Agronomy 2019, 9, 482. [Google Scholar] [CrossRef] [Green Version]
- Pohl, A.; Grabowska, A.; Kalisz, A.; Sekara, A. The eggplant yield and fruit composition as affected by genetic factor and biostimulant application. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 929–938. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Cheng, Z.-H.; Hayat, S.; Ahmad, H.; Ghani, M.I.; Liu, T. Foliar spraying of aqueous garlic bulb extract stimulates growth and antioxidant enzyme activity in eggplant (Solanum melongena L.). J. Integr. Agric. 2019, 18, 1001–1013. [Google Scholar] [CrossRef]
- Abbas, S.M. The influence of biostimulants on the growth and on the biochemical composition of Vicia faba CV. Giza 3 beans. Rom. Biotechnol. Lett. 2013, 18, 8061–8068. [Google Scholar]

| Plant Biostimulants | Key Points | References |
|---|---|---|
| Protein hydrolysates (PHs) and other N-containing compounds (amino acids) | a. Mixtures of peptides and amino acids which are produced via enzymatic, chemical or thermal hydrolysis of animal- or plant-derived proteins. | [34,84] |
| b. Effective in increasing yield and quality of various crop products. | [85] | |
| c. Categorization based on proteins, sources and the hydrolysis system; PHs boost both primary and secondary plant metabolism biochemical and physiological procedures. | [86,87] | |
| d. Effective in alleviating negative abiotic stress effects. | [24] | |
| Humic substances | a. Include fulvic acids and humic acids which they differ in color, molecular weight, carbon content and the degree of polymerization. | [88] |
| b. They could increase cationic exchange capacity (CEC) of the soil and interact with root membrane transporters. | [65] | |
| Seaweed extracts | a. Extracts from brown seaweeds, e.g., Ascophyllum, Fucus, and Laminaria genera. | [89] |
| b. They are rich in polysaccharides, polyphenols and compounds with hormonal activity that affect plant growth and development. | [90,91] | |
| Biopolymers (Chitosans and other polymers) | a. Chitosans are naturally occurring components in fungi nematodes, insects and crustaceans. | [68] |
| b. They regulate plant-defense mechanisms related to phytoalexins biosynthesis, reactive oxygen species, and pathogenesis-related proteins making plants more resistant to biotic and abiotic stressors. | [92] | |
| Microbial biostimulants (Mycorrhizal and non-mycorrhizal fungi, Rhizobium, Trichoderma, and Plant Growth-Promoting Rhizobacteria (PGPR)) | a. Symbiotic fungi, especially arbuscular mycorrhizal fungi (AMF) within the genus Glomus. | [14,16] |
| b. Trichoderma genus | [44] | |
| c. Beneficial bacteria with plant growth promoting properties also known as PGPBs (Bacillus, Rhizobium, Pseudomonas, Azospirillum, Azotobacter, and many others). | [48] | |
| Phosphite (Phi) | a. A phosphate (H2PO4−) analog which affects various plant growth and development processes. | [93] |
| b. Several beneficial effects have been reported in various vegetable crops. | [69,94,95,96,97] | |
| c. Biostimulatory impacts on fruit such as avocado, banana, citrus, peach, raspberry and strawberry. | [69,98,99,100] | |
| Silicon | a. Effective against abiotic and biotic stressors. | [11] |
| Vermicomposts | a. Hormonal activity of vermicompost leachates due to content in trace elements of hormones such as cytokinins, indolo-acetic acid (IAA), eighteen gibberellins (GAs) and brasinosteroids. | [101] |
| b. Phytohormones from three different classes, including cytokinins, auxins and gibberellins provide plant growth promoting activities in vermicompost | [102] |
| Plant | Common Name | Key Points | Effects | References |
|---|---|---|---|---|
| Allium cepa L. | Onion | a. Biostimulants containing humic acids, organic substances, amino acids, carbon and boron or algae extracts | Improved plant growth and yield, and shelf life of bulbs | [205] |
| b. Application of diluted bee-honey extract (DHE) | Increased photosynthetic parameters, biomass production and yield, and antioxidants activity | [206] | ||
| Allium cepa var. aggregatum L. | Shallot | a. Application of seaweed extracts, vermicompost and mixture of animal waste | Improved yield and bulb traits | [207] |
| b. Soaking of seeds in PGPB biostimulants | Increased germination percentage, plant growth and bulb parameters | [208] | ||
| Allium sativum L. | Garlic | a. Foliar application of liquid humic substances obtained from vermicompost extracts | Improved yield and quality parameters of bulbs | [160] |
| Amaranthus hybridus L. | Amaranth | a. Foliar application of vermicompost leachate, smoke-water, karrikinolide, eckol and Kelpak | Increased growth, higher chlorophylls, carotenoids and proteins content | [209] |
| b. Combination of plant growth-promoting rhizobacteria (PGPRs), and Ecklonia maxima extracts | Improved plant growth and photosynthetic pigment content, stress relief | [203] | ||
| Brassica juncea L. | Mustard green | a. Foliar application of vermicompost leachate, smoke-water, indole-3-butyric acid and Ecklonia maxima extracts on seedlings grown in soils from goldmines | Increased phytoremediative activities though the accumulation of heavy metals | [210] |
| Brassica oleraceae L. | Broccoli | a. Combination of foliar spraying with Ascophyllum nodosum extracts and watering with amino acids on broccoli plants subjected to water stress and re-watering | Increased photosynthetic parameters under water stress conditions | [152] |
| b. Combination of foliar spraying with Ascophyllum nodosum extracts and watering with amino acids on broccoli plants | Increased total phenolic compounds, sinapic acid and quercetin content | [211] | ||
| Brassica oleraceae L. | Cabbage | a. Foliar application of eckol from Ecklonia maxima extracts | Increased root and shoot length, photosynthetic pigments and proteins, proline and iridoid glycosides; inhibition of infestation from aphids | [39] |
| b. Thiosulfate application through the nutrient solution in cabbage plants subjected to Cd toxicity | Improved phytoremediative properties of Cd without biostimulant effects on cabbage plants | [212] | ||
| Capparis spinosa L. | Caper | a. Incorporation of crushed maize seeds in growing medium of caper plants subjected to salinity stress | Increased activity of soil enzymes, Na exclusion from plant tissues | [213] |
| Capsicum annuum L. | Pepper | a. Application of a lipo-complex biostimulant containing mainly polysaccharides, polypeptides and vitamins | Increased phenylalanine and metabolites associated with fruit ripening (organic acids, monosaccharides, carotenoids) | [214] |
| Capsicum frutescens L. | Chilli pepper | a. Foliar application of oligochitosan | Increased plant growth, chlorophyll content and fruit weight | [215] |
| Coriandrum sativum L. | Coriander | a. Seed inoculation with Azotobacter chroococcum and Azospirillum lipoferum | Increased biomass production | [216] |
| b. Foliar spraying with biostimulants (Asahi SL or Goemar Goteo) on plants subjected to chilling stress | Increased photosynthetic parameters, L-ascorbic acid and total phenolic compounds content and total antioxidant activity | [217] | ||
| Cynara scolymus L. | Globe artichoke | a. Application of A. nodosum extracts and trace elements. | Increased number and weight of heads | [218] |
| Daucus carota subsp. sativus | Carrot | a. Foliar application of Kelpak SL and Asahi SL | Kelpak SL improved nutritional value and increased storage life of carrots | [219] |
| Lactuca sativa L. | Lettuce | a. Foliar and root application of protein hydrolysates in lettuce plants grown under salinity conditions | Mitigation of oxidative stress, increased osmolytes and glucosinolates content | [220] |
| b. Foliar application of liquid humic substances obtained from vermicompost | Improved earliness of plants, increased the number of leaves per plant and total yield | [221] | ||
| c. Application of crude seaweed extracts (Gracilaria caudate and Gracilaria domingensis) on lettuce seedlings | Increased root growth | [222] | ||
| d. Inoculation of growth substrate with Bacillus spp. | Positive effects on plant growth nitrate content | [223] | ||
| Manihot esculenta Crantz | Cassava | a. Foliar application of Moringa oleifera leaves extracts. The plant height and leaf number of cassava plant were increased because of foliar application of MLE. | Improved plant growth and decreased incidence of Zonocerus variegatus attacks | [219] |
| Nasturtium officinale | Watercress | a. Foliar spraying of algal biostimulant on watercress plants grown in Cd contaminated soil | Increased plant growth and reduced Cd accumulation in plant tissues | [208] |
| Ocimum basilicum L. | Basil | a. Foliar application of Moringa oleifera leaves extracts | Increased growth and yield, and estragole and eucalyptol contents | [224] |
| b. Foliar spraying with palm pollen grains extract alleviated the negative effects of deficit irrigation on basil plants. | Improved plant growth and essential oils content and antioxidant enzyme activities; maintained relative water content, electrolyte leakage and water use efficiency; improved leaf and stem anatomy | [225] | ||
| Phaseolus vulgaris L. | Common bean | a. Foliar application of protein hydrolysates from pumkin seeds on Phaseolus vulgaris plants grown under saline conditions | Maintained plant growth, yield and anatomical features; mitigated negative effects of salt stress on macronutrients, photosynthetic pigments, relative water content and stability of cell membranes | [135] |
| b. Foliar and soil application of Nomoren, EKOprop, Veramin Ca on Phaseouls vulgaris plants grown under normal irrigation and water stress conditions | Positive effects on yield, nutritional parameters, chemical composition and bioactive properties of fresh pods and seeds | [14,15] | ||
| c. Seed soaking and foliar spraying with licorice root extract on common bean plants subjected to salinity stress | Improved growth, yield and physicochemical parameters | [226] | ||
| d. Seed soaking and foliar spraying with salicylic acid and Moringa oleifera leaves extracts on common bean plants subjected to salinity stress | Improved growth, yield and physicochemical parameters | [227] | ||
| Phaseolus vulgaris L. | Snap bean | a. Foliar spraying with Moringa oleifera leaves extracts | Improved plant growth and yield components, increased total phenolic compounds and minerals content in pods | [228] |
| b. Foliar spraying with garlic cloves extracts | Improved plant growth parameters, yield and chemical composition of pods | [229] | ||
| Pisum sativum L. | Pea | a. Foliar spraying with Moringa oleifera leaves extracts | Increased biomass production, pod and seed yield, proteins and nutrients content in seeds | [230] |
| b. Seed soaking in licorice root extract | Increased seedling growth, photosynthetic activity and antioxidant enzymes activity | [231] | ||
| Solanum lycopersicum L. | Tomato | a. Incorporation of humic acids and/or crushed maize grain | Improved shoot and root growth, increased relative water content and membrane stability of transplants, improved macronutrients uptake | [232] |
| b. Seed pretreatment with liquid extracts of Chaetomorpha antennina green seaweed | Increased germination percentage and vegetative growth, improved biochemical profile | [233] | ||
| c. Foliar spraying with Chitosan microparticles | Improved seed germination and seedling vigor, modulation of antioxidant enzymes activities | [234] | ||
| d. Foliar treatment with saffron extract | Improvement in morphological and biochemical parameters, antifungal effects against Phytophthora infestans | [234] | ||
| e. Foliar application of humic (Megafol) and amino acids (Viva) biostimulants | Improved plant growth under normal fertilization rates and minimized yield loses under nutrients deprivation | [153] | ||
| f. Foliar application of Tecamin Brix® and/or Tecamin Flower ® in tomato plants grown in saline conditions. | Improved yield and fruit quality | [235] | ||
| g. Deed treatment and foliar spraying of microalgal extracts | Improved germination and seedling growth rates | [236] | ||
| h. Soil application of compost and arbuscular mycorrhizal fungi | Improved plant growth and photosynthetic parameters, reduced incidence of Verticillium dahliae infestations | [237] | ||
| i. Soil application of biostimulants containing plant extracts, Ascophyllum nodosum extracts or animal derived protein hydrolysates in tomato plants after transplantation | Reduced transplantation shock through the increase of root and shoot development | [238] | ||
| j. Fertigation with microalgae polysaccharides | Improved vegetative growth, increased nutrients, protein and sterols content in leaves | [239] | ||
| k. Foliar application of brown seaweed extracts from A. nodosum and Sargassum sp. | Induced flower formation and fruit setting | [115] | ||
| Solanum melongena L. | Eggplant | a. Foliar application of A. nodosum extracts | Improved flower and fruit set, fruit yield and chemical composition | [240,241] |
| b. Foliar application of aqueous garlic bulb | Single application increased plant growth, photosynthetic parameters and antioxidant enzymes activity | [242] | ||
| Solanum tuberosum L. | Potato | a. Combined application of Ecklonia maxima extracts and Asahi SL with herbicides | Increased content of true and total proteins, increased marketable yield and yield parameters | [120,123] |
| b. Soil spraying of biostimulant containing N-fixing microbes (NFM0 combined or not with an amino acid blend | Unintended impacts on nitrogen losses | [82] | ||
| c. Potato seed pretreatment and foliage spraying with phosphite | Induced structural and biochemical changes in tuber periderm and cortex, increased tolerance to UV-B, enhanced sprouts emergence and early growth | [94,95,97] | ||
| d. Foliar application of biostimulants containing A. nodosum extracts, E. maxima extracts or humic and fulvic acids | Increased yield under drought stress, increased marketable yield | [156] | ||
| Spinacia oleracea L. | Spinach | a. Foliar spraying of smoke-water and Ecklonia maxima extracts, | Increased growth and biochemical parameters (antioxidant enzymes activity and sinapic acid content) | [119] |
| b. Application of various biostimulants (Megafol, Aminovert, Veramin Ca, Twin Antistress and irrigation treatments on spinach plants grown under normal and water stress conditions | Improved nutritional value and bioactive properties | [16] | ||
| Vicia faba L. | Broad bean | a. Foliar spraying with Bacillus licheniformis, yeast (5 g/L), extracts form algae and humic acid (20 g/L), increased pigments, carotenoids concentrations and total carbohydrates. | Improved photosynthesis and nutrients uptake, induced endogenous hormones and protein biosynthesis | [243] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables. Biomolecules 2021, 11, 698. https://doi.org/10.3390/biom11050698
Shahrajabian MH, Chaski C, Polyzos N, Petropoulos SA. Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables. Biomolecules. 2021; 11(5):698. https://doi.org/10.3390/biom11050698
Chicago/Turabian StyleShahrajabian, Mohamad Hesam, Christina Chaski, Nikolaos Polyzos, and Spyridon A. Petropoulos. 2021. "Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables" Biomolecules 11, no. 5: 698. https://doi.org/10.3390/biom11050698






