Stevia Genus: Phytochemistry and Biological Activities Update
Abstract
1. Introduction
2. Ethnobotany
3. Phytochemistry
3.1. General Aspects
3.2. Advances in the Chemistry of Stevia
4. Biological Activity
4.1. Biological Activity of Stevia Extracts
4.2. Biological Activity of Compounds Isolated from Stevia Species
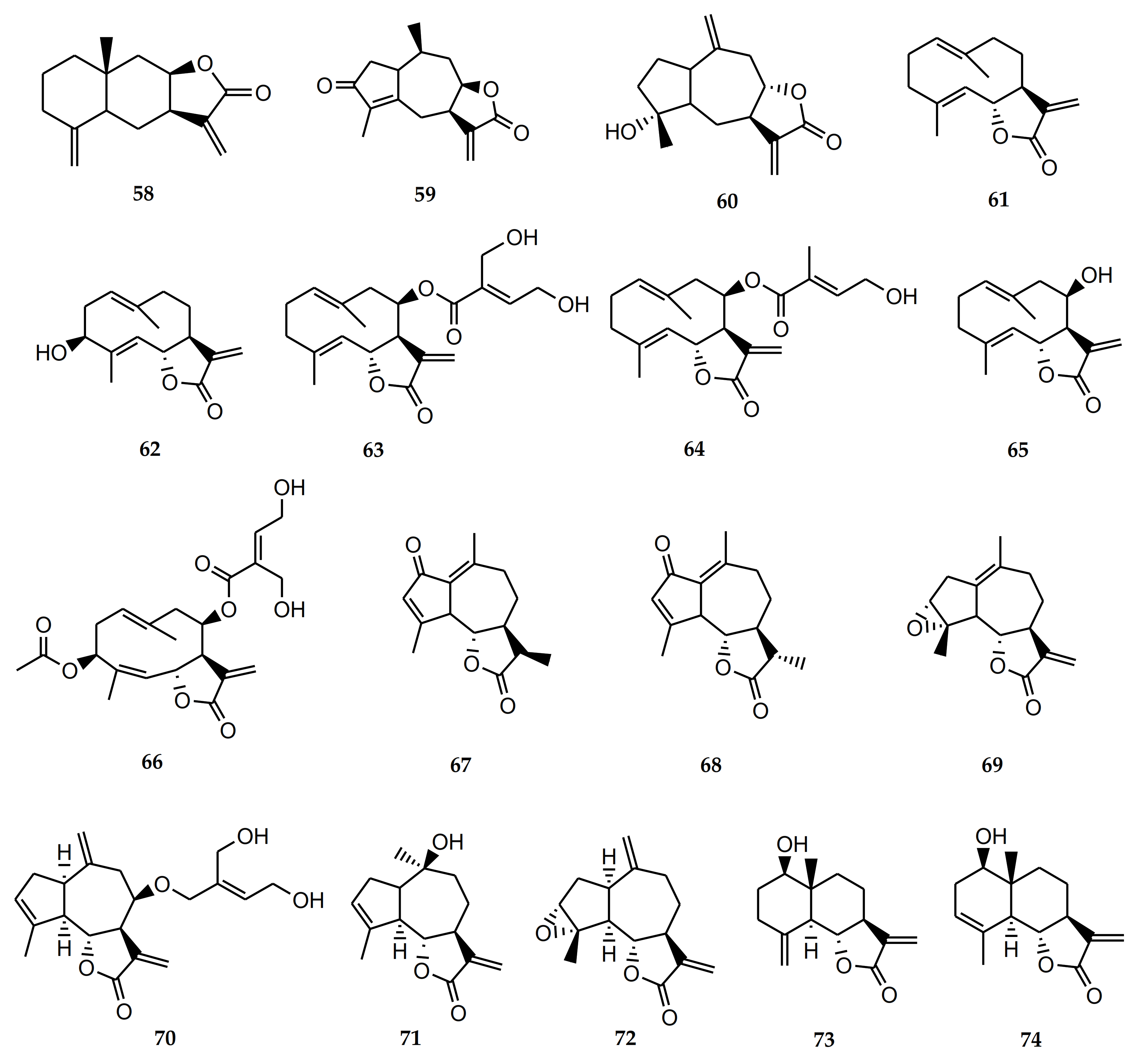
4.2.1. Biological Activity of Sesquiterpene Lactones
| Comp. N° | Common Name | Species | Reported Activity |
|---|---|---|---|
| 58 | Isoalantolactone | S. polyphylla, S. lucida | Antineoplastic. Antitumor. Antimicrobial. Anti-inflammatory. Anti-Trypanosoma cruzi and T. brucei. Inhibits osteoclastogenesis [85,86,87,88,89,90]. |
| 59 | Achalensolide | S. achalensis, S. polyphylla, S. satureifolia | Anti-inflammatory [39]. |
| 60 | Inuviscolide | S. achalensis, S. isomeca, S. ovata | Anti-inflammatory. Cytotoxic against melanoma cells [40,41,42] |
| 61 | Costunolide | S. amambayensis | Anti-inflammatory. Antitumor. Anti-Trypanosoma. Anti-Leishmania. Antioxidant. Antipyretic. Neuroprotecive. Antiallergic. Osteoporosis prevention. Antimycobacterial. Anti-Helicobacter pylori. Normoglycemic. Hypolipidemic [41,42,43,44,45,46,47,48,49,50]. |
| 62 | Hanphyllin | S. grisebachiana | Antitumoral. Antioxidant [51,52]. |
| 63 | Eupatoriopicrin | S. alpina var. glutinosa, S. maimarensis, S. procumbens, S. sarensis | Anti-Trypanosoma cruzi. Anti-T. brucei. Anti-Leishmania. Anti-P. falciparum. Anti-inflammatory. Antitumor. Antibacterial [46,53,54,55,56,57,58,59]. |
| 64 | 5′deoxy-eupatoriopicrin | S. chamaedrys | Anti-inflammatory [56]. |
| 65 | Eupatolide | S. alpina var. glutinosa | Antimetastatic. Antineoplastic [60,61]. |
| 66 | Eucannabinolide | S. origanoides, S. sarensis | Anti-T. brucei. Anti-inflammatory. Antibacterial. Antimetastatic [55,62,63,64,65]. |
| 67 | Achillin | S. alpina var. alpina | Anti-Trypanosoma cruzi. Antineoplastic. Antitumor. Anti-inflammatory. Antiallergic [43,67,68]. |
| 68 | Leukodin o desacetoxymatricarin | S. pilosa | Antiallergic. Inhibitory activity on melanoma cells. Meiosis inhibition in oocytes of amphibians [67,69,70]. |
| 69 | Ludartin | S. yaconensis var. subeglandulosa | Antineoplastic. Anti-inflammatory. Gastric cytoprotective. Aromatase inhibition [71,72,73,74]. |
| 70 | Eupahakonenin B | S. alpina var. glutinosa, S. chamaedrys, S. gilliesii, S. mercedensis, S. procumbens, S. sarensis, S. satureiifolia, S. setifera | Anti-T. cruzi [53,54]. |
| 71 | 10- epi-8- deoxycumambrin B | S. grisebachiana, S. yaconensis var. subeglandulosa | Inhibition of aromatase [73,75]. |
| 72 | Estafietin | S. alpina var. alpina, S. boliviensis, S. grisebachiana, S. yaconensis | Anti-T. cruzi. Anti-Leishmania brasiliensis. Immunomodulator [53,54,76,77]. |
| 73 | Reynosin | S. chamaedrys | Anti-inflammatory. Antimycobacterial. Hepatoprotective. Protective effect against dopamine-induced neuronal cell death [91,92,93,94]. |
| 74 | Santamarine | S. chamaedrys | Antitumor. Anti-inflammatory. Antimycobacterial [91,92,95,96]. |
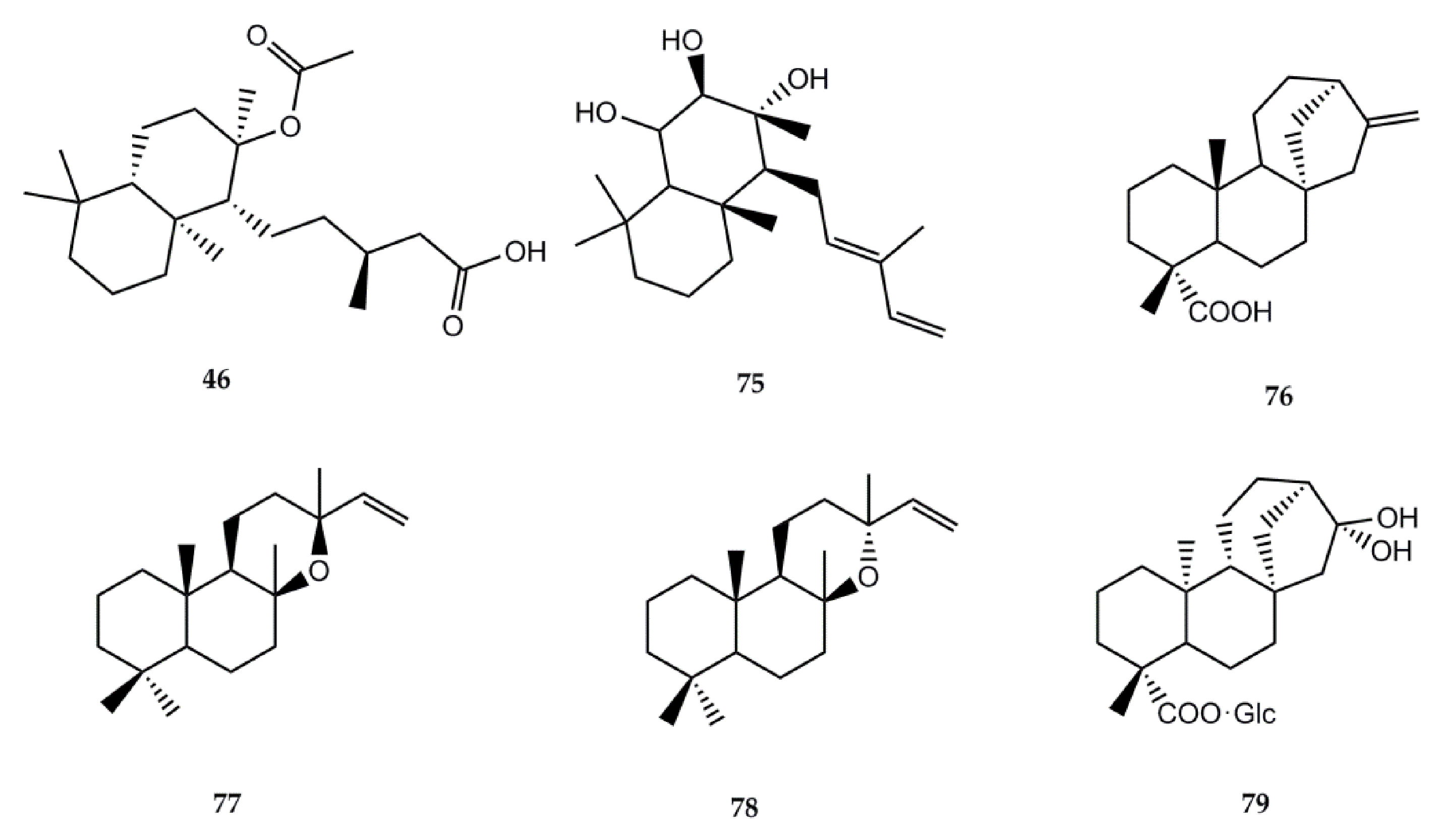
4.2.2. Biological Activity of Diterpenes
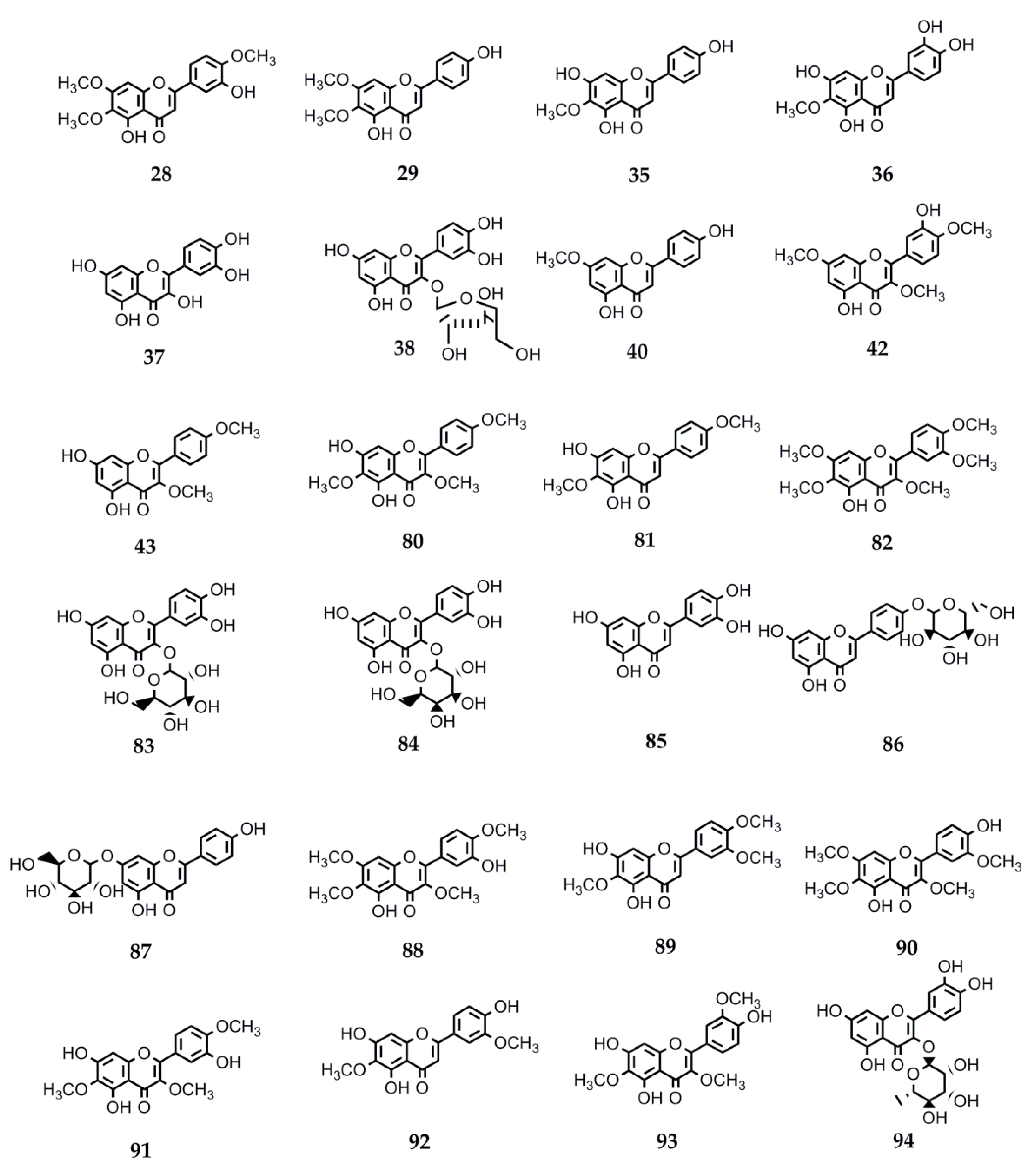
4.2.3. Biological Activity of Flavonoids
| Comp. N° | Common Name | Species | Reported Activity |
|---|---|---|---|
| 28 | Eupatorin | S. satureiifolia var. satureiifolia, S. breviaristata, S. procumbens, S. vaga | Anti-T. cruzi and Anti-L. braziliensis. Antibreast cancer. Antidiabetic. Angiotensin-converting enzyme inhibition. Antitumor. Vasorelaxant. Anti-M. tuberculosis [25,110,111,112,113,114,115]. |
| 29 | Cirsimaritin | S. satureiifolia var. satureiifolia, S. maimarensis | Trypanocidal and leishmanicidal. Antioxidant activity. Anti-E. hystolica. Antiproliferative and antimetastatic. Anti-HIV. Antidiabetic. Anti-influenza A virus. Anti-inflammatory. Cardiac remodeling and ventricular dysfunction improvement. Antidepressant. Anxiolytic. Antinociceptive. Antiepileptic. Antigiardial activity. Melanogenesis-inducing effect [131,144,145,146,147,148,149,150,151,152,153,154,155,156,157]. |
| 35 | Hispidulin | S. urticifolia, S. sanguinea | Anticancer. Anti-T. cruzi. Antidiabetic. Antiepileptic. Antihypnotic. Anti-influenza. Antiosteoporotic. Platelet aggregation inhibition. Antimetastatic (breast cancer cells) [117,127,151,158,159,160,161,162,163,164,165,166,167]. |
| 36 | Nepetin | S. urticifolia | Alpha-glucosidase inhibition. Osteoclastogenesis inhibition. Tyrosinase inhibition. Anti-inflammatory. Antiangiogenic and antitumor. Antiviral [198,211,246,247,248,249,250,251]. |
| 37 | Quercetin | S. urticifolia, S. pilosa, S. aupatoria S. rebaudiana | Leishmanicidal. Antimalarial. Antioxidant. Anti-influenza: neuraminidase inhibitor. Anti-H. pylori. Anti-inflammatory. Antiallergic. Antihypertensive. Antidiabetic. Neuroprotective. Antitumor [129,144,160,176,177,178,179,180,181,182,183,184]. |
| 38 | Avicularin | S. urticifolia | Anti-inflammatory. Anticancer. Rheumatoid arthritis protector. Antidepressive. Adipogenic genes expression inhibitor [252,253,254,255,256]. |
| 40 | Sakuranetin | S. subpubescens var. subpubescens | Anti-T. cruzi and anti-L. braziliensis. Anti-E. hystolitica. Antiproliferative. Antifungal. Anti- H. pylori. Anti-influenza B. Antiviral. Anti-inflammatory. Antioxidant. Antiasthmatic. Alzheimer’s disease treatment. Cytotoxic against melanoma cells [13,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141]. |
| 42 | Ayanin | S. subpubescens var. subpubescens | Antiasthmatic. Cardiovascular protector. Vasorelaxant. Antiallergic. Anti-inflammatory. Anticancer. Anti-L. donovani, -P. falciparum, and -T. b. rhodesiense [257,258,259,260,261,262]. |
| 43 | Ermanin | S. subpubescens var. subpubescens | Anti-inflammatory. Antitumor. Antiviral. Anti-L. donovani and -P. falciparum [263,264,265,266] |
| 80 | Santin | Stevia microchaeta, S. monardifolia, S. origanoides | Anti-T. cruzi. Anti- L. braziliensis. Antiplasmodial. Antibacterial. Anti-influenza A. Anti-T. brucei gambiense [116,117,118,119,120,121]. |
| 81 | Pectolinaringenin | S. lucida | Anti-T. cruzi. Larvicidal against A. aegypti and Culex quinquefasciatus [142,143]. |
| 82 | Artemetin | S. procumbens S. jujuyensis | Anti-inflammatory activity. Antimalarial. Antioxidant. Antiapoptotic. Endothelial function protection. Anti T. brucei rhodisiense. Hypotensive. Antitumor [168,169,170,171,172,173,174,175]. |
| 83 | Quercetin-3-O-B-D-Glc | S. rebaudiana S. nepetifolia | Anti-plasmodium and anti-L. donovani [176]. |
| 84 | Quercetin-3-O-B-D-Gal | S. nepetifolia, S. serrata, S. soratensis | Anti-L. donovani [176]. |
| 85 | Luteolin | S. pilosa S. eupatoria S. rebaudiana | Antiprotozoal against P. falciparum, L. donovani, and T. cruzi. Antiviral against Dengue virus type 1. Anti-influenza. Antidiabetic. Antiinflammatory. Xanthine oxidase inhibition [144,149,160,176,186,187]. |
| 86 | Apigenin-4′-O-glucoside | S. rebaudiana | Antioxidant [188]. |
| 87 | Apigetrin | S. soratensis | Anti-inflammatory. Anticomplementary. Antiproliferative and proapoptotic. Alpha-glucosidase inhibitor. Reduction of intestinal cholesterol uptake [189,190,191,192]. |
| 88 | Casticin | S. breviaristata S. vaga | Anticancer. Antiasthmatic. Antihyperprolactinemia and antinociceptive. Analgesic. Spasmolytic. Osteoarthritis-related cartilage degeneration attenuation [193,194,195,196,197,198]. |
| 89 | Eupatilin | S. gilliesii S. maimarensis S. lucida | Anticancer. Anti-inflammatory. Antiasthmatic. Antinociceptive. Chondroprotective properties. Antioxidant. Neuroprotective. Antidiabetic. Antiatherogenic. Antixanthine oxidase activity [115,199,200,201,202,203,204,205]. |
| 90 | Chrysosplenetin | S. jujuyensis | Cytotoxic activity. Anti-inflammatory. Antiacetylcholinesterase. Antiallergic. Antiviral against SARS-CoV-2 (COVID-19) and enterovirus 71. Antiprotozoal against T. brucei brucei, P. falciparum, and T. congolense. Tyrosinase inhibition. Neuraminidase inhibition. Pg-P inhibition. Osteogenesis activation [206,207,208,209,210,211,212,213,214]. |
| 91 | Centaureidin | S. rebaudiana S. nepetifolia S. cuzcoensis S. galeopsidifolia | Cytotoxic. Tumor cell growth inhibition. Anti-inflammatory. Antioxidant. Immunomodulatory. Anti-Dengue virus 4. Melanin pigmentation reduction [186,215,216,217,218,219,220]. |
| 92 | Jaceosidin | S. jujuyensis | Anticancer. Antioxidant. Antidiabetic. Antiallergic. Anti-inflammatory. Osteoarthritic cartilage damage attenuation. Antibacterial. Angiogenesis stimulation [221,222,223,224,225,226,227,228,229,230,231]. |
| 93 | Jaceidin | S. cuzcoensis | Anti-T. cruzi. Anti-L. infantum. Anti-E. coli activity. Anti-Dengue virus 4. Antitumor. Antiplatelet. Antioxidant activity. Xanthine oxidase inhibition [187,230,232,233,234]. |
| 94 | Quercitrin | S. rebaudiana | Oteoarthritis alleviation. Platelet activation inhibition. Antioxidant and anti-inflammatory. Hyperlipidemia. Hepatic steatosis amelioration. Hair growth stimulation. Alpha-glycosidase inhibition. Antiallergic. Antimalarial. Antileishmanial. Anti-Dengue virus. Osteoporosis attenuation. Anticancer [176,235,236,237,238,239,240,241,242,243,244,245]. |
5. Final Remarks and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hernández, L.R.; Catalán, C.A.N.; Joseph-Natan, P. The chemistry of the genus Stevia (Asteraceae). Rev. Acad. Colomb. Ciencias 1998, 22, 229–279. [Google Scholar]
- Rodríguez-Cravero, J.F.; Gutiérrez, D.G.; Katinas, L.; Grossi, M.A.; Bonifacino, J.M.; Marchesi, E. A revision and morphological analysis of the Uruguayan species of Stevia (Compositae, Eupatorieae). Rodriguésia 2019, 70, e01532018. [Google Scholar] [CrossRef]
- Soejarto, D.D. Botany of Stevia and Stevia rebaudiana. In Stevia. The Genus Stevia; Kinghorn, A.D., Ed.; Taylor and Francis: London, UK, 2002; Chapter 2; pp. 18–39. [Google Scholar]
- King, R.M.; Robinson, H. The Genera of the Eupatorieae (Asteraceae); Monographs in Systematic Botany vol 22; King, R.M., Robinson, H., Eds.; Allen Press, Inc.: Lawrence, KS, USA, 1987. [Google Scholar]
- Ruiz-Ruiz, J.C.; Moguel-Ordoñez, Y.B.; Segura-Campos, M.R. Biological activity of Stevia rebaudiana Bertoni and their relationship to health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Soejarto, D.D. Ethnobotany of Stevia and Stevia rebaudiana. In Stevia. The Genus Stevia; Kinghorn, A.D., Ed.; Taylor and Francis: London, UK, 2002; Chapter 3; pp. 40–67. [Google Scholar]
- Cerda-García-Rojas, C.M.; Pereda-Miranda, R. The phytochemistry of Stevia: A general survey. In Stevia. The Genus Stevia; Kinghorn, A.D., Ed.; Taylor and Francis: London, UK, 2002; Chapter 5; pp. 86–118. [Google Scholar]
- Rodríguez-Cravero, J.; Gutiérrez, D. Stevia Cav. In Plantas Cultivadas de la Argentina: Asteráceas-Compuestas, 1st ed.; Hurrell, J.A., Bayón, N.D., Delucchi, G., Eds.; Hemisferio Sur: Buenos Aires, Argentina, 2017; pp. 273–278. [Google Scholar]
- Cantero, J.J.; Núñez, C.O.; Bernardello, G.; Amuchastegui, A.; Mulko, J.; Brandolin, P.; Palchetti, M.V.; Iparraguirre, J.; Virginil, N.; y Ariza Espinar, L. Las Plantas de Importancia Económica en Argentina, 1st ed.; UniRío Editora: Rio Cuarto, Argentina, 2019. [Google Scholar]
- Soejarto, D.D.; Compadre, C.M.; Kinghorn, A.D. Ethnobotanical notes on Stevia. Bot. Mus. Leafl. Harv. Univ. 1983, 29, 1–25. [Google Scholar]
- Cariño-Cortés, R.; Hernández-Ceruelos, A.; Torres-Valencia, J.M.; González-Avila, M.; Arriaga-Alba, M.; Madrigal-Bujaidar, E. Antimutagenicity of Stevia pilosa and Stevia eupatoria evaluated with the Ames test. Toxicol. Vitro 2007, 21, 691–697. [Google Scholar] [CrossRef]
- Perez-Perez, I.; Valencia, J.M.T. Metabolitos secundarios aislados de las raíces y las hojas de Stevia jorullensis H.B.K. Bachelor’s Thesis, Universidad Autónoma del Estado de Hidalgo, Pachuca de Soto, Mexico, 2016. [Google Scholar]
- Brown, A.E.; Moritán, M.G.; Ventura, B.; Hilgert, N.I.; Malizia, L.R. Plantas silvestres, ámbito doméstico y subsistencia. In Finca San Andrés. Un Espacio de Cambios Ambientales y Sociales en el Alto Bermejo; Brown, A.E., Moritán, M.G., Ventura, B., Hilgert, N.I., Malizia, L.R., Eds.; Subtrópico: Tucumán, Argentina, 2007; Chapter 7; p. 210. [Google Scholar]
- Cordeiro, M.S.; Simas, D.L.R.; Pérez-Sabino, J.F.; Mérida-Reyes, M.S.; Muñoz-Wug, M.A.; Oliva-Hernández, B.E.; Da Silva, A.J.R.; Fernandes, P.D.; Giorno, T.B.S. Characterization of the antinociceptive activity from Stevia serrata Cav. Biomedicines 2020, 8, 79. [Google Scholar] [CrossRef]
- Sülsen, V.; Martino, V. Overview. In Sesquiterpene Lactones. Advances in Their Chemistry and Biological Aspects; Sülsen, V., Martino, V., Eds.; Springer: Cham, Switzerland, 2018; pp. 3–17. [Google Scholar]
- Sülsen, V.; Elso, O.; Borgo, J.; Laurella, L.C.; Catalan, C.A.N. Recent patents involving sesquiterpene lactones with therapeutic application. In Studies in Natural Product Chemistry (Bioactive Natural Products); Atta-ur-Rahman, Ed.; Elseiver Science Publisher: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Román, L.U.; Morales, N.R.; Hernandez, J.D.; Cerda-Garcia-Rojas, C.M.; Zepeda, L.G.; Flores Sandoval, C.A.; Joseph-Nathan, P. Generation of the new quirogane skeleton by a vinylogous retro-Michael type rearrangement of longipinene derivatives. Tetrahedron 2001, 57, 7269–7275. [Google Scholar] [CrossRef]
- Sánchez-Arreola, E.; Cerda-García-Rojas, C.M.; Román, L.U.; Hernández, J.D.; Joseph-Nathan, P. Longipinane Derivatives from Stevia connata. J. Nat. Prod. 2000, 63, 12–15. [Google Scholar] [CrossRef]
- Román, L.U.; Cambrón, J.I.; Del Río, R.E.; Hernández, J.D.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P. Grindelane diterpenoids from Stevia subpubescens. J. Nat. Prod. 2000, 63, 226–229. [Google Scholar] [CrossRef]
- Román, L.U.; Guerra-Ramírez, D.; Morán, G.; Martínez, I.; Hernández, J.D.; Cerda-García-Rojas, C.M.; Torres-Valencia, J.M.; Joseph-Nathan, P. First seco-C Oleananes from Nature. Org. Lett. 2004, 6, 173–176. [Google Scholar] [CrossRef]
- Álvarez-García, R.; Torres-Valencia, J.M.; Román, L.U.; Hernández, J.D.; Cerda-Garciía-Rojas, C.M.; Joseph-Nathan, P. Absolute configuration of the α-methylbutyryl residue in longipinene derivatives from Stevia pilosa. Phytochemistry 2005, 66, 639–642. [Google Scholar] [CrossRef]
- Rojas-Pérez, R.E.; Cedillo-Portugal, E.; Joseph-Nathan, P.; Burgueño-Tapia, E. A New Longipinene Diester from Stevia monardifolia Kunth. Nat. Prod. Commun. 2009, 4, 757–762. [Google Scholar] [CrossRef]
- Valdez-Calderón, A.; Torres-Valencia, J.M.; Manríquez-Torres, J.J.; Velázquez-Jiménez, R.; Román-Marín, L.U.; Hernández-Hernández, J.D.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P. An unusual diepoxyguaianolide from Stevia tomentosa. Tetrahedron Lett. 2013, 54, 3286–3289. [Google Scholar] [CrossRef]
- Ceunen, S.; Wim, D.B.; Compernolle, F.; Mai, A.H.; Geuns, J.M.C. Diterpene glycosides from Stevia phlebophylla A. Gray. Carbohydr. Res. 2013, 379, 1–6. [Google Scholar] [CrossRef]
- Beer, M.F.; Frank, F.M.; Elso, O.G.; Ernesto Bivona, A.; Cerny, N.; Giberti, G.; Malchiodi, E.L.; Martino, V.S.; Alonso, M.R.; Sülsen, V.P.; et al. Trypanocidal and leishmanicidal activities of flavonoids isolated from Stevia satureiifolia var. satureiifolia. Pharm. Biol. 2016, 54, 2188–2195. [Google Scholar] [CrossRef]
- Simas, D.L.R.; Mérida-Reyes, M.S.; Muñoz-Wug, M.A.; Cordeiro, M.S.; Giorno, T.B.S.; Taracena, E.A.; Oliva-Hernández, B.E.; Martínez-Arévalo, J.V.; Fernandes, P.D.; Pérez-Sabino, J.F.; et al. Chemical composition and evaluation of antinociceptive activity of the essential oil of Stevia serrata Cav. from Guatemala. Nat. Prod. Res. 2019, 33, 577–579. [Google Scholar] [CrossRef]
- Machado, K.N.; Tasco, A.J.H.; Salvador, M.J.; Rodrigues, I.V.; Pessoa, C.; Sousa, I.J.O.; Ferreira, P.M.P.; do Nascimento, A.M. Flavonoids, Antioxidant, and Antiproliferative Activities of Stevia urticifolia. Chem. Nat. Compd. 2017, 53, 1167–1169. [Google Scholar] [CrossRef]
- Perez-Castorena, A.L.; Arciniegas, A.; Nieto-Camacho, A.; Villasenor, J.L.; de Vivar, A.R. Chemical Constituents of Stevia subpubescens var. subpubescens and Evaluation of the Anti-Inflammatory Activity. Chem. Nat. Compd. 2019, 55, 538–539. [Google Scholar] [CrossRef]
- Pérez-Castorena, A.L.; Nieto-Camacho, A.; Maldonado, E. Sesquiterpene lactones and other constituents from Stevia jorullensis. Biochem. Syst. Ecol. 2020, 89. [Google Scholar] [CrossRef]
- Chacón-Morales, P.A.; Dugarte, C.S.; Amaro-Luis, J.M. Helenin from Stevia lucida. The first report of this natural eudesmanolide mixture in Eupatorieae tribe. Nat. Prod. Res. 2020, 1–4. [Google Scholar] [CrossRef]
- Fournet, A.; Barrios, A.A.; Muñoz, V. Leishmanicidal and trypanocidal activities of Bolivian medicinal plants. J. Ethnopharmacol. 1994, 41, 19–37. [Google Scholar] [CrossRef]
- Kedik, S.A.; Yartsev, E.I.; Stanishevskaya, I.E. Antiviral activity of dried extract of Stevia. Pharm. Chem. J. 2009, 43, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Mehta, A.; Mehta, P.; Bajpai, V.K. Antioxidant ability and total phenolic content of aqueous leaf extract of Stevia rebaudiana Bert. Exp. Toxicol. Pathol. 2012, 64, 807–811. [Google Scholar] [CrossRef]
- Moselhy, S.S.; Ghonieim, M.A.; Khan, J.A. In vitro and in vivo evaluation of antimicrobial and antioxidant potential of stevia extract. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 18–21. [Google Scholar] [CrossRef]
- Medina-Medrano, J.R.; Torres-Contreras, J.E.; Valiente-Banuet, J.I.; Mares-Quiñones, M.D.; Vázquez-Sánchez, M.; Álvarez-Bernal, D. Effect of the solid–liquid extraction solvent on the phenolic content and antioxidant activity of three species of Stevia leaves. Sep. Sci. Technol. 2019, 54, 2283–2293. [Google Scholar] [CrossRef]
- Farhat, G.; Berset, V.; Moore, L. Effects of stevia extract on postprandial glucose response, satiety and energy intake: A three-arm crossover trial. Nutrients 2019, 11, 3036. [Google Scholar] [CrossRef]
- Martínez-Rojo, E.; Cariño-Cortés, R.; Berumen, L.C.; García-Alcocer, G.; Escobar-Cabrera, J. Stevia eupatoria and Stevia pilosa extracts inhibit the proliferation and migration of prostate cancer cells. Medicina 2020, 56, 90. [Google Scholar] [CrossRef]
- Gonzales, M.; Villena, G.K.; Kitazono, A.A. Evaluation of the antioxidant activities of aqueous extracts from seven wild plants from the Andes using an in vivo yeast assay. Results Chem. 2021, 3, 100098. [Google Scholar] [CrossRef]
- Ríos, V.E.; León, A.; Chávez, M.I.; Torres, Y.; Ramírez-Apan, M.T.; Toscano, R.A.; Bravo-Monzón, Á.E.; Espinosa-García, F.J.; Delgado, G. Sesquiterpene lactones from Mikania micrantha and Mikania cordifolia and their cytotoxic and anti-inflammatory evaluation. Fitoterapia 2014, 94, 155–163. [Google Scholar] [CrossRef]
- Máñez, S.; Hernández, V.; Giner, R.M.; Ríos, J.L.; del Carmen Recio, M. Inhibition of pro-inflammatory enzymes by inuviscolide, a sesquiterpene lactone from Inula viscosa. Fitoterapia 2007, 78, 329–331. [Google Scholar] [CrossRef]
- Moujir, L.; Callies, O.; Sousa, P.M.C.; Sharopov, F.; Seca, A.M.L. Applications of sesquiterpene lactones: A review of some potential success cases. Appl. Sci. 2020, 10, 3001. [Google Scholar] [CrossRef]
- Rozenblat, S.; Grossman, S.; Bergman, M.; Gottlieb, H.; Cohen, Y.; Dovrat, S. Induction of G2/M arrest and apoptosis by sesquiterpene lactones in human melanoma cell lines. Biochem. Pharmacol. 2008, 75, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Luna-Herrera, J.; Costa, M.C.; González, H.G.; Rodrigues, A.I.; Castilho, P.C. Synergistic antimycobacterial activities of sesquiterpene lactones from Laurus Spp. J. Antimicrob. Chemother. 2007, 59, 548–552. [Google Scholar] [CrossRef]
- Asaruddin, M.R.; Honda, G.; Tsubouchi, A.; Nakajima-Shimada, J.; Aoki, T.; Kiuchi, F. Trypanocidal constituents from Michelia alba. J. Nat. Med. 2003, 57, 61–63. [Google Scholar]
- Sánchez, L.A.; Capitan, Z.; Romero, L.I.; Ortega-Barría, E.; Gerwick, W.H.; Cubilla-Rios, L. Bio-Assay Guided Isolation of Germacranes with Anti-Protozoan Activity from Magnolia sororum. Nat. Prod. Commun. 2007, 2, 1065–1069. [Google Scholar] [CrossRef]
- Julianti, T.; Hata, Y.; Zimmermann, S.; Kaiser, M.; Hamburger, M.; Adams, M. Antitrypanosomal sesquiterpene lactones from Saussurea costus. Fitoterapia 2011, 82, 955–959. [Google Scholar] [CrossRef]
- Lee, B.K.; Park, S.J.; Nam, S.Y.; Kang, S.; Hwang, J.; Lee, S.J.; Im, D.S. Anti-allergic effects of sesquiterpene lactones from Saussurea costus (Falc.) Lipsch. determined using in vivo and in vitro experiments. J. Ethnopharmacol. 2018, 213, 256–261. [Google Scholar] [CrossRef]
- Eliza, J.; Daisy, P.; Ignacimuthu, S. Antioxidant activity of costunolide and eremanthin isolated from Costus speciosus (Koen ex. Retz) Sm. Chem. Biol. Interact. 2010, 188, 467–472. [Google Scholar] [CrossRef]
- Lee, Y.S.; Choi, E.M. Costunolide stimulates the function of osteoblastic MC3T3-E1 cells. Int. Immunopharmacol. 2011, 11, 712–718. [Google Scholar] [CrossRef]
- Ham, A.; Lee, S.J.; Shin, J.; Kim, K.H.; Mar, W. Regulatory effects of costunolide on dopamine metabolism-associated genes inhibit dopamine-induced apoptosis in human dopaminergic SH-SY5Y cells. Neurosci. Lett. 2012, 507, 101–105. [Google Scholar] [CrossRef]
- Hajdú, Z.; Zupkó, I.; Réthy, B.; Forgo, P.; Hohmann, J. Bioactivity-guided isolation of cytotoxic sesquiterpenes and flavonoids from anthemis ruthenica. Planta Med. 2010, 76, 94–96. [Google Scholar] [CrossRef]
- Fischedick, J.T.; Standiford, M.; Johnson, D.A.; De Vos, R.H.; Todorović, S.; Banjanac, T.; Verpoorte, R.; Johnson, J.A. Activation of antioxidant response element in mouse primary cortical cultures with sesquiterpene lactones isolated from Tanacetum parthenium. Planta Med. 2012, 78, 1725–1730. [Google Scholar] [CrossRef]
- Fabian, L.; Sülsen, V.; Frank, F.; Cazorla, S.; Malchiodi, E.; Martino, V. In silico study of structural and geometrical requirements of natural sesquiterpene lactones with trypanocidal activity. Mini Rev. Med. Chem. 2013, 13, 407–1414. [Google Scholar] [CrossRef]
- Elso, O.G.; Bivona, A.E.; Alberti, A.S.; Cerny, N.; Fabian, L.; Morales, C.; Catalán, C.A.N.; Malchiodi, E.L.; Cazorla, S.I.; Sülsen, V.P. Trypanocidal activity of four sesquiterpene lactones isolated from Asteraceae species. Molecules 2020, 25, 2014. [Google Scholar] [CrossRef] [PubMed]
- Kimani, N.M.; Matasyoh, J.C.; Kaiser, M.; Brun, R.; Schmidt, T.J. Antiprotozoal Sesquiterpene Lactones and Other Constituents from Tarchonanthus camphoratus and Schkuhria pinnata. J. Nat. Prod. 2018, 81, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Michalak, B.; Piwowarski, J.P.; Granica, S.; Waltenberger, B.; Atanasov, A.G.; Khan, S.Y.; Breuss, J.M.; Uhrin, P.; Zyzynska-Granica, B.; Stojakowska, A.; et al. Eupatoriopicrin inhibits pro-inflammatory functions of neutrophils via suppression of il-8 and tnf-Alpha production and p38 and erk 1/2 map kinases. J. Nat. Prod. 2019, 82, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Bachelier, A.; Mayer, R.; Klein, C.D. Sesquiterpene lactones are potent and irreversible inhibitors of the antibacterial target enzyme MurA. Bioorganic Med. Chem. Lett. 2006, 16, 5605–5609. [Google Scholar] [CrossRef] [PubMed]
- Rucker, G.; Heiden, K.; Schenkel, E. Antitumor-active lactones from Kaunia rufescens and Eupatorium cannabinum. J. Indian Inst. Sci. 2001, 81, 333–334. [Google Scholar]
- Beekman, A.C.; Woerdenbag, H.J.; Harm, H.; Kampinga, H.H.; Antonius, W.T.; Konings, A.W.T. Cytotoxicity of Artemisinin, a Dimer of Dihydroartemisinin, Artemisitene and Eupatoriopicrin as Evaluated by the MTT and Clonogenic Assay. Phytother. Res. 1996, 10, 140–144. [Google Scholar] [CrossRef]
- Wu, X.D.; Ding, L.F.; Tu, W.C.; Yang, H.; Su, J.; Peng, L.Y.; Li, Y.; Zhao, Q.S. Bioactive sesquiterpenoids from the flowers of Inula japonica. Phytochemistry 2016, 129, 68–76. [Google Scholar] [CrossRef]
- Boldbaatar, A.; Lee, S.; Han, S.; Jeong, A.L.; Ka, H.I.; Buyanravjikh, S.; Lee, J.H.; Lim, J.S.; Lee, M.S.; Yang, Y. Eupatolide inhibits the TGF-β1-induced migration of breast cancer cells via downregulation of SMAD3 phosphorylation and transcriptional repression of ALK5. Oncol. Lett. 2017, 14, 6031–6039. [Google Scholar] [CrossRef]
- Elso, O.G.; Clavin, M.; Hernandez, N.; Sgarlata, T.; Bach, H.; Catalan, C.A.N.; Aguilera, E.; Alvarez, G.; Sülsen, V.P. Antiprotozoal Compounds from Urolepis hecatantha (Asteraceae). Evid. Based Complement Alternat. Med. 2021, 2021, 6622894. [Google Scholar] [CrossRef]
- Kimani, S.; Backhaus, J.; Matasyoh, J.C.; Kaiser, M.; Herrmann, F.C.; Schmidt, T.J.; Langer, K. Preparation of sesquiterpene lactone-loaded PLA nanoparticles and evaluation of their antitrypanosomal activity. Molecules 2019, 24, 2110. [Google Scholar] [CrossRef]
- Woerdenbag, H.; Hendriks, H.J.; Malingr’e, T.M.; Van Stralen, R.; Van den Berg, K.J.; Konings, A.W.T. In vitro cytotoxicity of sesquiterpene lactones from Eupatorium cannabinum L. and semi-synthetic derivatives from eupatoriopicrin. Phytother. Res. 1988, 2, 109–114. [Google Scholar] [CrossRef]
- Kudumela, R.G.; Mazimba, O.; Masoko, P. Isolation and characterisation of sesquiterpene lactones from Schkuhria pinnata and their antibacterial and anti-inflammatory activities. S. Afr. J. Bot. 2019, 126, 340–344. [Google Scholar] [CrossRef]
- Zhu, Z.; Yuan, J.; Xu, X.; Wei, Y.; Yang, B.; Zhao, H. Eucannabinolide, a novel sesquiterpene lactone, suppresses the growth, metastasis and BCSCS-like traits of TNBC via inactivation of STAT3. Neoplasia 2021, 23, 36–48. [Google Scholar] [CrossRef]
- Liu, S.J.; Liao, Z.X.; Tang, Z.S.; Cui, C.L.; Liu, H.B.; Liang, Y.N.; Zhang, Y.; Shi, H.X.; Liu, Y.R. Phytochemicals and biological activities of Artemisia sieversiana. Phytochem. Rev. 2017, 16, 441–460. [Google Scholar] [CrossRef]
- Sanchez-Carranza, J.N.; González-Maya, L.; Razo-Hernández, R.S.; Salas-Vidal, E.; Nolasco-Quintana, N.Y.; Clemente-Soto, A.F.; García-Arizmendi, L.; Sánchez-Ramos, M.; Marquina, S.; Alvarez, L. Achillin increases chemosensitivity to paclitaxel, overcoming resistance and enhancing apoptosis in human hepatocellular carcinoma cell line resistant to paclitaxel (Hep3B/PTX). Pharmaceutics 2019, 11, 512. [Google Scholar] [CrossRef]
- Woo, S.M.; Choi, W.R.; Lee, D.R.; Kim, H.S.; Yi, C.; Kim, K.H.; Kim, H.L.; Cheng, J.; Le, B.; Yang, S.H.; et al. Leukodin isolated from Artemisia capillaris inhibits alpha-melanocyte stimulating hormone induced melanogenesis in B16F10 melanoma cells. Eur. J. Integr. Med. 2019, 25, 85–91. [Google Scholar] [CrossRef]
- Zapata-Martínez, J.; Sánchez-Toranzo, G.; Chaín, F.; Cataláan, C.A.N.; Bühler, M.I. Effect of guaianolides in the meiosis reinitiation of amphibian oocytes. Zygote 2016, 25, 10–16. [Google Scholar] [CrossRef]
- Zhang, S.L.; Li, B.L.; Li, W.; Lu, M.; Ni, L.Y.; Ma, H.L.; Meng, Q.G. The effects of ludartin on cell proliferation, cell migration, cell cycle arrest and apoptosis are associated with upregulation of p21WAF1 in Saos-2 osteosarcoma cells in vitro. Med. Sci. Monit. 2018, 24, 4926–4933. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Miao, S.; Feng, Y. Ludartin exhibits therapeutic effect on spinal cord injury through inhibition of apoptosis and inflammation. Bangladesh J. Pharmacol. 2019, 14, 54–60. [Google Scholar] [CrossRef]
- Blanco, J.G.; Gil, R.R.; Alvarez, C.I.; Patrito, L.C.; Genti-Raimondi, S.; Flury, A. A novel activity for a group of sesquiterpene lactones: Inhibition of aromatase. FEBS Lett. 1997, 409, 396–400. [Google Scholar] [CrossRef]
- Giordano, O.S.; Guerreiro, E.; Pestchanker, M.J.; Guzman, J.; Pastor, D.; Guardia, T. The gastric cytoprotective effect of Several sesquiterpene lactones. J. Nat. Prod. 1990, 53, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.J.; Wang, J.Z.; Deng, W.Q.; Zou, K. DFT calculations and docking study on sesquiterpene lactones: Inhibition of aromatase. Procedia Environ. Sci. 2011, 8, 446–450. [Google Scholar] [CrossRef]
- Sülsen, V.P.; Lizarraga, E.F.; Elso, O.G.; Cerny, N.; Alberti, A.S.; Bivona, A.E.; Malchiodi, E.L.; Cazorla, S.I.; Catalán, C.A.N. Activity of estafietin and analogues on Trypanosoma cruzi and Leishmania braziliensis. Molecules 2019, 24, 1209. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Kirpotina, L.N.; Mitchell, P.T.; Kishkentaeva, A.S.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Quinn, M.T. The natural sesquiterpene lactones arglabin, grosheimin, agracin, parthenolide, and estafiatin inhibit T cell receptor (TCR) activation. Phytochemistry 2018, 146, 36–46. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, Q.; Jing, L.; Feng, L.; Zhou, Z.; Ni, Z. 11, 13-Dehydro Lactone Moiety in Gynecologic Cancer Cells. Iran J. Public Health. 2020, 49, 2103–2110. [Google Scholar] [CrossRef]
- Cai, H.; Meng, X.; Li, Y.; Yang, C.; Liu, Y. Growth inhibition effects of isoalantolactone on K562/A02 cells: Caspase-dependent apoptotic pathways, S phase arrest, and downregulation of Bcr/Abl. Phytother. Res. 2014, 28, 1679–1686. [Google Scholar] [CrossRef]
- Fan, Y.; Weng, Z.; Gao, H.; Hu, J.; Wang, H.; Li, L. Isoalantolactone Enhances the Radiosensitivity of UMSCC-10A Cells via Specific Inhibition of Erk1/2 Phosphorylation. PLoS ONE 2015, 10. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Cui, L.; Feng, L.; Zhang, Z.; Song, J.; Liu, D.; Jia, X. Isoalantolactone inhibits the migration and invasion of human breast cancer MDA-MB-231 cells via suppression of the p38 MAPK/NF-κB signaling pathway. Oncol. Rep. 2016, 36, 1269–1376. [Google Scholar] [CrossRef]
- Weng, Z.; Gao, H.; Hu, J.; Fan, Y.; Wang, H.; Li, L. Isoalantolactone induces autophagic cell death in SKOV₃ human ovarian carcinoma cells via upregulation of PEA-15. Oncol. Rep. 2016, 35, 833–840. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, G.; Zhang, Y.; Hua, P.; Song, G.; Sun, M.; Li, X.; Tong, T.; Li, B.; Zhang, X. Isoalantolactone induces intrinsic apoptosis through p53 signaling pathway in human lung squamous carcinoma cells. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Khan, M.; Ding, C.; Rasul, A.; Yi, F.; Li, T.; Gao, H.; Gao, R.; Zhong, L.; Zhang, K.; Fang, X.; et al. Isoalantolactone induces reactive oxygen species mediated apoptosis in pancreatic carcinoma PANC-1 cells. Int. J. Biol. Sci. 2012, 8, 533–647. [Google Scholar] [CrossRef]
- Rasul, A.; Khan, M.; Ali, M.; Li, J.; Li, X. Targeting apoptosis pathways in cancer with alantolactone and isoalantolactone. Sci. World J. 2013, 2013, 248532. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Zhang, Q.; Zhang, B.; Yang, B.; Lin, N.M. Active ingredients of Inula helenium L. exhibits similar anti-cancer effects as isoalantolactone in pancreatic cancer cells. Nat. Prod. Res. 2020, 34, 2539–2544. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, Y.; Wen, Z.; Ci, X.; Xia, L.; Wang, Y.; Deng, X.; Wang, J. Isoalantolactone enhances the antimicrobial activity of penicillin g against Staphylococcus aureus by inactivating β-lactamase during protein translation. Pathogens 2020, 9, 161. [Google Scholar] [CrossRef]
- Lu, J.; Kuang, Z.; Chen, T.; Ye, C.; Hou, W.; Tang, L.; Chen, Y.; He, R. Isoalantolactone inhibits RANKL-induced osteoclast formation via multiple signaling pathways. Int. Immunopharmacol. 2020, 84, 106550. [Google Scholar] [CrossRef]
- Yuan, C.-B.; Tian, L.; Yang, B.; Zhou, H.-Y. Isoalantolactone protects LPS-induced acute lung injury through Nrf2 activation. Microb. Pathog. 2018, 123, 213–218. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Brun, R.; Willuhn, G.; Khalid, S.A. Anti-trypanosomal Activity of Helenalin and Some Structurally Related Sesquiterpene Lactones. Planta Med. 2002, 68, 750–751. [Google Scholar] [CrossRef]
- Turk, A.; Ahn, J.H.; Jo, Y.H.; Song, J.Y.; Khalife, H.K.; Gali-Muhtasib, H.; Kim, Y.; Hwang, B.Y.; Lee, M.K. NF-κB inhibitory sesquiterpene lactones from Lebanese Laurus nobilis. Phytochem. Lett. 2019, 30, 120–123. [Google Scholar] [CrossRef]
- Coronado-Aceves, E.W.; Velázquez, C.; Robles-Zepeda, R.E.; Jiménez-Estrada, M.; Hernández-Martínez, J.; Gálvez-Ruiz, J.C.; Garibay-Escobar, A. Reynosin and santamarine: Two sesquiterpene lactones from Ambrosia confertiflora with bactericidal activity against clinical strains of Mycobacterium tuberculosis. Pharm. Biol. 2016, 54, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Lee, S.J.; Nam, K.W.; Kim, K.H.; Mar, W. Hepatoprotective effects of reynosin against thioacetamide-induced apoptosis in primary hepatocytes and mouse liver. Arch. Pharm. Res. 2013, 36, 485–494. [Google Scholar] [CrossRef]
- Ham, A.; Kim, D.W.; Kim, K.H.; Lee, S.J.; Oh, K.B.; Shin, J.; Mar, W. Reynosin protects against neuronal toxicity in dopamine-induced SH-SY5Y cells and 6-hydroxydopamine-lesioned rats as models of Parkinson’s disease: Reciprocal up-regulation of E6-AP and down-regulation of α-synuclein. Brain Res. 2013, 1524, 54–61. [Google Scholar] [CrossRef]
- Mehmood, T.; Maryam, A.; Tian, X.; Khan, M.; Ma, T. Santamarine inhibits NF-κB and STAT3 activation and induces apoptosis in HepG2 liver cancer cells via oxidative stress. J. Cancer 2017, 8, 3707–3717. [Google Scholar] [CrossRef]
- Choi, H.G.; Lee, D.S.; Li, B.; Choi, Y.H.; Lee, S.H.; Kim, Y.C. Santamarin, a sesquiterpene lactone isolated from Saussurea lappa, represses LPS-induced inflammatory responses via expression of heme oxygenase-1 in murine macrophage cells. Int. Immunopharmacol. 2012, 13, 271–279. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Alexander-Lindo, R.L.; DeWitt, D.L.; Nair, M.G. Terpenoids from Stinking toe (Hymneae courbaril) fruits with cyclooxygenase and lipid peroxidation inhibitory activities. Food Chem. 2007, 105, 485–490. [Google Scholar] [CrossRef]
- Cho, B.O.; Ryu, H.W.; So, Y.; Cho, J.K.; Woo, H.S.; Jin, C.H.; Seo, K.I.; Park, J.C.; Jeong, I.Y. Anti-inflammatory effect of austroinulin and 6-O-acetyl-austroinulin from Stevia rebaudiana in lipopolysaccharide-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 62, 638–644. [Google Scholar] [CrossRef]
- Byun, M. Anti-Inflammatory Activity of Austroinulin from Stevia rebaudiana in LPS-induced RAW264.7. Cells J. Korean Soc. Food Sci. Nutr. 2012, 41, 456–461. [Google Scholar] [CrossRef]
- Vieira, H.S.; Takahashi, J.A.; De Oliveira, A.B.; Chiari, E.; Boaventura, M.A.D. Novel derivatives of kaurenoic acid: Preparation and evaluation of their trypanocidal activity. J. Braz. Chem. Soc. 2002, 13, 151–157. [Google Scholar] [CrossRef]
- Brito, S.; Crescente, O.; Fernández, A.; Coronado, A.; Rodriguez, N. Efficacy of a kaurenic acid extracted from the Venezuelan plant Wedelia trilobata (Asteracea) against Leishmania (Viannia) braziliensis. Biomédica 2006, 26, 180–187. [Google Scholar] [CrossRef][Green Version]
- Villasmil, T.; Rojas, J.; Aparicio, R.; Gamboa, N.; Acosta, M.E.; Rodrigues, J.; Usubillaga, A. Antimalarial activity of some kaurenes. Nat. Prod. Commun. 2017, 12, 217–220. [Google Scholar] [CrossRef]
- Mendoza, C.; Márquez, A.; Matheus, N.; Sosa, S.M.; López-Ortega, A. Acción protectora del ácido kaurénico en el estrés oxidativo hepático. Rev. Vet. 2017, 28, 27. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Xia, Y.X.; Liang, Z.M.; Tsang, S.W.; Zhang, H.J. Mechanistic pathways and molecular targets of plant-derived anticancer ent-kaurane diterpenes. Biomolecules 2020, 10, 144. [Google Scholar] [CrossRef]
- Sosa-Sequera, M.C.; Suárez, O.; Daló, N.L. Kaurenic acid: An in vivo experimental study of its anti-inflammatory and antipyretic effects. Indian J. Pharmacol. 2010, 42, 293–296. [Google Scholar] [CrossRef]
- Venditti, A.; Maggi, F.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Ornano, L.; Ballero, M.; Sanna, C.; Bruno, M.; Rosselli, S.; et al. Bioactive Constituents of Juniperus turbinata Guss. from La Maddalena Archipelago. Chem. Biodivers. 2018, 15. [Google Scholar] [CrossRef]
- De Las Heras, B.; Hoult, J.R.S. Non-cytotoxic inhibition of macrophage eicosanoid biosynthesis and effects on leukocyte functions and reactive oxygen species of two novel anti-inflammatory plant diterpenoids. Planta Med. 1994, 60, 501–506. [Google Scholar] [CrossRef]
- Balaei-Kahnamoei, M.; Eftekhari, M.; Ardekani, M.R.S.; Akbarzadeh, T.; Saeedi, M.; Jamalifar, H.; Safavi, M.; Sam, S.; Zhalehjoo, N.; Khanavi, M. Phytochemical constituents and biological activities of Salvia macrosiphon Boiss. BMC Chem. 2021, 15, 1–7. [Google Scholar] [CrossRef]
- Rasool, N.; Rashid, M.A.; Khan, S.S.; Ali, Z.; Zubair, M.; Ahmad, V.U.; Khan, S.N.; Choudhary, M.I.; Tareen, R.B. Novel α-glucosidase activator from Pulicaria undulata. Nat. Prod. Commun. 2013, 8, 757–759. [Google Scholar] [CrossRef]
- Castellar, A.; Coelho, T.S.; Silva, P.E.A.; Ramos, D.F.; Lourenço, M.C.S.; Lage, C.L.S.; Julião, L.S.; Barbosa, Y.G.; Leitão, S.G. The activity of flavones and oleanolic acid from Lippia lacunosa against susceptible and resistant Mycobacterium tuberculosis strains. Rev. Bras. Farm. 2011, 21, 835–840. [Google Scholar] [CrossRef]
- Shafaei, A.; Khan, M.S.S.; Aisha, A.F.A.; Majid, A.M.S.A.; Hamdan, M.R.; Mordi, M.N.; Ismail, Z. Flavonoids-rich Orthosiphon stamineus extract as new candidate for angiotensin I-converting enzyme inhibition: A molecular docking study. Molecules 2016, 21, 1500. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Tan, C.S.; Ahmad, M.; Shibao, R. Mechanism of vasorelaxation induced by eupatorin in the rats aortic ring. Eur. J. Pharmacol. 2016, 789, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, D.H.; Jung, Y.J.; Shin, S.Y.; Lee, Y.H. The natural flavone eupatorin induces cell cycle arrest at the G2/M phase and apoptosis in HeLa cells. Appl. Biol. Chem. 2016, 59, 193–199. [Google Scholar] [CrossRef]
- Razak, N.A.; Yeap, S.K.; Alitheen, N.B.; Ho, W.Y.; Yong, C.Y.; Tan, S.W.; Tan, W.S.; Long, K. Eupatorin Suppressed Tumor Progression and Enhanced Immunity in a 4T1 Murine Breast Cancer Model. Integr. Cancer Ther. 2020, 19. [Google Scholar] [CrossRef]
- Gulçin, İ.; Taslimi, P.; Aygün, A.; Sadeghian, N.; Bastem, E.; Kufrevioglu, O.I.; Turkan, F.; Şen, F. Antidiabetic and antiparasitic potentials: Inhibition effects of some natural antioxidant compounds on α-glycosidase, α-amylase and human glutathione S-transferase enzymes. Int. J. Biol. Macromol. 2018, 119, 741–746. [Google Scholar] [CrossRef]
- Rajbhandari, A.; Roberts, M.F. The Flavonoids of Stevia microchaeta, Stevia monardifolia, and Stevia origanoides. J. Nat. Prod. 1985, 48, 502–503. [Google Scholar] [CrossRef]
- Sülsen, V.P.; Cazorla, S.I.; Frank, F.M.; Redko, F.C.; Anesini, C.A.; Coussio, J.D.; Malchiodi, E.L.; Martino, V.S.; Muschietti, L.V. Trypanocidal and leishmanicidal activities of flavonoids from Argentine medicinal plants. Am. J. Trop. Med. Hyg. 2007, 77, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Melaku, Y.; Worku, T.; Tadesse, Y.; Mekonnen, Y.; Schmidt, J.; Arnold, N.; Dagne, E. Antiplasmodial Compounds from Leaves of Dodonaea angustifolia. Curr. Bioact. Compd. 2017, 13, 268–273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teffo, L.S.; Aderogba, M.A.; Eloff, J.N. Antibacterial and antioxidant activities of four kaempferol methyl ethers isolated from Dodonaea viscosa Jacq. var. angustifolia leaf extracts. S. Afr. J. Bot. 2010, 76, 25–29. [Google Scholar] [CrossRef]
- Mai, L.H.; Chabot, G.G.; Grellier, P.; Quentin, L.; Dumontet, V.; Poulain, C.; Espindola, L.S.; Michel, S.; Vo, H.T.B.; Deguin, B.; et al. Antivascular and anti-parasite activities of natural and hemisynthetic flavonoids from New Caledonian Gardenia species (Rubiaceae). Eur. J. Med. Chem. 2015, 93, 93–100. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, H.Q.; Yan, H.Y.; Wu, S.; Gu, Z.Y.; Li, Y.H. Santin inhibits influenza A virus replication through regulating MAPKs and NF-κB pathways. J. Asian Nat. Prod. Res. 2019, 21, 1205–1214. [Google Scholar] [CrossRef]
- Stompor, M. A review on sources and pharmacological aspects of sakuranetin. Nutrients 2020, 12, 513. [Google Scholar] [CrossRef]
- Ugocsai, K.; Varga, A.; Molnar, P.; Antus, S.; Molnar, J. Effects of selected flavonoids and carotenoids on drug accumulation and apoptosis induction in multidrug-resistant colon cancer cells expressing MDR1/LRP. In Vivo 2005, 19, 433–438. [Google Scholar]
- Park, J.H.; Fu, Y.Y.; Chung, I.S.; Hahn, T.R.; Cho, M.H. Cytotoxic property of ultraviolet-induced rice phytoalexins to human colon carcinoma HCT-116 cell. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 237–241. [Google Scholar] [CrossRef]
- Drira, R.; Sakamoto, K. Sakuranetin induces melanogenesis in B16BL6 melanoma cells through inhibition of ERK and PI3K/AKT signaling pathways. Phytother. Res. 2016, 30, 997–1002. [Google Scholar] [CrossRef]
- Hong, L.; Ying, S.H. Ethanol extract and isolated constituents from Artemisia dracunculus inhibit esophageal squamous cell carcinoma and induce apoptotic cell death. Drug Res. 2015, 65, 101–106. [Google Scholar] [CrossRef]
- Grecco, D.S.; Dorigueto, A.C.; Landre, I.M.; Soares, M.G.; Martho, K.; Lima, R.; Pascon, R.C.; Vallim, M.A.; Capello, T.M.; Romoff, P. Structural crystalline characterization of sakuranetin—An antimicrobial flavanone from twigs of Baccharis retusa (Asteraceae). Molecules 2014, 19, 7528–7542. [Google Scholar] [CrossRef]
- Pacciaroni, A.V.; Gette, M.A.; Derita, M.; Luis Ariza-Espinar, L.; Gil, R.R.; Zacchino, S.A.; Silva, G.L. Antifungal Activity of Heterothalamus alienus Metabolites. Phytother. Res. 2008, 22, 524–528. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three flavonoids targeting the β-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: Crystal structure characterization with enzymatic inhibition assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef]
- Grecco, S.D.S.; Reimão, J.Q.; Tempone, A.G.; Sartorelli, P.; Cunha, R.L.O.R.; Romoff, P.; Ferreira, M.J.P.; Fávero, O.A.; Lago, J.H.G. In vitro antileishmanial and antitrypanosomal activities of flavanones from Baccharis retusa DC. (Asteraceae). Exp. Parasitol. 2012, 130, 141–145. [Google Scholar] [CrossRef]
- Quintanilla-Licea, R.; Vargas-Villarreal, J.; Verde-Star, M.J.; Rivas-Galindo, V.M.; Torres-Hernández, Á.D. Antiprotozoal Activity against Entamoeba histolytica of Flavonoids Isolated from Lippia graveolens Kunth. Molecules 2020, 25, 2464. [Google Scholar] [CrossRef]
- Kwon, D.H.; Ji, J.H.; Yim, S.H.; Kim, B.S.; Choi, H.J. Suppression of influenza B virus replication by sakuranetin and mode of its action. Phytother. Res. 2018, 32, 2475–2479. [Google Scholar] [CrossRef]
- Choi, H.J. In vitro antiviral activity of sakuranetin against human rhinovirus 3. Osong Public Health Res. Perspect. 2017, 8, 415–420. [Google Scholar] [CrossRef]
- Bittencourt-Mernak, M.I.; Pinheiro, N.M.; Santana, F.P.R.; Guerreiro, M.P.; Saraiva-Romanholo, B.M.; Grecco, S.S.; Caperuto, L.C.; Felizardo, R.J.F.; Câmara, N.O.S.; Tibério, I.F.L.C.; et al. Prophylactic and therapeutic treatment with the flavonone sakuranetin ameliorates LPS-induced acute lung injury. Am. J. Physiol. Lung C 2017, 312, L217–L230. [Google Scholar] [CrossRef]
- Sakoda, C.P.P.; de Toledo, A.C.; Perini, A.; Pinheiro, N.M.; Hiyane, M.I.; Grecco, S.d.S.; de Fátima Lopes Calvo Tibério, I.; Câmara, N.O.S.; de Arruda Martins, M.; Lago, J.G.H.; et al. Sakuranetin reverses vascular peribronchial and lung parenchyma remodeling in a murine model of chronic allergic pulmonary inflammation. Acta Histochem. 2016, 118, 615–624. [Google Scholar] [CrossRef]
- Taguchi, L.; Pinheiro, N.M.; Choqueta-Toledo, A.; Grecco, S.S.; Lopes, F.D.; Caperuto, L.C.; Martins, L.C.; Tiberio, I.F.; Câmara, N.O.; Lago, J.H.; et al. A flavanone from Baccharis retusa (Asteraceae) prevents elastase-induced emphysema in mice by regulating NF-κB, oxidative stress and metalloproteinases. Respir. Res. 2015, 16, 79. [Google Scholar] [CrossRef]
- Toledo, A.C.; Sakoda, C.P.P.; Perini, A.; Pinheiro, N.M.; Magalhães, R.M.; Grecco, S.; Tibério, I.F.L.C.; Cãmara, N.O.; Martins, M.A.; Lago, J.H.G.; et al. Flavanone treatment reverses airway inflammation and remodeling in an asthma murine model. Br. J. Pharmacol. 2013, 168, 1736–1749. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Okuyama, T.; Ishii, T.; Okumura, T.; Ikeya, Y.; Nishizawa, M. Sakuranetin downregulated inducible nitric oxide synthase expression by affecting interleukin-1 receptor and CCAAT/enhancer-binding protein β. J. Nat. Med. 2019, 73, 353–368. [Google Scholar] [CrossRef]
- Zhang, X.; Hung, T.M.; Phuong, P.T.; Ngoc, T.M.; Min, B.-S.; Song, K.-S.; Seong, Y.H.; Bae, K. Anti-inflammatory activity of flavonoids from Populus davidiana. Arch. Pharm. Res. 2006, 29, 1102–1108. [Google Scholar] [CrossRef]
- Hernández, V.; Recio, M.C.; Máñez, S.; Giner, R.M.; Rios, J.L. Effects of naturally occuring dihydroflavonols from Inula viscosa on inflammation and enzymes involved in the arachidonic acid metabolism. Life Sci. 2007, 81, 480–488. [Google Scholar] [CrossRef]
- Chen, L.; Hu, C. Protective effect of sakuranetin in brain cells of dementia model rats. Cell. Mol. Biol. 2019, 51, 1. [Google Scholar]
- Grecco, D.S.; Félix, M.J.P.; Lago, J.H.G.; Pinto, É.G.; Tempone, A.G.; Romoff, P.; Ferreira, M.J.P.; Sartorelli, P. Anti-trypanosomal phenolic derivatives from Baccharis uncinella. Nat. Prod. Commun. 2014, 9, 171–173. [Google Scholar] [CrossRef]
- Muthu, C.; Reegan, A.D.; Kingsley, S.; Ignacimuthu, S. Larvicidal activity of pectolinaringenin from Clerodendrum phlomidis L. against Culex quinquefasciatus Say and Aedes aegypti L. (Diptera: Culicidae). Parasitol. Res. 2012, 111, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Ruedi, P. Antitrypanosomal and Antileishmanial Activities of Flavonoids and Their Analogues: In Vitro, In Vivo, Structure-Activity Relationship, and Quantitative Structure-Activity Relationship Studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Chebib, M.; Aburjai, T.; Johnston, G.A.R.; Hanrahan, J.R. GABAA Receptor Modulation by Compounds Isolated from Salvia triloba L. Adv. Biol. Chem. 2014, 4, 148–159. [Google Scholar] [CrossRef][Green Version]
- Abdelhalim, A.; Karim, N.; Chebib, M.; Aburjai, T.; Khan, I.; Johnston, G.A.R.; Hanrahan, J.R. Antidepressant, anxiolytic and antinociceptive activities of constituents from Rosmarinus officinalis. J. Pharm. Pharm. Sci. 2015, 18, 448–459. [Google Scholar] [CrossRef]
- Hernández-Bolio, G.I.; Torres-Tapia, L.W.; Moo-Puc, R.; Peraza-Sánchez, S.R. Antigiardial activity of flavonoids from leaves of Aphelandra scabra. Rev. Bras. Farmacogn. 2015, 25, 233–237. [Google Scholar] [CrossRef][Green Version]
- Kim, H.J.; Kim, I.S.; Dong, Y.; Lee, I.S.; Kim, J.S.; Kim, J.S.; Woo, J.T.; Cha, B.Y. Melanogenesis-inducing effect of cirsimaritin through increases in microphthalmia-associated transcription factor and tyrosinase expression. Int. J. Mol. Sci. 2015, 16, 8772–8788. [Google Scholar] [CrossRef]
- Wu, Z.K.; Wang, J.J.; Zhu, S.S.; Zhang, J.Y.; Wei, J.H.; Li, L. Cirsimaritin ameliorates cardiac remodeling and dysfunction through promoting myocardial autophagy in rats with heart failure. Int. J. Clin. Exp. Pathol. 2016, 9, 509–520. [Google Scholar]
- Lee, D.; Jung, Y.; Baek, J.Y.; Shin, M.S.; Lee, S.; Hahm, D.H.; Lee, S.C.; Shim, J.S.; Kim, S.N.; Kang, K.S. Cirsimaritin Contributes to the Estrogenic Activity of Cirsium japonicum var. maackii through the Activation of Estrogen Receptor α. Bull. Korean Chem. Soc. 2017, 38, 1486–1490. [Google Scholar] [CrossRef]
- Abbas, G.; Al Harrasi, A.; Hussain, H.; Hamaed, A.; Supuran, C.T. The management of diabetes mellitus-imperative role of natural products against dipeptidyl peptidase-4, α-glucosidase and sodium-dependent glucose co-transporter 2 (SGLT2). Bioorganic Chem. 2019, 86, 305–315. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, H.Y.; Shibamoto, T.; Jang, T.S.; Lee, S.C.; Shim, J.S.; Hahm, D.H.; Lee, H.J.; Lee, S.; Kang, K.S. Beneficial effects of a medicinal herb, Cirsium japonicum var. maackii, extract and its major component, cirsimaritin on breast cancer metastasis in MDA-MB-231 breast cancer cells. Bioorg. Med. Chem. Lett. 2017, 27, 3968–3973. [Google Scholar] [CrossRef]
- Shin, M.S.; Park, J.Y.; Lee, J.; Yoo, H.H.; Hahm, D.H.; Lee, S.C.; Lee, S.; Hwang, G.S.; Jung, K.; Kang, K.S. Anti-inflammatory effects and corresponding mechanisms of cirsimaritin extracted from Cirsium japonicum var. Maackii Maxim. Bioorganic Med. Chem. Lett. 2017, 27, 3076–3080. [Google Scholar] [CrossRef]
- Yan, H.; Wang, H.; Ma, L.; Ma, X.; Yin, J.; Wu, S.; Huang, H.; Li, Y. Cirsimaritin inhibits influenza A virus replication by downregulating the NF-κB signal transduction pathway. Virol. J. 2018, 15, 1–9. [Google Scholar] [CrossRef]
- Manurung, K.; Sulastri, D.; Zubir, N.; Ilyas, S. In silico anticancer activity and in vitro antioxidant of flavonoids in Plectranthus amboinicus. Pharmacogn. J. 2020, 12, 1573–1577. [Google Scholar] [CrossRef]
- Pathak, G.; Singh, S.; Kumari, P.; Raza, W.; Hussain, Y.; Meena, A. Cirsimaritin, a lung squamous carcinoma cells (NCIH-520) proliferation inhibitor. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef]
- Thanasansurapong, S.; Tuchinda, P.; Reutrakul, V.; Pohmakotr, M.; Piyachaturawat, P.; Chairoungdua, A.; Suksen, K.; Akkarawongsapat, R.; Limthongkul, J.; Napaswad, C.; et al. Cytotoxic and anti-HIV-1 activities of triterpenoids and flavonoids isolated from leaves and twigs of Gardenia sessiliflora. Phytochem. Lett. 2020, 35, 46–52. [Google Scholar] [CrossRef]
- Bourdillat, B.; Delautier, D.; Labat, C.; Benveniste, J.; Potier, P.; Brink, C. Hispidulin, a natural flavone, inhibits human platelet aggregation by increasing cAMP levels. Eur. J. Pharmacol. 1988, 147, 1–6. [Google Scholar] [CrossRef]
- Marques, M.R.; Stüker, C.; Kichik, N.; Tarragó, T.; Giralt, E.; Morel, A.F.; Dalcol, I.I. Flavonoids with prolyl oligopeptidase inhibitory activity isolated from Scutellaria racemosa Pers. Fitoterapia 2010, 81, 552–556. [Google Scholar] [CrossRef]
- Mercader, A.G.; Pomilio, A.B. QSAR study of flavonoids and biflavonoids as influenza H1N1 virus neuraminidase inhibitors. Eur. J. Med. Chem. 2010, 45, 1724–1730. [Google Scholar] [CrossRef]
- Yu, C.Y.; Su, K.Y.; Lee, P.L.; Jhan, J.Y.; Tsao, P.H.; Chan, D.C.; Chen, Y.L.S. Potential therapeutic role of hispidulin in gastric cancer through induction of apoptosis via NAG-1 signaling. Evid. Based Complementary Altern. Med. 2013. [Google Scholar] [CrossRef]
- Xu, Q.; Xie, H.; Wu, P.; Wei, X. Flavonoids from the capitula of Eriocaulon australe. Food Chem. 2013, 139, 149–154. [Google Scholar] [CrossRef]
- Xie, W.; Li, H.; Zhu, J. Antitumor effect of hispidulin in vivo and in vitro. Mater. Med. 2007, 23, 21–22. [Google Scholar]
- Reutrakul, V.; Krachangchaeng, C.; Tuchinda, P.; Pohmarkotr, M.; Jaipetch, T.; Yoosook, C.; Kasisit, J.; Sophasan, S.; Sujarit, K.; Santisuk, T. Cytotoxic and anti-HIV-1 constituents from leaves and twigs of Gardenia tubifera. Tetrahedron 2004, 60, 1517–1523. [Google Scholar] [CrossRef]
- Nepal, M.; Choi, H.J.; Choi, B.Y.; Yang, M.S.; Chae, J.I.; Li, L.; Soh, Y. Hispidulin attenuates bone resorption and osteoclastogenesis via the RANKL-induced NF-κB and NFATc1 pathways. Eur. J. Pharmacol. 2013, 715, 96–104. [Google Scholar] [CrossRef]
- Kim, H.A.; Lee, J. Hispidulin modulates epithelial-mesenchymal transition in breast cancer cells. Oncol. Lett. 2021, 21. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, F.; Yan, J.; Xia, Z.; Jiang, D.; Maa, P. Hispidulin: A promising flavonoid with diverse anti-cancer properties. Life Sci. 2020, 259, 118395. [Google Scholar] [CrossRef]
- Serti‘e, J.A.A.; Basile, A.C.; Panizza, S.; Matida, A.K.; Zelnik, R. Anti-inflammatory activity and sub-acute toxicity of artemetin. Planta Med. 1990, 56, 36–40. [Google Scholar] [CrossRef]
- Grossini, E.; Marotta, P.; Farruggio, S.; Sigaudo, L.; Qoqaiche, F.; Raina, G.; De Giuli, V.; Mary, D.; Vacca, G.; Pollastro, F. Effects of Artemetin on Nitric Oxide Release and Protection against Peroxidative Injuries in Porcine Coronary Artery Endothelial Cells. Phytother. Res. 2015, 29, 1339–1348. [Google Scholar] [CrossRef]
- Hu, J.; Ma, W.; Li, N.; Wang, K.J. Antioxidant and anti-inflammatory flavonoids from the flowers of chuju, a medical cultivar of chrysanthemum morifolim ramat. J. Mex. Chem. Soc. 2017, 61, 282–289. [Google Scholar] [CrossRef]
- De Souza, P.; Gasparotto, A.; Crestani, S.; Élida, M.; Stefanello, A.; Consuelo, M.; Marques, A.; Eduardo, J.; Aparecida, C.; Kassuya, L. Phytomedicine Hypotensive mechanism of the extracts and artemetin isolated from Achillea millefolium L. (Asteraceae) in rats. Eur. J. Integr. Med. 2011, 18, 819–825. [Google Scholar] [CrossRef]
- Liu, K.C.; Yang, S.L.; Roberts, M.F.; Elford, B.C.; Phillipson, J.D. Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant. Cell Rep. 1992, 11, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Nwodo, N.; Okoye, F.; Lai, D.; Debbab, A.; Kaiser, M.; Brun, R.; Proksch, P. Evaluation of the in vitro trypanocidal activity of methylated flavonoid constituents of Vitex simplicifolia leaves. BMC Complement. Altern. Med. 2015, 15, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wee, H.N.; Neo, S.Y.; Singh, D.; Yew, H.C.; Qiu, Z.Y.; Tsai, X.R.C.; How, S.Y.; Yip, K.Y.C.; Tan, C.H.; Koh, H.L. Effects of Vitex trifolia L. Leaf extracts and phytoconstituents on cytokine production in human u937 macrophages. BMC Complement. Altern. Med. 2020, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Yanaka, T.; Yamamoto, M.; Ito, Y.; Nohara, T. New diterpenes and norditerpenes from the fruits of Vitex rotundifolia. J. Nat. Prod. 2002, 65, 537–541. [Google Scholar] [CrossRef]
- Boniface, P.K.; Ferreira, E.I. Flavonoids as efficient scaffolds: Recent trends for malaria, leishmaniasis, Chagas disease, and dengue. Phytother. Res. 2019, 33, 2473–2517. [Google Scholar] [CrossRef]
- Yuting, C.; Rongliang, Z.; Zhngjian, J.; Yong, J. Flavonoids as superoxide scavengers and antioxidants. Free Radic. Biol. Med. 1990, 9, 19–21. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and its anti-allergic immune response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Marunaka, Y.; Marunaka, R.; Sun, H.; Yamamoto, T.; Kanamura, N.; Inui, T.; Taruno, A. Actions of quercetin, a polyphenol, on blood pressure. Molecules 2017, 22, 209. [Google Scholar] [CrossRef]
- Eid, H.M.; Haddad, P.S. The Antidiabetic Potential of Quercetin: Underlying Mechanisms. Curr. Med. Chem. 2017, 24, 355–364. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I.A.; ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Asemi, Z. Quercetin: A natural compound for ovarian cancer treatment. J. Ovarian Res. 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective effects of quercetin in Alzheimer’s disease. Biomolecules 2020, 10, 59. [Google Scholar] [CrossRef]
- Li, J.; Jiang, H.; Shi, R. A new acylated quercetin glycoside from the leaves of Stevia rebaudiana Bertoni. Nat. Prod. Res. 2009, 23, 1378–1383. [Google Scholar] [CrossRef]
- Li, Y.; Di Frenz, C.M.; Chen, M.H.; Wang, Y.R.; Li, F.J.; Luo, C.; Liang, N.; Yang, H.; Bohlin, L.; Wang, C.L. Primary Virtual and in vitro Bioassay Screening of Natural Inhibitors from Flavonoids against COX-2. Chin. J. Nat. Med. 2011, 9, 156–160. [Google Scholar] [CrossRef]
- Nguyen, M.T.T.; Awale, S.; Tezuka, Y.; Ueda, J.Y.; Le Tran, Q.; Kadota, S. Xanthine oxidase inhibitors from the flowers of Chrysanthemum sinense. Planta Med. 2006, 72, 46–51. [Google Scholar] [CrossRef]
- Krasteva, I.; Bratkov, V.; Bucar, F.; Kunert, O.; Kollroser, M.; Kondeva-Burdina, M.; Ionkova, I. Flavoalkaloids and Flavonoids from Astragalus monspessulanus. J. Nat. Prod. 2015, 78, 2565–2571. [Google Scholar] [CrossRef] [PubMed]
- Karunarathne, W.A.H.M.; Lee, K.T.; Choi, Y.H.; Jin, C.Y.; Kim, G.Y. Anthocyanins isolated from Hibiscus syriacus L. attenuate lipopolysaccharide-induced inflammation and endotoxic shock by inhibiting the TLR4/MD2-mediated NF-κB signaling pathway. Phytomedicine 2020, 76, 153237. [Google Scholar] [CrossRef]
- Minda, D.; Avram, S.; Pavel, I.Z.; Kis, B.; Ghitu, A.; Zupko, I.; Dehelean, C.; Buda, V.; DIaconeasa, Z.; Scurtu, A.; et al. An in vitro evaluation of apigenin and apigenin-7-o-glucoside against hela human cervical cancer cell line. Rev. Chim. 2020, 71, 140–144. [Google Scholar] [CrossRef]
- Villa-Rodriguez, J.A.; Kerimi, A.; Tumova, S.; Williamson, G. Inhibition of intestinal glucose transport by polyphenols: A mechanism for indirect attenuation of cholesterol absorption. Food Funct. 2019, 10, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ma, Y.; Cheng, G.; Zhang, Y.; Cai, S. Comparative Study of Dietary Flavonoids with Different Structures as α-Glucosidase Inhibitors and Insulin Sensitizers. J. Agric. Food Chem. 2019, 67, 10521–10533. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, S.; Naz, I.; Lee, J.H.; Khan, M.R.; Ahn, K.S. An overview of the potential antineoplastic effects of casticin. Molecules 2020, 25, 1287. [Google Scholar] [CrossRef]
- Koh, D.J.; Ahn, H.S.; Chung, H.S.; Lee, H.; Kim, Y.; Lee, J.Y.; Kim, D.G.; Hong, M.; Shin, M.; Bae, H. Inhibitory effects of casticin on migration of eosinophil and expression of chemokines and adhesion molecules in A549 lung epithelial cells via NF-κB inactivation. J. Ethnopharmacol. 2011, 136, 399–405. [Google Scholar] [CrossRef]
- Bergendorff, O.; Sterner, O. Spasmolytic Flavonols from Artemisia abrotanum. Planta Med. 1995, 61, 370–371. [Google Scholar] [CrossRef]
- Hu, Y.; Xin, H.L.; Zhang, Q.Y.; Zheng, H.C.; Rahman, K.; Qin, L.P. Anti-nociceptive and anti-hyperprolactinemia activities of Fructus viticis and its effective fractions and chemical constituents. Phytomedicine 2007, 14, 668–674. [Google Scholar] [CrossRef]
- Webster, D.E.; He, Y.; Chen, S.N.; Pauli, G.F.; Farnsworth, N.R.; Wang, Z.J. Opioidergic mechanisms underlying the actions of Vitex agnus-castus L. Biochem. Pharmacol. 2011, 81, 170–177. [Google Scholar] [CrossRef]
- Chu, J.; Yan, B.; Zhang, J.; Peng, L.; Ao, X.; Zheng, Z.; Jiang, T.; Zhang, Z. Casticin Attenuates Osteoarthritis-Related Cartilage Degeneration by Inhibiting the ROS-Mediated NF-κB Signaling Pathway in vitro and in vivo. Inflammation 2020, 43, 810–820. [Google Scholar] [CrossRef]
- Nageen, B.; Sarfraz, I.; Rasul, A.; Hussain, G.; Rukhsar, F.; Irshad, S.; Riaz, A.; Selamoglu, Z.; Ali, M. Eupatilin: A natural pharmacologically active flavone compound with its wide range applications. J. Asian Nat. Prod. Res. 2018, 22, 1–16. [Google Scholar] [CrossRef]
- Li, Y.; Ren, R.; Wang, L.; Peng, K. Eupatilin alleviates airway remodeling via regulating phenotype plasticity of airway smooth muscle cells. Biosci. Rep. 2020, 40, 1–10. [Google Scholar] [CrossRef]
- Jeong, J.H.; Moon, S.J.; Jhun, J.Y.; Yang, E.J.; Cho, M.L.; Min, J.K. Eupatilin exerts antinociceptive and chondroprotective properties in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. PLoS ONE 2015, 10, e0130882. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, L.; Xie, J.; Li, J.; Wang, C. Eupatilin prevents behavioral deficits and dopaminergic neuron degeneration in a Parkinson’s disease mouse model. Life Sci. 2020, 253, 117745. [Google Scholar] [CrossRef]
- Kang, Y.J.; Jung, U.J.; Lee, M.K.; Kim, H.J.; Jeon, S.M.; Park, Y.B.; Chung, H.G.; Baek, N.I.; Lee, K.T.; Jeong, T.S.; et al. Eupatilin, isolated from Artemisia princeps Pampanini, enhances hepatic glucose metabolism and pancreatic β-cell function in type 2 diabetic mice. Diabetes Res. Clin. Pract. 2008, 82, 25–32. [Google Scholar] [CrossRef]
- Son, J.E.; Lee, E.; Seo, S.G.; Lee, J.; Kim, J.E.; Kim, J.; Lee, K.W.; Lee, H.J. Eupatilin, a major flavonoid of artemisia, attenuates aortic smooth muscle cell proliferation and migration by inhibiting PI3K, MKK3/6, and MKK4 activities. Planta Med. 2013, 79, 1009–1016. [Google Scholar] [CrossRef]
- Metoui, R.; Bouajila, J.; Znati, M.; Cazaux, S.; Neffati, M.; Akrout, A. Bioactive flavones isolated from Tunisian Artemisia campestris L. Leaves. Cell. Mol. Biol. 2017, 63, 86–91. [Google Scholar] [CrossRef]
- Chougouo, R.D.K.; Nguekeu, Y.M.M.; Dzoyem, J.P.; Awouafack, M.D.; Kouamouo, J.; Tane, P.; McGaw, L.J.; Eloff, J.N. Anti-inflammatory and acetylcholinesterase activity of extract, fractions and five compounds isolated from the leaves and twigs of Artemisia annua growing in Cameroon. SpringerPlus 2016, 5. [Google Scholar] [CrossRef]
- Ebada, S.S.; Al-Jawabri, N.A.; Youssef, F.S.; El-Kashef, D.H.; Knedel, T.O.; Albohy, A.; Korinek, M.; Hwang, T.L.; Chen, B.H.; Lin, G.H.; et al. Anti-inflammatory, antiallergic and COVID-19 protease inhibitory activities of phytochemicals from the Jordanian hawksbeard: Identification, structure-Activity relationships, molecular modeling and impact on its folk medicinal uses. RSC Adv. 2020, 10, 38128–38141. [Google Scholar] [CrossRef]
- Zhu, Q.C.; Wang, Y.; Liu, Y.P.; Zhang, R.Q.; Li, X.; Su, W.H.; Long, F.; Luo, X.D.; Peng, T. Inhibition of enterovirus 71 replication by chrysosplenetin and penduletin. Eur. J. Pharm. Sci. 2011, 44, 392–398. [Google Scholar] [CrossRef]
- Ortiz, S.; Dali-Yahia, K.; Vasquez-Ocmin, P.; Grougnet, R.; Grellier, P.; Michel, S.; Maciuk, A.; Boutefnouchet, S. Heme-binding activity of methoxyflavones from Pentzia monodiana Maire (Asteraceae). Fitoterapia 2017, 118, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nurbek, S.; Murata, T.; Suganuma, K.; Ishikawa, Y.; Buyankhishig, B.; Kikuchi, T.; Byambajav, T.; Davaapurev, B.O.; Sasaki, K.; Batkhuu, J. Isolation and evaluation of trypanocidal activity of sesquiterpenoids, flavonoids, and lignans in Artemisia sieversiana collected in Mongolia. J. Nat. Med. 2020, 74, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Arroo, R.R.J.; Sari, S.; Barut, B.; Özel, A.; Ruparelia, K.C.; Şöhretoğlu, D. Flavones as tyrosinase inhibitors: Kinetic studies in vitro and in silico. Phytochem. Anal. 2020, 31, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zang, Y.; Huang, X.; Cheng, Z. Chemical constituents from Artemisia rupestris and their neuraminidase inhibitory activity. Nat. Prod. Res. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wei, S.; Yang, B.; Ma, W.; Wu, X.; Ji, H.; Sui, H.; Chen, J. Chrysosplenetin inhibits artemisinin efflux in Pgp- over-expressing Caco-2 cells and reverses P-gp/MDR1 mRNA up-regulated expression induced by artemisinin in mouse small intestine. Pharm. Biol. 2017, 55, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; He, X.; Shen, Y.; Chen, X.; Yang, F.; Yang, P.; Pang, F.; Han, X.; He, W.; Wei, Q. Chrysosplenetin promotes osteoblastogenesis of bone marrow stromal cells via Wnt/β-catenin pathway and enhances osteogenesis in estrogen deficiency-induced bone loss. Stem Cell Res. Ther. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Forgo, P.; Zupkó, I.; Molnár, J.; Vasas, A.; Dombi, G.; Hohmann, J. Bioactivity-guided isolation of antiproliferative compounds from Centaurea jacea L. Fitoterapia 2012, 83, 921–925. [Google Scholar] [CrossRef]
- Ahmed, S.; Kamel, E.M. Cytotoxic activities of flavonoids from Centaurea scoparia. Sci. World J. 2014, 2014, 274207. [Google Scholar] [CrossRef]
- Jachak, S.M.; Gautam, R.; Selvam, C.; Madhan, H.; Srivastava, A.; Khan, T. Anti-inflammatory, cyclooxygenase inhibitory and antioxidant activities of standardized extracts of Tridax procumbens L. Fitoterapia 2011, 82, 173–177. [Google Scholar] [CrossRef]
- Chang, S.L.; Chiang, Y.M.; Chang, C.L.T.; Yeh, H.H.; Shyur, L.F.; Kuo, Y.H.; Wu, T.K.; Yang, W.C. Flavonoids, centaurein and centaureidin, from Bidens pilosa, stimulate IFN-γ expression. J. Ethnopharmacol. 2007, 112, 232–236. [Google Scholar] [CrossRef]
- Qaddir, I.; Majeed, A.; Hussain, W.; Mahmood, S.; Rasool, N. An in silico investigation of phytochemicals as potential inhibitors against non-structural protein 1 from dengue virus 4. Braz. J. Pharm. 2020, 56, 1–21. [Google Scholar] [CrossRef]
- Ito, Y.; Kanamaru, A.; Tada, A. Centaureidin promotes dendrite retraction of melanocytes by activating Rho. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 487–494. [Google Scholar] [CrossRef]
- Zater, H.; Huet, J.; Fontaine, V.; Benayache, S.; Stévigny, C.; Duez, P.; Benayache, F. Chemical constituents, cytotoxic, antifungal and antimicrobial properties of Centaurea diluta Ait. subsp. algeriensis (Coss. Dur.) Maire. Asian Pac. J. Trop. Med. 2016, 9, 554–561. [Google Scholar] [CrossRef]
- Şekerler, T.; Şen, A.; Bitiş, L.; Şener, A. In vitro antihepatocellular carcinoma activity of secondary metabolites of Centaurea kilaea boiss. J. Res. Pharm. 2020, 24, 479–486. [Google Scholar] [CrossRef]
- Lee, H.G.; Yu, K.A.; Oh, W.K.; Baeg, T.W.; Oh, H.C.; Ahn, J.S.; Jang, W.C.; Kim, J.W.; Lim, J.S.; Choe, Y.K.; et al. Inhibitory effect of jaceosidin isolated from Artemisia argyi on the function of E6 and E7 oncoproteins of HPV 16. J. Ethnopharmacol. 2005, 98, 339–343. [Google Scholar] [CrossRef]
- Kim, M.J.; Han, J.M.; Jin, Y.Y.; Baek, N.I.; Bang, M.H.; Chung, H.G.; Choi, M.S.; Lee, K.T.; Sok, D.E.; Jeong, T.S. In vitro antioxidant and anti-inflammatory activities of jaceosidin from Artemisia princeps Pampanini cv. Sajabal. Arch. Pharm. Res. 2008, 31, 429–437. [Google Scholar] [CrossRef]
- Park, E.; Hong, K.; Kwon, B.M.; Kim, Y.; Kim, J.H. Jaceosidin ameliorates insulin resistance and kidney dysfunction by enhancing insulin receptor signaling and the antioxidant defense system in type 2 diabetic mice. J. Med. Food 2020, 23, 1083–1092. [Google Scholar] [CrossRef]
- Lee, S.H.; Bae, E.A.; Park, E.K.; Shin, Y.W.; Baek, N.I.; Han, E.J.; Chung, H.G.; Kim, D.H. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps in IgE-induced hypersensitivity. Int. Immunopharmacol. 2007, 7, 1678–1684. [Google Scholar] [CrossRef]
- Min, S.W.; Kim, N.J.; Baek, N.I.; Kim, D.H. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps on carrageenan-induced inflammation in mice. J. Ethnopharmacol. 2009, 125, 497–500. [Google Scholar] [CrossRef]
- Lee, H.; Jang, D.; Jeon, J.; Cho, C.; Choi, S.; Han, S.J.; Oh, E.; Nam, J.; Park, C.H.; Shin, Y.S.; et al. Seomae mugwort and jaceosidin attenuate osteoarthritic cartilage damage by blocking IκB degradation in mice. J. Cell. Mol. Med. 2020, 24, 8126–8137. [Google Scholar] [CrossRef]
- Kumar, R.; Lu, Y.; Elliott, A.G.; Kavanagh, A.M.; Cooper, M.A.; Davis, R.A. Semi-synthesis and NMR spectral assignments of flavonoid and chalcone derivatives. Magn. Reson. Chem. 2016, 54, 880–886. [Google Scholar] [CrossRef]
- Allison, B.J.; Allenby, M.C.; Bryant, S.S.; Min, J.E.; Hieromnimon, M.; Joyner, P.M. Antibacterial activity of fractions from three Chumash medicinal plant extracts and in vitro inhibition of the enzyme enoyl reductase by the flavonoid jaceosidin. Nat. Prod. Res. 2017, 31, 707–712. [Google Scholar] [CrossRef]
- Lee, T.H.; Jung, H.; Park, K.H.; Bang, M.H.; Baek, N.I.; Kim, J. Jaceosidin, a natural flavone, promotes angiogenesis via activation of VEGFR2/FAK/PI3K/AKT/NF-iB signaling pathways in endothelial cells. Exp. Biol. Med. 2014, 239, 1325–1334. [Google Scholar] [CrossRef]
- Afifi, F.U.; Aburjai, T. Antiplatelet activity of Varthemia iphionoides. Fitoterapia 2004, 75, 629–633. [Google Scholar] [CrossRef]
- Aljančić, I.; Stanković, M.; Tešević, V.; Vujisić, L.; Vajs, V.; Milosavljević, S. Protective effect on human lymphocytes of some flavonoids isolated from two Achillea species. Nat. Prod. Commun. 2010, 5, 729–732. [Google Scholar] [CrossRef]
- Elhady, S.S.; Eltamany, E.E.; Shaaban, A.E.; Bagalagel, A.A.; Muhammad, Y.A.; El-Sayed, N.M.; Ayyad, S.N.; Ahmed, A.A.M.; Elgawish, M.S.; Ahmed, S.A. Jaceidin flavonoid isolated from Chiliadenus montanus attenuates tumor progression in mice via vegf inhibition: In vivo and in silico studies. Plants 2020, 9, 1031. [Google Scholar] [CrossRef]
- Camuesco, D.; Comalada, M.; Rodríguez-Cabezas, M.E.; Nieto, A.; Lorente, M.D.; Concha, A.; Zarzuelo, A.; Gálvez, J. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br. J. Pharmacol. 2004, 143, 908–918. [Google Scholar] [CrossRef]
- Cincin, Z.B.; Unlu, M.; Kiran, B.; Bireller, E.S.; Baran, Y.; Cakmakoglu, B. Molecular mechanisms of quercitrin-induced apoptosis in non-small cell lung cancer. Arch. Med. Res. 2014, 45, 445–454. [Google Scholar] [CrossRef]
- Chiow, K.H.; Phoon, M.C.; Putti, T.; Tan, B.K.H.; Chow, V.T. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Xing, L.-Z.; Ni, H.-J.; Wang, Y.-L. Quercitrin attenuates osteoporosis in ovariectomized rats by regulating mitogen-activated protein kinase (MAPK) signaling pathways. Biomed. Pharmacother. 2017, 89, 1136–1141. [Google Scholar] [CrossRef]
- Kim, J.; Re Kim, S.; Choi, Y.H.; Shin, J.Y.; Kim, C.D.; Kang, N.G.; Park, B.C.; Lee, S. Quercitrin Stimulates Hair Growth with Enhanced Expression of Growth Factors via Activation of MAPK/CREB Signaling Pathway. Molecules 2020, 25, 4004. [Google Scholar] [CrossRef] [PubMed]
- Hur, H.J.; Jeong, Y.H.; Lee, S.H.; Sung, M.J. Quercitrin ameliorates hyperlipidemia and hepatic steatosis in ovariectomized mice. Life 2020, 10, 243. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, B.; Liu, X.; Li, Y.; Hou, J.; Chen, S.; Chen, J.; Li, S. Screening of α-Glucosidase Inhibitors from Houttuynia cordata and Evaluation of the Binding Mechanisms. Chem. Sel. 2020, 5, 8440–8446. [Google Scholar] [CrossRef]
- Jegal, J.; Park, N.J.; Lee, S.Y.; Jo, B.G.; Bong, S.K.; Kim, S.N.; Yang, M.H. Quercitrin, the Main Compound in Wikstroemia indica, Mitigates Skin Lesions in a Mouse Model of 2,4-Dinitrochlorobenzene-Induced Contact Hypersensitivity. Evid. Based Complement. Altern. Med. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Razafin-drabazo, F.; Donno, D.; Tombozara, N.; Razafindrakoto, Z.R.; Rajaonarison, J.F.; Andrianjara, C.; Ramanitrahasimbola, D.; Beccaro, G.L. Phyto-compounds and pharmacological activities of Lygodium lanceolatum Desv. (Schizaeaceae). S. Afr. J. Bot. 2020, 135, 225–232. [Google Scholar] [CrossRef]
- Oh, T.W.; Do, H.J.; Jeon, J.H.; Kim, K. Quercitrin inhibits platelet activation in arterial thrombosis. Phytomedicine 2021, 80, 153363. [Google Scholar] [CrossRef]
- Guo, H.; Yin, W.; Zou, Z.; Zhang, C.; Sun, M.; Min, L.; Yang, L.; Kong, L. Quercitrin alleviates cartilage extracellular matrix degradation and delays ACLT rat osteoarthritis development: An in vivo and in vitro study. J. Adv. Res. 2021, 28, 255–267. [Google Scholar] [CrossRef]
- Militão, G.C.G.; Albuquerque, M.R.J.R.; Pessoa, O.D.L.; Pessoa, C.; Moraes, M.E.A.; De Moraes, M.O.; Costa-Lotufo, L.V. Cytotoxic activity of nepetin, a flavonoid from Eupatorium ballotaefolium HBK. Pharmazie 2004, 59, 965–966. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, D.; Xu, D.; Yu, H.; Zhao, Z.; Ma, D.; Jin, J. Eupafolin exhibits potent anti-angiogenic and antitumor activity in hepatocellular carcinoma. Int. J. Biol. Sci. 2017, 13, 701–711. [Google Scholar] [CrossRef]
- Wang, C.Y.; Huang, S.C.; Lai, Z.R.; Ho, Y.L.; Jou, Y.J.; Kung, S.H.; Zhang, Y.; Chang, Y.S.; Lin, C.W. Eupafolin and ethyl acetate fraction of Kalanchoe gracilis stem extract show potent antiviral activities against enterovirus 71 and coxsackievirus A16. Evid. Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Zhang, C.; Ma, L.; Wei, T.; Zhao, Y.; Peng, X. Comparative study of inhibition mechanisms of structurally different flavonoid compounds on α-glucosidase and synergistic effect with acarbose. Food Chem. 2021, 347, 129056. [Google Scholar] [CrossRef]
- Lee, C.W.; Lin, Z.C.; Hsu, L.F.; Fang, J.Y.; Chiang, Y.C.; Tsai, M.H.; Lee, M.H.; Li, S.Y.; Hu, S.C.S.; Lee, I.T.; et al. Eupafolin ameliorates COX-2 expression and PGE2 production in particulate pollutants-exposed human keratinocytes through ROS/MAPKs pathways. J. Ethnopharmacol. 2016, 189, 300–309. [Google Scholar] [CrossRef]
- Chen, X.; Han, R.; Hao, P.; Wang, L.; Liu, M.; Jin, M.; Kong, D.; Li, X. Nepetin inhibits IL-1β induced inflammation via NF-κB and MAPKs signaling pathways in ARPE-19 cells. Biomed. Pharmacother. 2018, 101, 87–93. [Google Scholar] [CrossRef]
- Vo, V.A.; Lee, J.W.; Chang, J.E.; Kim, J.Y.; Kim, N.H.; Lee, H.J.; Kim, S.S.; Chun, W.; Kwon, Y.S. Avicularin inhibits lipopolysaccharide-induced inflammatory response by suppressing ERK phosphorylation in RAW264.7 macrophages. Biomol. Ther. 2012, 20, 532–537. [Google Scholar] [CrossRef]
- Wang, Z.; Li, F.; Quan, Y.; Shen, J. Avicularin ameliorates human hepatocellular carcinoma via the regulation of NF-κB/COX-2/PPAR-γ activities. Mol. Med. Rep. 2019, 19, 5417–5423. [Google Scholar] [CrossRef]
- Wang, W.E.I.; Zheng, H.; Zheng, M.; Liu, X.; Yu, J. Protective effect of avicularin on rheumatoid arthritis and its associated mechanisms. Exp. Ther. Med. 2018, 16, 5343–5349. [Google Scholar] [CrossRef]
- Shen, Z.; Xu, Y.; Jiang, X.; Wang, Z.; Guo, Y.; Pan, W.; Hou, J. Avicularin relieves depressive-like behaviors induced by chronic unpredictable mild stress in mice. Med. Sci. Monit. 2019, 25, 2777–2784. [Google Scholar] [CrossRef]
- Fujimori, K.; Shibano, M. Avicularin, a plant flavonoid, suppresses lipid accumulation through repression of C/EBPα-activated GLUT4-mediated glucose uptake in 3T3-L1 cells. J. Agric. Food Chem. 2013, 61, 5139–5147. [Google Scholar] [CrossRef]
- Ko, W.C.; Kuo, S.W.; Sheu, J.; Lin, C.; Tzeng, S.; Chen, C. Relaxant Effects of Quercetin Methyl Ether Derivatives in Isolated Guinea Pig Trachea and their Structure-Activity Relationships. Planta Med. 1999, 65, 273–275. [Google Scholar] [CrossRef]
- Guerrero, M.F.; Puebla, P.; Martín, M.L.; Carrón, R.; San Román, L.; Reguero, M.T.; Arteaga, L. Inhibitory effect of N(G)-nitro-L-arginine methyl ester on the anti-adrenergic response elicited by ayanin in the pithed rat. Planta Med. 2002, 68, 322–325. [Google Scholar] [CrossRef]
- Guerrero, M.F.; Puebla, P.; Carrón, R.; Martín, M.L.; Román, L.S. Quercetin 3,7-dimethyl ether: A vasorelaxant flavonoid isolated from Croton schiedeanus Schlecht. J. Pharm. Pharmacol. 2002, 54, 1373–1378. [Google Scholar] [CrossRef]
- Kawai, M.; Hirano, T.; Higa, S.; Arimitsu, J.; Maruta, M.; Kuwahara, Y.; Ohkawara, T.; Hagihara, K.; Yamadori, T.; Shima, Y.; et al. Flavonoids and related compounds as anti-allergic substances. Allergol. Int. 2007, 56, 113–123. [Google Scholar] [CrossRef]
- Pick, A.; Müller, H.; Mayer, R.; Haenisch, B.; Pajeva, I.K.; Weigt, M.; Bönisch, H.; Müller, C.E.; Wiese, M. Structure-activity relationships of flavonoids as inhibitors of breast cancer resistance protein (BCRP). Bioorg. Med. Chem. 2011, 19, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.B.; Danton, O.; Kaiser, M.; Khalid, S.; Hamburger, M.; Mäser, P. HPLC-Based Activity Profiling for Antiprotozoal Compounds in Croton gratissimus and Cuscuta hyalina. Front. Pharmacol. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guerra, J.A.; Molina, M.F.; Abad, M.J.; Villar, A.M.; Paulina, B. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids isolated from Tanacetum microphyllum. Int. Immunopharmacol. 2006, 6, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Castillo, Q.A.; Triana, J.; Eiroa, J.L.; Padrón, J.M.; Plata, G.B.; Abel-Santos, E.V.; Báez, L.A.; Rodríguez, D.C.; Jiménez, M.A.; Pérez-Pujols, M.F. Flavonoids from eupatorium illitum and their antiproliferative activities. Pharmacogn. J. 2015, 7, 178–181. [Google Scholar] [CrossRef]
- Saepou, S.; Pohmakotr, M.; Reutrakul, V.; Yoosook, C.; Kasisit, J.; Napaswad, C.; Tuchinda, P. Anti-HIV1 Diterpenoids from Leaves and Twigs of Polyalthia sclerophylla. Planta Med. 2010, 76, 721–725. [Google Scholar] [CrossRef]
- Filho, A.A.d.S.; Resende, D.O.; Fukui, M.J.; Santos, F.F.; Pauletti, P.M.; Cunha, W.R.; Silva, M.L.A.; Gregório, L.E.; Bastos, J.K.; Nanayakkara, N.P.D. In vitro antileishmanial, antiplasmodial and cytotoxic activities of phenolics and triterpenoids from Baccharis dracunculifolia D. C. (Asteraceae). Fitoterapia 2009, 80, 478–482. [Google Scholar] [CrossRef]
| Species | Common Name | Ethnobotanical Use | Location | Refs. |
|---|---|---|---|---|
| S. achalensis | Comadre | Ornamental. | Argentina | [8,9] |
| S. balansae Hieron. | - | Antidiarrheal. | Paraguay | [10] |
| S. bogotensis Tr. ex Cortés | Jarilla, Clavito, eupatoria | Febrifugue. Diaphoretic. | Colombia | [6,10] |
| S. cardiatica Perkins | - | Heart diseases. | Bolivia | [6,10] |
| S. collina Gardn. | Caá-ehé | Sweetener. As stomachic. | Brazil | [6] |
| S. connata Lag. | Pericón de monte | Stomachache treatment. | Guatemala | [6,10] |
| S. elatior HBK. | A-cí | To soothe burns and scratches | Mexico | [6,10] |
| S. eupatoria (Spreng.) Wild | Hierba del borrego, yerba del borrego, cola del borrego, estevia | Diuretic. Antimalarial. For stomachache. Hypoglycemiant. Analgesic. Anti-inflammatory. Antihypertensive. | Cuba | [6,10,11] |
| S. fiebrigii Hieron. var. vattuonei (Hicken) Cabrera | - | Ornamental. | Argentina | [8] |
| S. glandulosa Hook. et Arn. | Hierba de la pulga | Antipyretic. | Mexico | [6,10] |
| S. linoides Sch. Bip. | - | Astringent. | - | [12] |
| S. lucida Lag. | Yerba del aire, hierba de la araña, ma-li-too, kebuj, mariposa, chirca, chilca, javillo, golondrina de la sabanera | To cure wounds. To soothe pains. Rheumatism treatment. Anti-inflammatory. | Mexico, Guatemala, Colombia, Venezuela | [6,10] |
| S. macbridei B. L. Robins var. anomala B. L. Robins | Jauja-huancayo | Used as a bath by women. | Peru | [6,10] |
| S. mercedensis Hieron. var. mercedensis | Comadre | Ornamental. | Bolivia, Argentina | [9,13] |
| S. nepetifolia HBK | Zazal, anis de ratón, peracón | Dysmenorrhea treatment. | Mexico, Guatemala | [6,10] |
| S. palmeri Gray | Raniweri, raniwori | Odoriferous. | Mexico | [6] |
| S. petiolata (Cass) Sch. Bip. | Guarme-guarmi | To give flavor to meat. | Peru | [6,10] |
| S. pilosa Lag. | Flor de María | Antimalarial. Antipyretic. Cathartic. Diuretic. | Mexico | [6,10] |
| S. plummerae Gray | Ronino | To make washes and poultices for open wounds. | Mexico | [6] |
| S. puberula Hook. | Lima-lima | Used as tea substitute and stomach medicine. | Peru | [6,10] |
| S. rebaudiana Bertoni | Hierba dulce del Paraguay, estevia, stevia | Sweetener. Food additive. Contraceptive. Antidiabetic. Used to regulate arterial pressure. | Paraguay, Brazil | [6,10] |
| S. rhombifolia HKB var. stepphanocoma Sch. Bip. | Manka pak’I, pirq’a | Stomachache treatment. As an emetic. Used for mate. | Peru | [6] |
| S. salicifolia Cav. | Hierba del aire, hierba de la mula, la envidia, zazale de olor, yerba de la mula. Hierba de la Santa Rita | Rheumatism treatment. Cathartic. For intestinal upset due to parasites. Purgative. For fevers and colds. | Mexico, USA | [6,10] |
| S. sanguinea Hieron. | Malvisco | Ornamental. | Argentina | [8] |
| S. satureiifolia (Lam.) Sch. Bip. ex Klotzsch var. satureiifolia | Romerillo | Ornamental. | Argentina, Brazil, Uruguay | [8] |
| S. serrata Cav. | Ronino, Uriki, Otoninawa, Chapo, yerba picante, hipericón, Q’ang’aj, anis silvestre, hipericon arrie | To make washes and poultices for open wounds. Applied to cuts on feet and on snake bites. As cough remedy. For gastrointestinal disorders. | Guatemala, Mexico | [6,10,14] |
| S. subpubescens Lag. | Hierba de la mula, Zazal | As a bath after parturition. Stomachache treatment. To treat joint pains. | Mexico | [6,10] |
| S. trifida Lag. | Manzanilla de agua | Dysentery treatment. | Mexico | [6,10] |
| S. yalae Cabrera | - | Ornamental. | Argentina | [8] |
| Comp. N° | Common Name | Species | Reported Activity |
|---|---|---|---|
| 46 | Labdanolic acid | S. salicifolia, S. subpubescens var. subpubescens | Anti-inflammatory [97]. |
| 75 | Austroinulin | S. rebaudiana | Anti-inflammatory [98,99]. |
| 76 | Kaurenic acid/kaurenoic acid | S. monardaefolia S. setifera | Anti-T. cruzi. Anti-Leishmania. Antimalarial. Antioxidant. Antitumor. Anti-inflammatory. Antipyretic [100,101,102,103,104,105]. |
| 77 | Manoyl oxide | S. berlandieri | Radical scavenger. Inhibits PgE2 production [106,107]. |
| 78 | Epi-manoyl oxide | S. salicifolia | Cytotoxic [108]. |
| 79 | Paniculoside IV | S. paniculata | Alpha-glucosidase activator [109]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgo, J.; Laurella, L.C.; Martini, F.; Catalán, C.A.N.; Sülsen, V.P. Stevia Genus: Phytochemistry and Biological Activities Update. Molecules 2021, 26, 2733. https://doi.org/10.3390/molecules26092733
Borgo J, Laurella LC, Martini F, Catalán CAN, Sülsen VP. Stevia Genus: Phytochemistry and Biological Activities Update. Molecules. 2021; 26(9):2733. https://doi.org/10.3390/molecules26092733
Chicago/Turabian StyleBorgo, Jimena, Laura C. Laurella, Florencia Martini, Cesar A. N. Catalán, and Valeria P. Sülsen. 2021. "Stevia Genus: Phytochemistry and Biological Activities Update" Molecules 26, no. 9: 2733. https://doi.org/10.3390/molecules26092733
APA StyleBorgo, J., Laurella, L. C., Martini, F., Catalán, C. A. N., & Sülsen, V. P. (2021). Stevia Genus: Phytochemistry and Biological Activities Update. Molecules, 26(9), 2733. https://doi.org/10.3390/molecules26092733