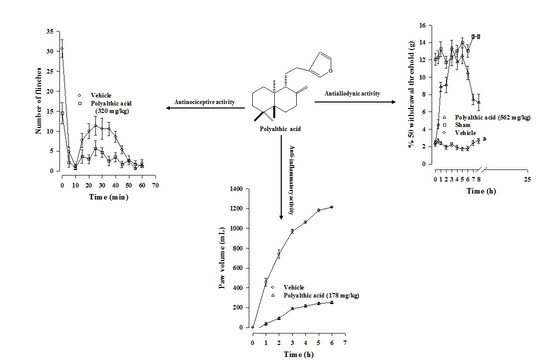
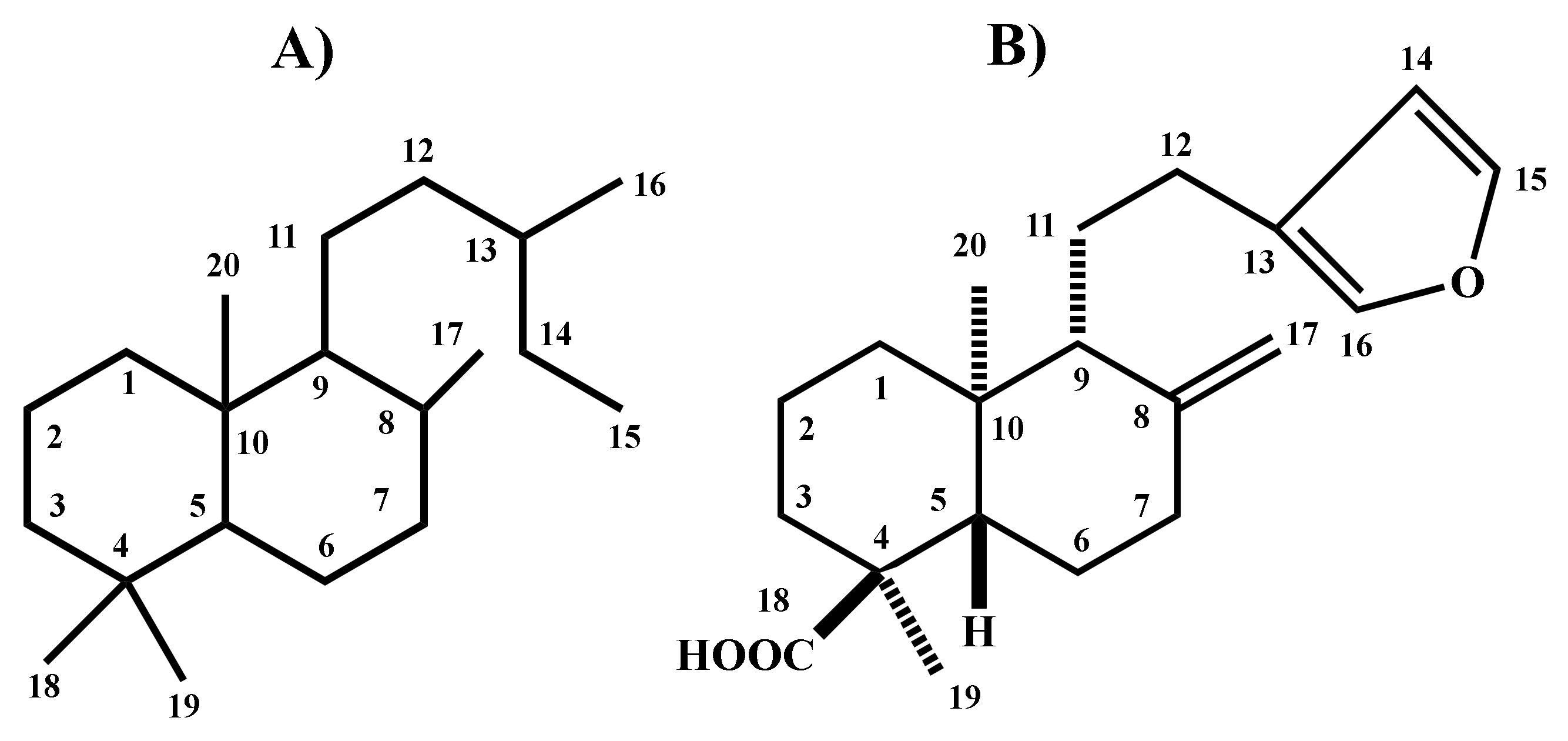
Evaluation of the Antinociceptive, Antiallodynic, Antihyperalgesic and Anti-Inflammatory Effect of Polyalthic Acid
Abstract
1. Introduction
2. Results
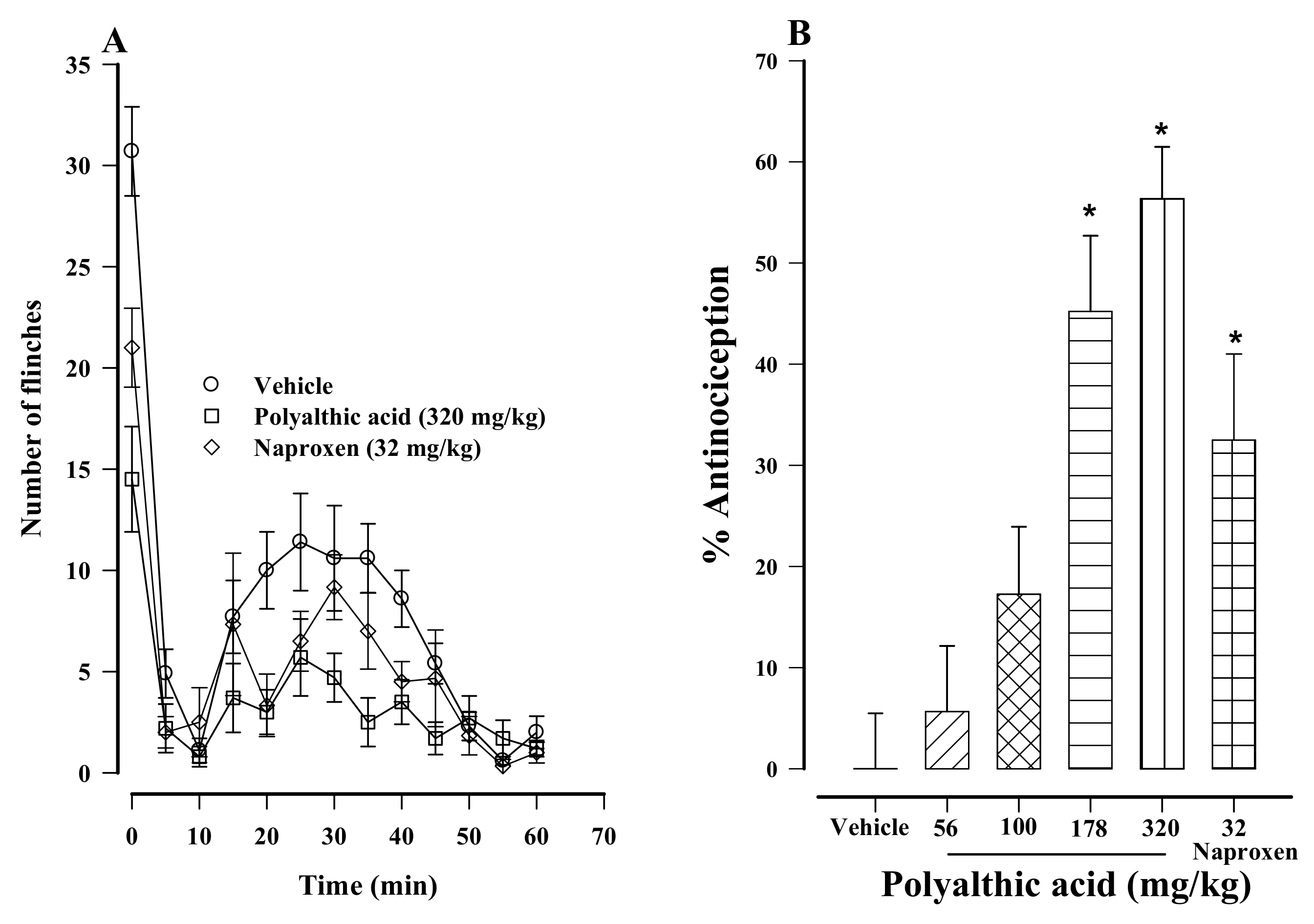
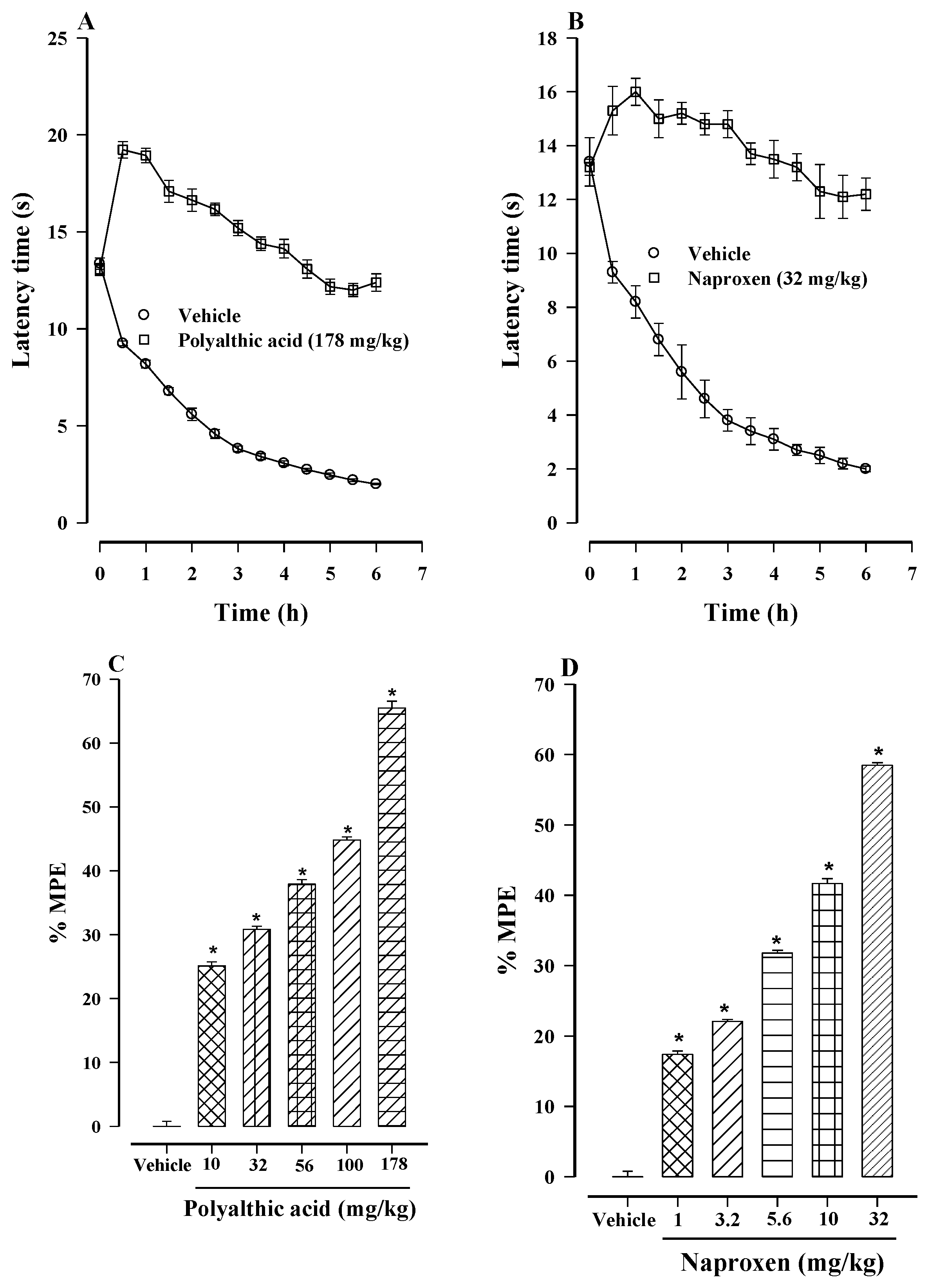
2.1. Antinociceptive Effect of Polyalthic Acid
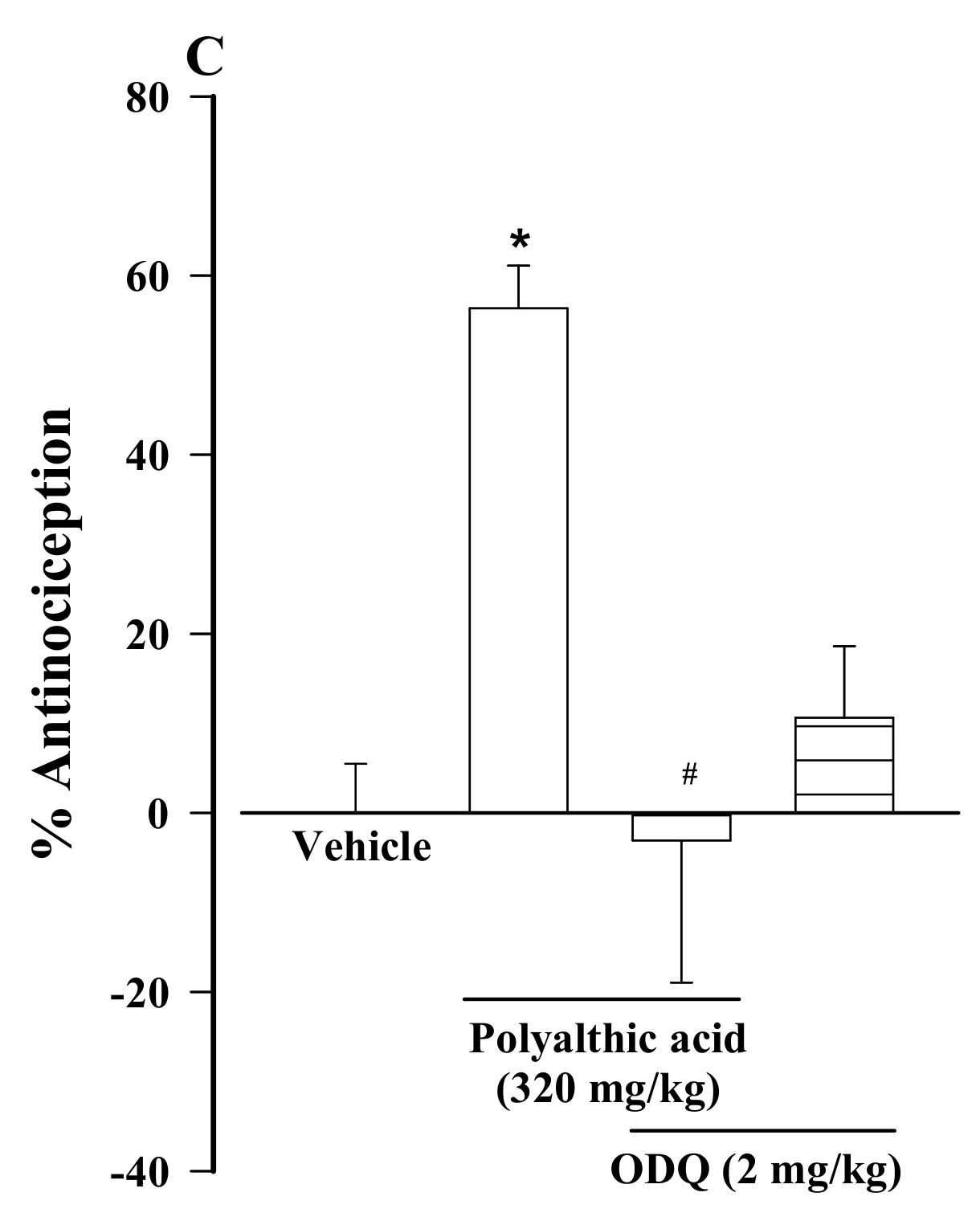
2.2. Possible Mechanisms of the Antinociceptive Effect of Polyalthic Acid
2.3. Antiallodynic Effect of Polyalthic Acid on Rats with an L5/L6 Spinal Nerve Ligation
2.4. Antihyperalgesic Effect of Polyalthic Acid and Naproxen
2.5. Synergistic Interaction between Polyalthic Acid and Naproxen
2.6. Anti-Inflammatory Effect of Polyalthic Acid
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Formalin Test
4.4. L5/L6 Spinal Nerve Ligation
4.5. Thermal Hyperalgesia
4.6. Isobologram
4.7. Carrageenan-Induced Paw Edema
4.8. Experimental Design
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Millan, M.J. The induction of pain: An integrative review. Prog. Neurobiol. 1999, 57, 1–164. [Google Scholar] [CrossRef]
- Williams, A.C.D.C.; Craig, K.D. Updating the definition of pain. Pain 2016, 157, 2420–2423. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; Olivieri, J.; Allison, J.J.; Gaffo, A.; Juarez, L.; Kovac, S.H.; Person, S.; Saag, K.G. A group randomized trial to improve safe use of nonsteroidal anti-inflammatory drugs. Am. J. Manag. Care 2005, 11, 537–543. [Google Scholar]
- Simon, L.S. Nonsteroidal anti-inflammatory drugs and their risk: A story still in development. Arthritis Res. Ther. 2013, 15, 1–2. [Google Scholar] [CrossRef]
- Wallace, J.L.; Viappiani, S.; Bolla, M. Cyclooxygenase-inhibiting nitric oxide donators for osteoarthritis. Trends Pharmacol. Sci. 2009, 30, 112–117. [Google Scholar] [CrossRef]
- Carlo, P. Cardiovascular effects of cyclooxygenase-2 inhibitors: A mechanistic and clinical perspective. Br. J. Clin. Pharmacol. 2016, 82, 957–964. [Google Scholar]
- Reza, T. Risk of Cardiovascular Events and Cyclooxygenase-2 Inhibitors. Vasc. Health. Risk. Manag. 2006, 2, 95. [Google Scholar]
- Babu, T.H.; Manjulatha, K.; Kumar, G.S.; Hymavathi, A.; Tiwari, A.K.; Purohit, M.; Rao, J.M.; Babu, K.S. Gastroprotective flavonoid constituents from Oroxylum indicum Vent. Bioorganic Med. Chem. Lett. 2010, 20, 117–120. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Frade, R.F.M.; Afonso, C.A.M. Isolation, Chemical, and Biotransformation Routes of Labdane-type Diterpenes. Chem. Rev. 2011, 111, 4418–4452. [Google Scholar] [CrossRef]
- Reyes-Trejo, B.; Sánchez-Mendoza, M.E.; Becerra-García, A.A.; Cedillo-Portugal, E.; Castillo-Henkel, C.; Arrieta, J. Bioassay-guided isolation of an anti-ulcer diterpenoid from Croton reflexifolius: Role of nitric oxide, prostaglandins and sulfhydryls. J. Pharm. Pharmacol. 2008, 60, 931–936. [Google Scholar] [CrossRef]
- Sánchez-Mendoza, M.E.; Reyes-Trejo, B.; De La Rosa, L.; Rodríguez-Silverio, J.; Castillo-Henkel, C.; Arrieta, J. Polyalthic Acid Isolated from Croton reflexifolius has Relaxing Effect in Guinea Pig Tracheal Smooth Muscle. Pharm. Biol. 2008, 46, 800–807. [Google Scholar] [CrossRef][Green Version]
- Huang, D.; Qing, S.; Zeng, G.; Wang, Y.; Guo, H.; Tan, J.; Zhou, Y. Lipophilic components from Fructus Viticis Negundo and their anti-tumor activities. Fitoterapia 2013, 86, 144–148. [Google Scholar] [CrossRef]
- Borges, C.H.G.; Cruz, M.G.; Carneiro, L.J.; Da Silva, J.J.M.; Bastos, J.K.; Tavares, D.C.; De Oliveira, P.F.; Rodrigues, V.; Veneziani, R.C.S.; Parreira, R.L.T.; et al. Copaifera duckei Oleoresin and Its Main Nonvolatile Terpenes: In Vitro Schistosomicidal Properties. Chem. Biodivers. 2016, 13, 1348–1356. [Google Scholar] [CrossRef]
- Bardají, D.K.; da Silva, J.J.; Bianchi, T.C.; de Souza, E.D.; de Oliveira, P.F.; Leandro, L.F.; Rogez, H.L.; Venezianni, R.C.; Ambrosio, S.R.; Tavares, D.C.; et al. Copaifera reticulata oleoresin: Chemical characterization and antibacterial properties against oral pathogens. Anaerobe 2016, 40, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Misiewicz, B.; Zelazowska, E.; Raybourne, R.B.; Cizza, G.; Sternberg, E.M. Inflammatory responses to carrageenan injection in LEW/N and F344/N rats: LEW/N rats show sex- and age-dependent changes in inflammatory reactions. Neuroimmunomodulation 1996, 3, 93–101. [Google Scholar] [CrossRef]
- Tall, J.M.; Crisp, T. Effects of gender and gonadal hormones on nociceptive responses to intraplantar carrageenan in the rat. Neurosci. Lett. 2004, 354, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Yaksh, T.L.; Ozaki, G.; McCumber, D.; Rathbun, M.; Svensson, C.; Malkmus, S.; Yaksh, M.C. An automated flinch detecting system for use in the formalin nociceptive bioassay. J. Appl. Physiol. 2001, 90, 2386–2402. [Google Scholar] [CrossRef]
- Vincler, M.; Maixner, W.; Vierck, C.J.; Light, A.R. Estrous cycle modulation of nociceptive behaviors elicited by electrical stimulation and formalin. Pharmacol. Biochem. Behav. 2001, 69, 315–324. [Google Scholar] [CrossRef]
- Aloisi, A.M.; Albonetti, M.E.; Carli, G. Behavioural effects of different intensities of formalin pain in rats. Physiol. Behav. 1995, 58, 603–610. [Google Scholar] [CrossRef]
- Aloisi, A.M.; Sacerdote, P.; Albonetti, M.E.; Carli, G. Sex-related effects on behaviour and beta-endorphin of different intensities of formalin pain in rats. Brain Res. 1995, 699, 242–249. [Google Scholar] [CrossRef]
- Caram-Salas, N.L.; Reyes-García, G.; Bartoszyk, G.D.; Araiza-Saldaña, C.I.; Ambriz-Tututi, M.; Rocha-González, H.I.; Arreola-Espino, R.; Cruz, S.L.; Granados-Soto, V. Subcutaneous, intrathecal and periaqueductal grey administration of asimadoline and ICI-204448 reduces tactile allodynia in the rat. Eur. J. Pharmacol. 2007, 573, 75–83. [Google Scholar] [CrossRef]
- Lima, F.O.; Souza, G.R.; Verri, W.A.; Parada, C.A.; Ferreira, S.H.; Cunha, F.Q.; Cunha, T.M. Direct blockade of inflammatory hypernociception by peripheral A1 adenosine receptors: Involvement of the NO/cGMP/PKG/KATP signaling pathway. Pain 2010, 151, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.C.; Duarte, I.D. Dibutyryl-cyclic GMP induces peripheral antinociception via activation of ATP-sensitive K+ channels in the rat PGE2 -induced hyperalgesic paw. Br. J. Pharmacol. 2001, 134, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.Q.; Teixeira, M.M.; Ferreira, S.H. Pharmacological modulation of secondary mediator systems-cyclic AMP and cyclic GMP-on inflammatory hyperalgesia. Br. J. Pharmacol. 1999, 127, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Noble, M.; Treadwell, J.R.; Tregear, S.J.; Coates, V.H.; Wiffen, P.J.; Akafomo, C.; Schoelles, K.M.; Chou, R. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst. Rev. 2010, 2010, CD006605. [Google Scholar] [CrossRef] [PubMed]
- Meert, T.F.; Vermeirsch, H.A. A preclinical comparison between different opioids: Antinociceptive versus adverse effects. Pharmacol. Biochem. Behav. 2005, 80, 309–326. [Google Scholar] [CrossRef]
- Hashemi, M.; Karami, M.; Zarrindast, M.R.; Sahebgharani, M. Role of nitric oxide in the rat hippocampal CA1 in morphine antinociception. Brain Res. 2010, 1313, 79–88. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Duarte, I.D.; Lorenzetti, B.B. The molecular mechanism of action of peripheral morphine analgesia: Stimulation of the cGMP system via nitric oxide release. Eur. J. Pharmacol. 1991, 201, 121–122. [Google Scholar] [CrossRef]
- Florentino, I.F.; Galdino, P.M.; De Oliveira, L.P.; Silva, D.P.; Pazini, F.; Vanderlinde, F.A.; Lião, L.M.; Menegatti, R.; Costa, E.A. Involvement of the NO/cGMP/KATP pathway in the antinociceptive effect of the new pyrazole 5-(1-(3-fluorophenyl)-1H-pyrazol-4-yl)-2H-tetrazole (LQFM-021). Nitric Oxide. 2015, 47, 17–24. [Google Scholar] [CrossRef]
- Cunha, T.M.; Roman-Campos, D.; Lotufo, C.M.; Duarte, H.L.; Souza, G.R.; Verri, W.A.; Funez, M.I.; Dias, Q.M.; Schivo, I.R.; Domingues, A.C.; et al. Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 4442–4447. [Google Scholar] [CrossRef]
- Cunha, T.M.; Souza, G.R.; Domingues, A.C.; Carreira, E.U.; Lotufo, C.M.; Funez, M.I.; Verri, W.A.; Cunha, F.Q.; Ferreira, S.H. Stimulation of Peripheral Kappa Opioid Receptors Inhibits Inflammatory Hyperalgesia via Activation of the PI3Kγ/AKT/nNOS/NO Signaling Pathway. Mol. Pain 2012, 8, 10. [Google Scholar] [CrossRef]
- Duggan, K.C.; Walters, M.J.; Musee, J.; Harp, J.M.; Kiefer, J.R.; Oates, J.A.; Marnett, L.J. Molecular basis for cyclooxygenase inhibition by the non-steroidal anti-inflammatory drug naproxen. J. Biol. Chem. 2010, 285, 34950–34959. [Google Scholar] [CrossRef]
- Ishiguro, H.; Kawahara, T. Nonsteroidal Anti-Inflammatory Drugs and Prostatic Diseases. BioMed. Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Robich, M.P.; Chu, L.M.; Feng, J.; Burgess, T.A.; Laham, R.J.; Bianchi, C.; Sellke, F.W. Effects of selective cyclooxygenase-2 and nonselective cyclooxygenase inhibition on ischemic myocardium. J. Thorac. Cardiovasc. Surg. 2010, 140, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.I.; González-García, M.P.; Ponce-Monter, H.A.; Castañeda-Hernández, G.; Aguilar-Robles, P. Synergistic effect of the interaction between naproxen and citral on inflammation in rats. Phytomedicine 2010, 18, 74–79. [Google Scholar] [CrossRef]
- Wu, X.; Xie, J.; Qiu, L.; Zou, L.; Huang, Y.; Xie, Y.; Xu, H.; He, S.; Zhang, Q. The anti-inflammatory and analgesic activities of the ethyl acetate extract of Viburnum taitoense Hayata. J. Ethnopharmacol. 2021, 6, 113742. [Google Scholar] [CrossRef]
- Salvemini, D.; Kim, S.F.; Mollace, V. Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: Relevance and clinical implications. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R473–R487. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Wheeler-Aceto, H.; Cowan, A. Standardization of the rat paw formalin test for the evaluation of analgesics. Psychopharmacology 1991, 104, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chung, J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Ortega-Varela, L.F.; Herrera, J.E.; Caram-Salas, N.L.; Rocha-González, H.I.; Granados-Soto, V. Isobolographic Analyses of the Gabapentin-Metamizol Combination after Local Peripheral, Intrathecal and Oral Administration in the Rat. Pharmacology 2007, 79, 214–222. [Google Scholar] [CrossRef]
- Tallarida, R. Calculations for Combination Drug Analysis. In Drug Synergism and Dose-Effect Data Analysis; Chapman and Hall/CRC: Boca Raton, FL, USA, 2000; pp. 57–73. [Google Scholar]
- Winder, C.W.; Wax, J.; Been, M.A. Rapid foot volume measurements on unanesthetized rats, and the question of a phe-nylbutazone effect on anaphylactoid edema. Arch. Int. Pharmacodyn. Ther. 1957, 112, 174–187. [Google Scholar] [PubMed]




| Combination | Oral Dose (mg/kg) | ||
|---|---|---|---|
| Polyalthic Acid | Naproxen | Total Dose in the Combination | |
| 1 | 3.2 | 0.6 | 3.8 |
| 2 | 6.4 | 1.3 | 7.7 |
| 3 | 12.7 | 2.5 | 15.2 |
| 4 | 25.4 | 5.0 | 30.4 |
| 5 | 50.8 | 10 | 60.8 |
| Drug | ED50 (mg/kg) |
|---|---|
| Naproxen | 20.1 ± 4.8 |
| Polyalthic acid | 101.6 ± 30.9 |
| Theoretical ED50 of the polyalthic acid–naproxen combination | 60.9 ± 15.7 CI (32.4–114.3) |
| Experimental ED50 of the polyalthic acid-–naproxen combination | 2.4 ± 0.02 * CI (0.9–6.6) |
| Interaction index (γ) | 0.04 ± 0.02 CI (0.02–0.10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Silverio, J.; Sánchez-Mendoza, M.E.; Rocha-González, H.I.; Reyes-García, J.G.; Flores-Murrieta, F.J.; López-Lorenzo, Y.; Quiñonez-Bastidas, G.N.; Arrieta, J. Evaluation of the Antinociceptive, Antiallodynic, Antihyperalgesic and Anti-Inflammatory Effect of Polyalthic Acid. Molecules 2021, 26, 2921. https://doi.org/10.3390/molecules26102921
Rodríguez-Silverio J, Sánchez-Mendoza ME, Rocha-González HI, Reyes-García JG, Flores-Murrieta FJ, López-Lorenzo Y, Quiñonez-Bastidas GN, Arrieta J. Evaluation of the Antinociceptive, Antiallodynic, Antihyperalgesic and Anti-Inflammatory Effect of Polyalthic Acid. Molecules. 2021; 26(10):2921. https://doi.org/10.3390/molecules26102921
Chicago/Turabian StyleRodríguez-Silverio, Juan, María Elena Sánchez-Mendoza, Héctor Isaac Rocha-González, Juan Gerardo Reyes-García, Francisco Javier Flores-Murrieta, Yaraset López-Lorenzo, Geovanna Nallely Quiñonez-Bastidas, and Jesús Arrieta. 2021. "Evaluation of the Antinociceptive, Antiallodynic, Antihyperalgesic and Anti-Inflammatory Effect of Polyalthic Acid" Molecules 26, no. 10: 2921. https://doi.org/10.3390/molecules26102921
APA StyleRodríguez-Silverio, J., Sánchez-Mendoza, M. E., Rocha-González, H. I., Reyes-García, J. G., Flores-Murrieta, F. J., López-Lorenzo, Y., Quiñonez-Bastidas, G. N., & Arrieta, J. (2021). Evaluation of the Antinociceptive, Antiallodynic, Antihyperalgesic and Anti-Inflammatory Effect of Polyalthic Acid. Molecules, 26(10), 2921. https://doi.org/10.3390/molecules26102921