Entropy Analysis of Electroencephalography for Post-Stroke Dysphagia Assessment
Abstract
1. Introduction
1.1. Dysphagia
1.2. Actual Diagnosis and Treatment of Dysphagia
1.3. Entropy in EEG Signals
2. Materials and Methods
2.1. Database
2.2. Recording Protocol
2.3. Preprocessing
2.4. Obtaining Biomarkers Based on Sample Entropy (SampEn)
2.5. Statistical Analysis
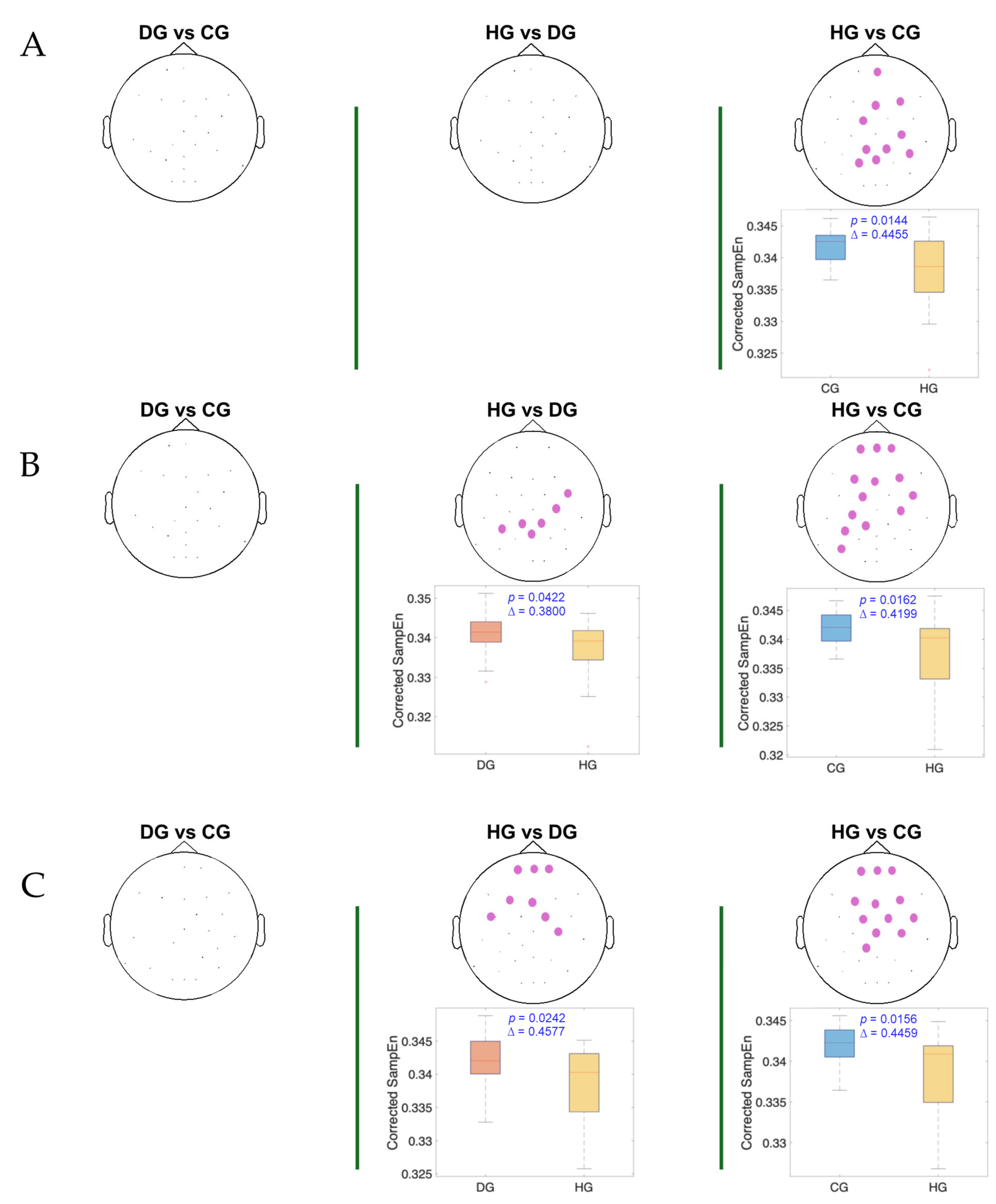
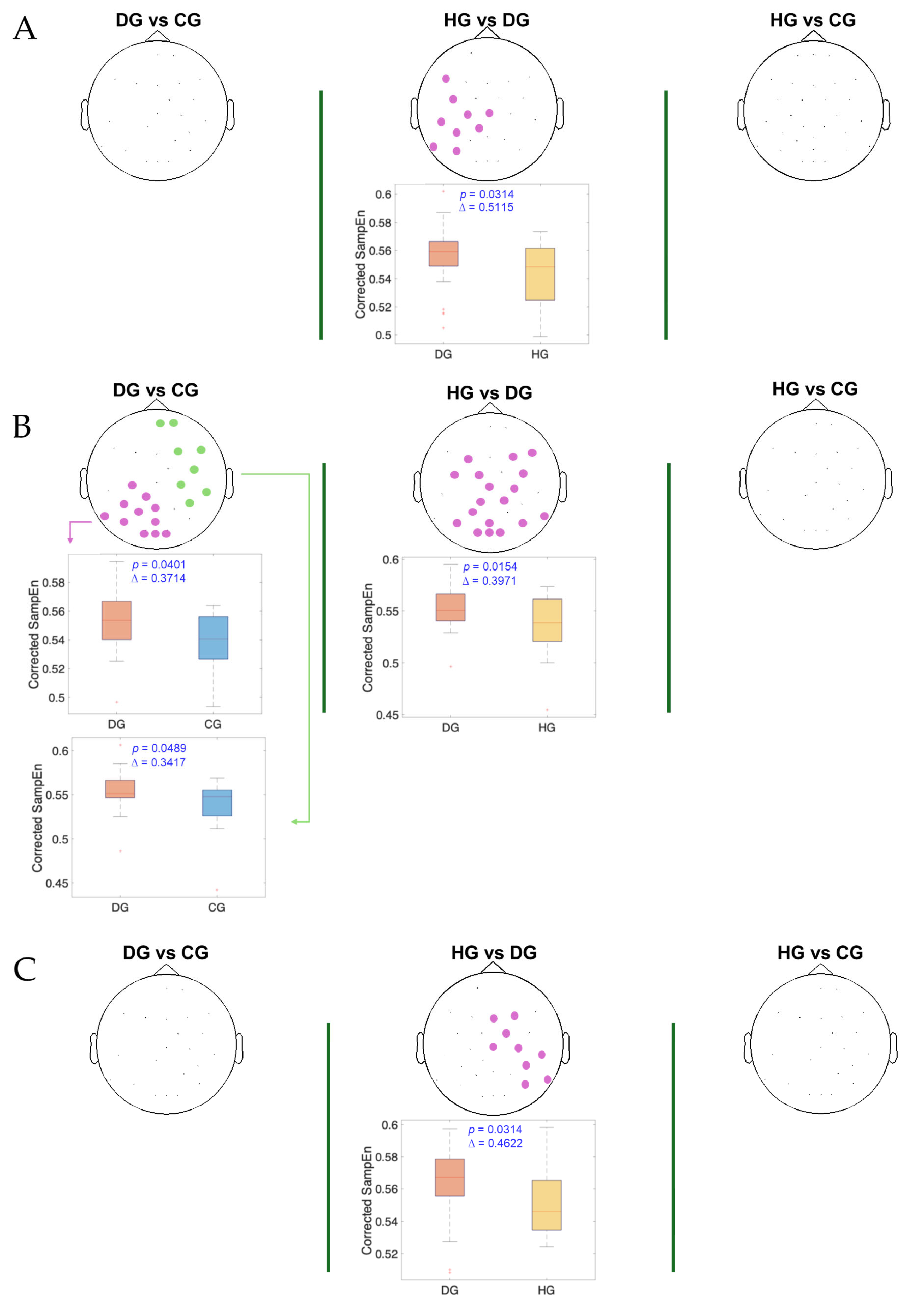
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panara, K.; Ahangar, E.R.; Padalia, D. Physiology, Swallowing. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ujiki, M.B.; Hedberg, H.M. Dysphagia. In Dysphagia: A Clinical Guide; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–217. [Google Scholar] [CrossRef]
- Wang, E.Q. Dysphagia. In Encyclopedia of Movement Disorders, Three-Volume Set; Academic Press: Cambridge, MA, USA, 2010; pp. V1-365–V1-367. [Google Scholar] [CrossRef]
- Clavé, P.; Shaker, R. Dysphagia: Current Reality and Scope of the Problem. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 259–270. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, M.; Ottenstein, L.; Atanelov, L.; Christian, A.B. Dysphagia after Stroke: An Overview. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 187. [Google Scholar] [CrossRef] [PubMed]
- Attrill, S.; White, S.; Murray, J.; Hammond, S.; Doeltgen, S. Impact of Oropharyngeal Dysphagia on Healthcare Cost and Length of Stay in Hospital: A Systematic Review. BMC Health Serv. Res. 2018, 18, 594. [Google Scholar] [CrossRef]
- Clavé, P.; Arreola, V.; Velasco, M.; Quer, M.; Castellví, J.M.; Almirall, J.; Peris, P.G.; Carrau, R. Diagnóstico y Tratamiento de La Disfagia Orofaríngea Funcional. Aspectos de Interés Para El Cirujano Digestivo. Cir. Esp. 2007, 82, 62–76. [Google Scholar] [CrossRef]
- Ertekin, C.; Aydogdu, I.; Yüceyar, N.; Tarlaci, S.; Kiylioglu, N.; Pehlivan, M.; Çelebi, G. Electrodiagnostic Methods for Neurogenic Dysphagia. Electroencephalogr. Clin. Neurophysiol./Electromyogr. Mot. Control. 1998, 109, 331–340. [Google Scholar] [CrossRef]
- Simons, A.; Hamdy, S. The Use of Brain Stimulation in Dysphagia Management. Dysphagia 2017, 32, 209–215. [Google Scholar] [CrossRef]
- Furlong, P.L.; Hobson, A.R.; Aziz, Q.; Barnes, G.R.; Singh, K.D.; Hillebrand, A.; Thompson, D.G.; Hamdy, S. Dissociating the Spatio-Temporal Characteristics of Cortical Neuronal Activity Associated with Human Volitional Swallowing in the Healthy Adult Brain. Neuroimage 2004, 22, 1447–1455. [Google Scholar] [CrossRef]
- Zald, D.H.; Pardo, J.V. Cortical Activation Induced by Intraoral Stimulation with Water in Humans. Chem. Senses 2000, 25, 267–275. [Google Scholar] [CrossRef]
- Vasant, D.H.; Hamdy, S. Cerebral Cortical Control of Deglutition. In Principles of Deglutition: A Multidisciplinary Text for Swallowing and Its Disorders; Springer: Berlin/Heidelberg, Germany, 2013; pp. 55–65. [Google Scholar] [CrossRef]
- Hamdy, S.; Aziz, Q.; Rothwell, J.C.; Singh, K.D.; Barlow, J.; Hughes, D.G.; Tallis, R.C.; Thompson, D.G. The Cortical Topography of Human Swallowing Musculature in Health and Disease. Nat. Med. 1996, 2, 1217–1224. [Google Scholar] [CrossRef]
- Rangarathnam, B.; Kamarunas, E.; McCullough, G.H. Role of Cerebellum in Deglutition and Deglutition Disorders. Cerebellum 2014, 13, 767–776. [Google Scholar] [CrossRef]
- Hamdy, S.; Aziz, Q.; Rothwell, J.C.; Power, M.; Singh, K.D.; Nicholson, D.A.; Tallis, R.C.; Thompson, D.G. Recovery of Swallowing after Dysphagic Stroke Relates to Functional Reorganization in the Intact Motor Cortex. Gastroenterology 1998, 115, 1104–1112. [Google Scholar] [CrossRef]
- Bein, B. Entropy. Best Pr. Res. Clin. Anaesthesiol. 2006, 20, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Nolan, H.; Whelan, R.; Reilly, R.B. FASTER: Fully Automated Statistical Thresholding for EEG Artifact Rejection. J. Neurosci. Methods 2010, 192, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.; Cattaneo, R.; Spadaro, A.; Giannoni, M. Surface Electromyography Pattern of Human Swallowing. BMC Oral Health 2008, 8, 6. [Google Scholar] [CrossRef]
- Roubeau, B.; Morinière, S.; Périé, S.; Martineau, A.; Falières, J.; St Guily, J.L. Use of Reaction Time in the Temporal Analysis of Normal Swallowing. Dysphagia 2008, 23, 102–109. [Google Scholar] [CrossRef]
- Suntrup, S.; Teismann, I.; Wollbrink, A.; Warnecke, T.; Winkels, M.; Pantev, C.; Dziewas, R. Altered Cortical Swallowing Processing in Patients with Functional Dysphagia: A Preliminary Study. PLoS ONE 2014, 9, e89665. [Google Scholar] [CrossRef]
- Omari, T.I.; Ferris, L.; Schar, M.; Cock, C.; Doeltgen, S. Multiple Swallow Behaviour during High Resolution Pharyngeal Manometry: Prevalence and Sub-Typing in Healthy Adults. Speech Lang. Hear. 2022, 25, 1–7. [Google Scholar] [CrossRef]
- Imaz-Higuera, J.; Prats-Boluda, G.; Ye-Lin, Y.; Martinez-de-Juan, J.L.; Gutierrez-Delgado, M.; Prieto-House, J.; Más-Sesé, G.; Belda-Calabuig, A.; Garcia-Casado, J. Post-Stroke Dysphagia: Evaluating Myoelectric Alterations in Swallowing Muscles. Measurement 2025, 249, 117010. [Google Scholar] [CrossRef]
- Imaz-Higuera, J.; Beltran-Sanchez, J.; Garcia-Casado, J.; Ye-Lin, Y.; Martinez-de-Juan, J.L.; Gutierrez-Delgado, M.; Prieto-House, J.; Más-Sesé, G.; Belda-Calabuig, A.; Prats-Boluda, G. Comparison of Supra and Infrahyoid Muscle Activity in Healthy and Dysphagic Elderly Populations. IFMBE Proc. 2024, 110, 221–229. [Google Scholar] [CrossRef]
- Molina Picó, A. Caracterizacion de Medidas de Regulariadad En Señales Biomedicas. Ph.D. Thesis, En la Universitat Politècnica de València, Valencia, Spain, 2014. [Google Scholar]
- Song, Y.; Crowcroft, J.; Zhang, J. Automatic Epileptic Seizure Detection in EEGs Based on Optimized Sample Entropy and Extreme Learning Machine. J. Neurosci. Methods 2012, 210, 132–146. [Google Scholar] [CrossRef]
- Thomas, K.P.; Vinod, A.P. Biometric Identification of Persons Using Sample Entropy Features of EEG during Rest State. In Proceedings of the 2016 IEEE International Conference on Systems, Man, and Cybernetics, SMC 2016—Conference Proceedings, Budapest, Hungary, 9–12 October 2016; pp. 3487–3492. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, L.; Fan, S.Z.; Abbod, M.F.; Shieh, J.S. Sample Entropy Analysis for the Estimating Depth of Anaesthesia through Human EEG Signal at Different Levels of Unconsciousness during Surgeries. PeerJ 2018, 6, e4817. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Y.F.; Fan, S.Z.; Abbod, M.F.; Shieh, J.S. EEG Signals Analysis Using Multiscale Entropy for Depth of Anesthesia Monitoring during Surgery through Artificial Neural Networks. Comput. Math. Methods Med. 2015, 2015, 232381. [Google Scholar] [CrossRef]
- Li, P.; Karmakar, C.; Yearwood, J.; Venkatesh, S.; Palaniswami, M.; Liu, C. Detection of Epileptic Seizure Based on Entropy Analysis of Short-Term EEG. PLoS ONE 2018, 13, e0193691. [Google Scholar] [CrossRef] [PubMed]
- Dastgoshadeh, M.; Rabiei, Z. Detection of Epileptic Seizures through EEG Signals Using Entropy Features and Ensemble Learning. Front. Hum. Neurosci. 2022, 16, 1084061. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Takahashi, K.; Kameda, S.; Yoshida, F.; Maezawa, H.; Oshino, S.; Tani, N.; Khoo, H.M.; Yanagisawa, T.; Yoshimine, T.; et al. Swallowing-related Neural Oscillation: An Intracranial EEG Study. Ann. Clin. Transl. Neurol. 2021, 8, 1224. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.F.; Frank, M.J. Frontal Theta as a Mechanism for Cognitive Control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R. Generalized Additive Models. Stat. Sci. 1986, 1, 297–318. [Google Scholar] [CrossRef]
- Maris, E.; Oostenveld, R. Nonparametric Statistical Testing of EEG- and MEG-Data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
- Kitchenham, B.; Madeyski, L. Recommendations for Analysing and Meta-Analysing Small Sample Size Software Engineering Experiments. Empir. Softw. Eng. 2024, 29, 137. [Google Scholar] [CrossRef]
- Sato, Y.; Schmitt, O.; Ip, Z.; Rabiller, G.; Omodaka, S.; Tominaga, T.; Yazdan-Shahmorad, A.; Liu, J. Pathological Changes of Brain Oscillations Following Ischemic Stroke. J. Cereb. Blood Flow Metab. 2022, 42, 1753. [Google Scholar] [CrossRef] [PubMed]
- Schleiger, E.; Sheikh, N.; Rowland, T.; Wong, A.; Read, S.; Finnigan, S. Frontal EEG Delta/Alpha Ratio and Screening for Post-Stroke Cognitive Deficits: The Power of Four Electrodes. Int. J. Psychophysiol. 2014, 94, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Luu, P.; Tucker, D.M.; Englander, R.; Lockfeld, A.; Lutsep, H.; Oken, B. Localizing Acute Stroke-Related EEG Changes: Assessing the Effects of Spatial Undersampling. J. Clin. Neurophysiol. 2001, 18, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Teismann, I.K.; Steinstraeter, O.; Stoeckigt, K.; Suntrup, S.; Wollbrink, A.; Pantev, C.; Dziewas, R. Functional Oropharyngeal Sensory Disruption Interferes with the Cortical Control of Swallowing. BMC Neurosci. 2007, 8, 62. [Google Scholar] [CrossRef]
- Teismann, I.K.; Steinsträter, O.; Warnecke, T.; Suntrup, S.; Ringelstein, E.B.; Pantev, C.; Dziewas, R. Tactile Thermal Oral Stimulation Increases the Cortical Representation of Swallowing. BMC Neurosci. 2009, 10, 71. [Google Scholar] [CrossRef]
- Yamamura, K.; Kurose, M.; Okamoto, K. Guide to Enhancing Swallowing Initiation: Insights from Findings in Healthy Subjects and Dysphagic Patients. Curr. Phys. Med. Rehabil. Rep. 2018, 6, 178–185. [Google Scholar] [CrossRef]
- Frank, U.; Radtke, J.; Nienstedt, J.C.; Pötter-Nerger, M.; Schönwald, B.; Buhmann, C.; Gerloff, C.; Niessen, A.; Flügel, T.; Koseki, J.C.; et al. Dysphagia Screening in Parkinson’s Disease. A Diagnostic Accuracy Cross-Sectional Study Investigating the Applicability of the Gugging Swallowing Screen (GUSS). Neurogastroenterol. Motil. 2021, 33, e14034. [Google Scholar] [CrossRef]
- Martin, R.E.; MacIntosh, B.J.; Smith, R.C.; Barr, A.M.; Stevens, T.K.; Gati, J.S.; Menon, R.S. Cerebral Areas Processing Swallowing and Tongue Movement Are Overlapping but Distinct: A Functional Magnetic Resonance Imaging Study. J. Neurophysiol. 2004, 92, 2428–2443. [Google Scholar] [CrossRef]
- Hager, B.M.; Yang, A.C.; Gutsell, J.N. Measuring Brain Complexity During Neural Motor Resonance. Front. Neurosci. 2018, 12, 758. [Google Scholar] [CrossRef]
- Dziewas, R.; Sörös, P.; Ishii, R.; Chau, W.; Henningsen, H.; Ringelstein, E.B.; Knecht, S.; Pantev, C. Neuroimaging Evidence for Cortical Involvement in the Preparation and in the Act of Swallowing. Neuroimage 2003, 20, 135–144. [Google Scholar] [CrossRef]
- Chung, J.W.; Ofori, E.; Misra, G.; Hess, C.W.; Vaillancourt, D.E. Beta-Band Activity and Connectivity in Sensorimotor and Parietal Cortex Are Important for Accurate Motor Performance. Neuroimage 2017, 144, 164–173. [Google Scholar] [CrossRef]
- Tzagarakis, C.; Ince, N.F.; Leuthold, A.C.; Pellizzer, G. Beta-Band Activity during Motor Planning Reflects Response Uncertainty. J. Neurosci. 2010, 30, 11270. [Google Scholar] [CrossRef]
- Coleman, S.C.; Seedat, Z.A.; Whittaker, A.C.; Lenartowicz, A.; Mullinger, K.J. Beyond the Beta Rebound: Post-Task Responses in Oscillatory Activity Follow Cessation of Working Memory Processes. Neuroimage 2023, 265, 119801. [Google Scholar] [CrossRef]
- Schmidt, R.; Ruiz, M.H.; Kilavik, B.E.; Lundqvist, M.; Starr, P.A.; Aron, A.R. Beta Oscillations in Working Memory, Executive Control of Movement and Thought, and Sensorimotor Function. J. Neurosci. 2019, 39, 8231. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco-Hernandez, A.; Imaz-Higuera, J.; Martinez-de-Juan, J.L.; Ye-Lin, Y.; Garcia-Casado, J.; Gutierrez-Delgado, M.; Prieto-House, J.; Mas-Sese, G.; Belda-Calabuig, A.; Prats-Boluda, G. Entropy Analysis of Electroencephalography for Post-Stroke Dysphagia Assessment. Entropy 2025, 27, 818. https://doi.org/10.3390/e27080818
Velasco-Hernandez A, Imaz-Higuera J, Martinez-de-Juan JL, Ye-Lin Y, Garcia-Casado J, Gutierrez-Delgado M, Prieto-House J, Mas-Sese G, Belda-Calabuig A, Prats-Boluda G. Entropy Analysis of Electroencephalography for Post-Stroke Dysphagia Assessment. Entropy. 2025; 27(8):818. https://doi.org/10.3390/e27080818
Chicago/Turabian StyleVelasco-Hernandez, Adrian, Javier Imaz-Higuera, Jose Luis Martinez-de-Juan, Yiyao Ye-Lin, Javier Garcia-Casado, Marta Gutierrez-Delgado, Jenny Prieto-House, Gemma Mas-Sese, Araceli Belda-Calabuig, and Gema Prats-Boluda. 2025. "Entropy Analysis of Electroencephalography for Post-Stroke Dysphagia Assessment" Entropy 27, no. 8: 818. https://doi.org/10.3390/e27080818
APA StyleVelasco-Hernandez, A., Imaz-Higuera, J., Martinez-de-Juan, J. L., Ye-Lin, Y., Garcia-Casado, J., Gutierrez-Delgado, M., Prieto-House, J., Mas-Sese, G., Belda-Calabuig, A., & Prats-Boluda, G. (2025). Entropy Analysis of Electroencephalography for Post-Stroke Dysphagia Assessment. Entropy, 27(8), 818. https://doi.org/10.3390/e27080818






