Computing Entropy for Long-Chain Alkanes Using Linear Regression: Application to Hydroisomerization
Abstract
1. Introduction
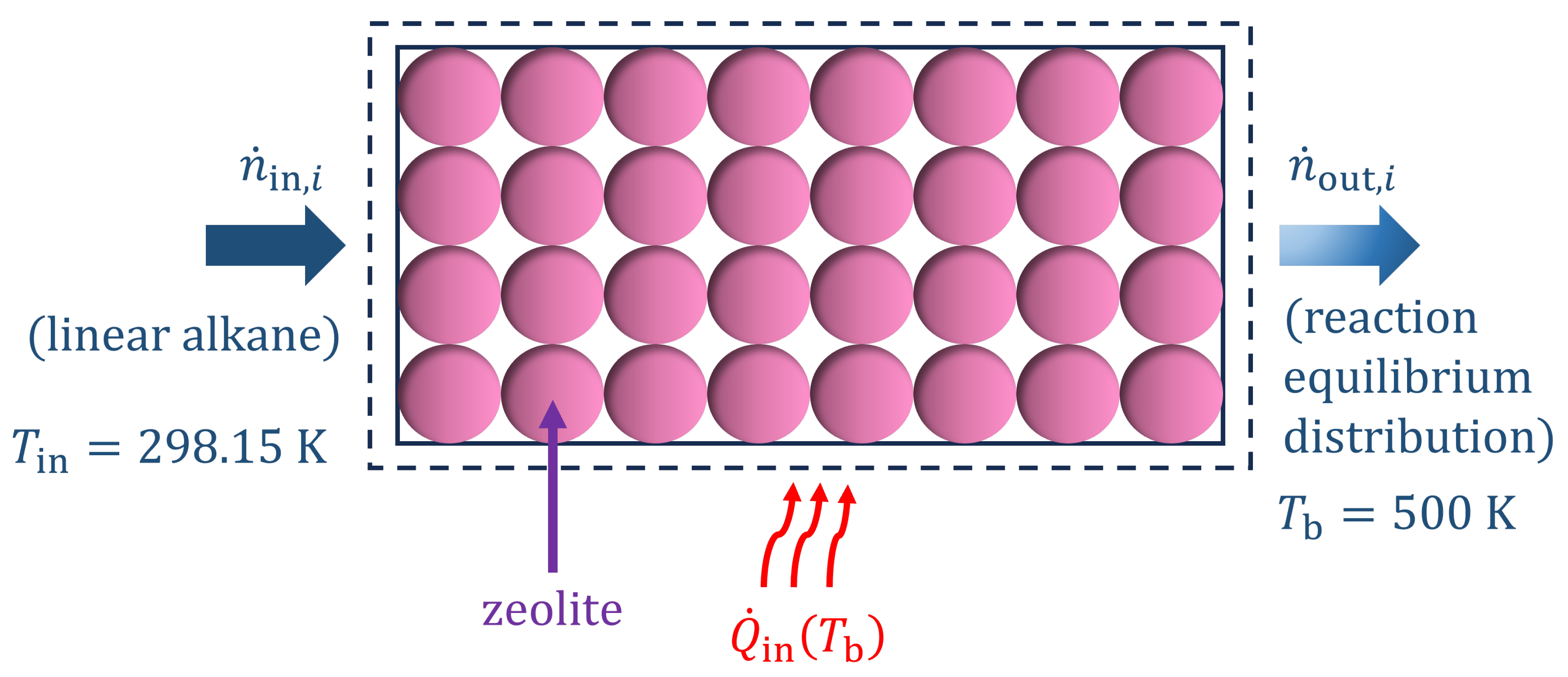
2. Theory
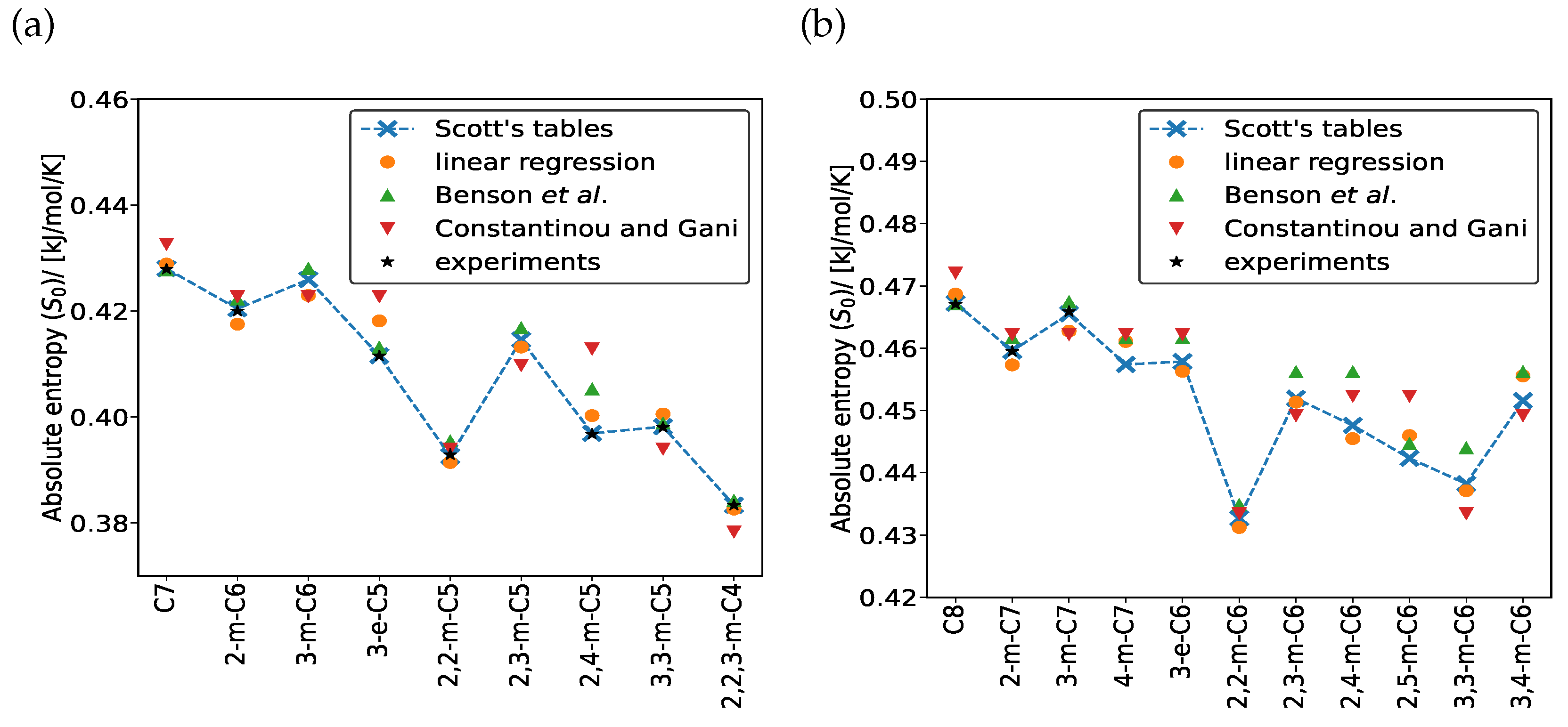
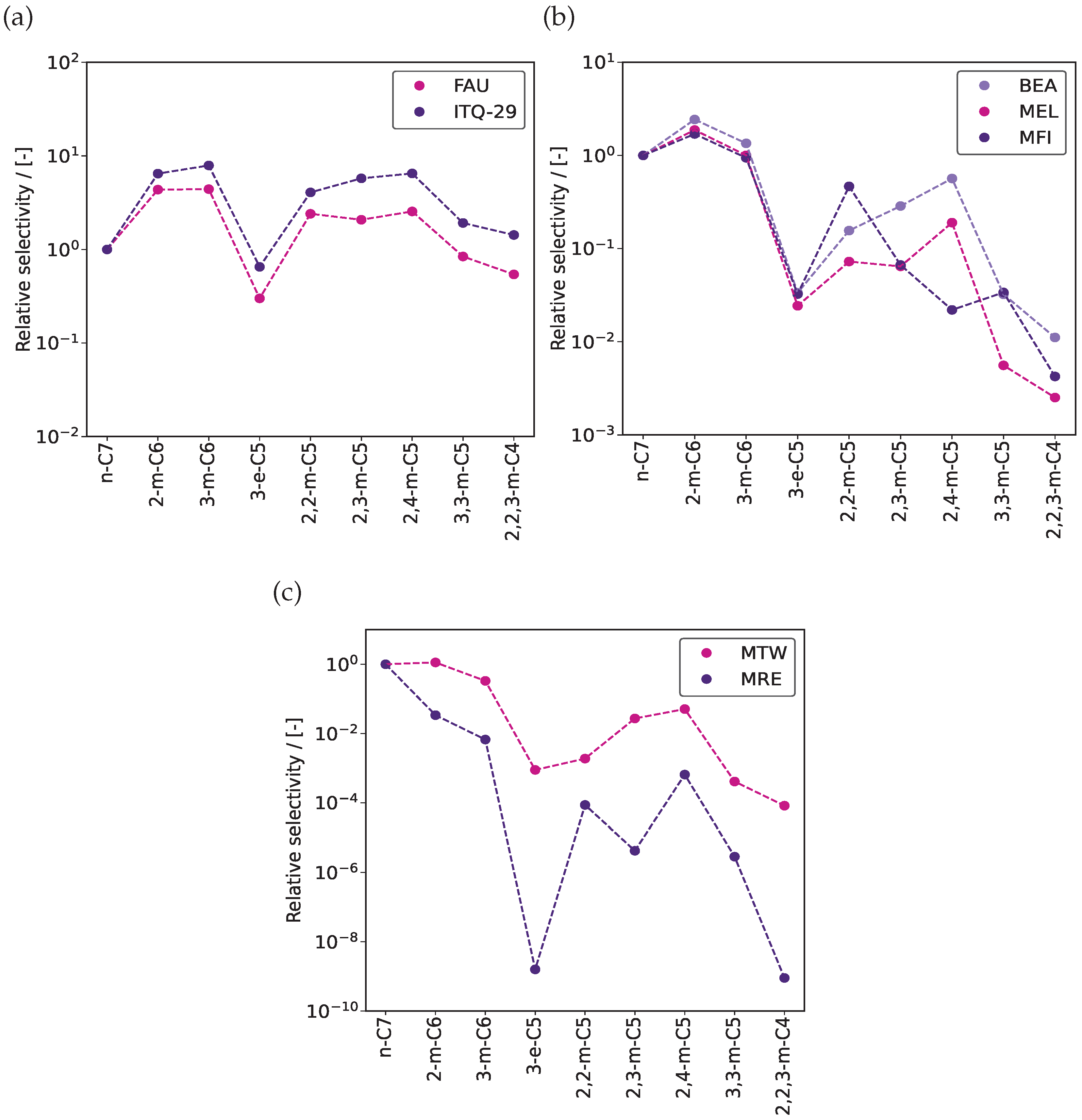
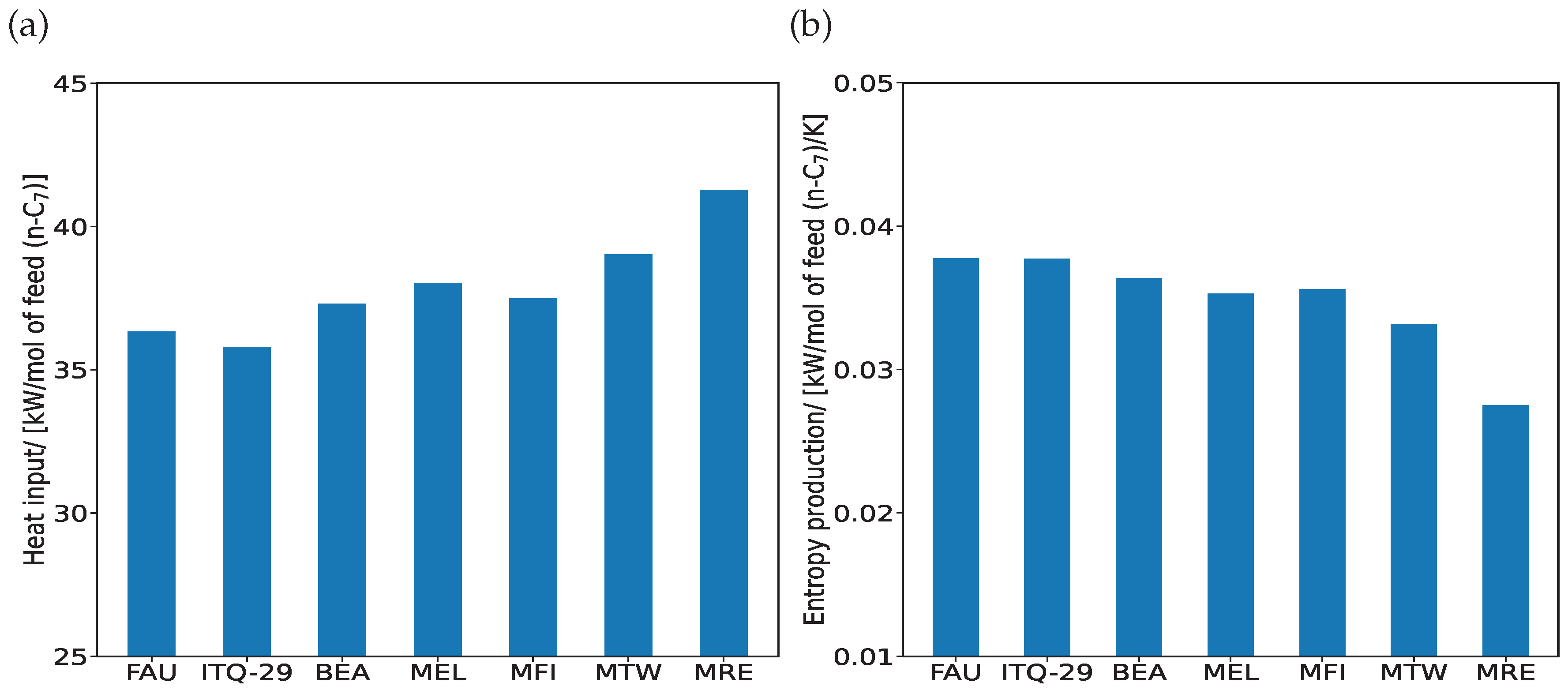
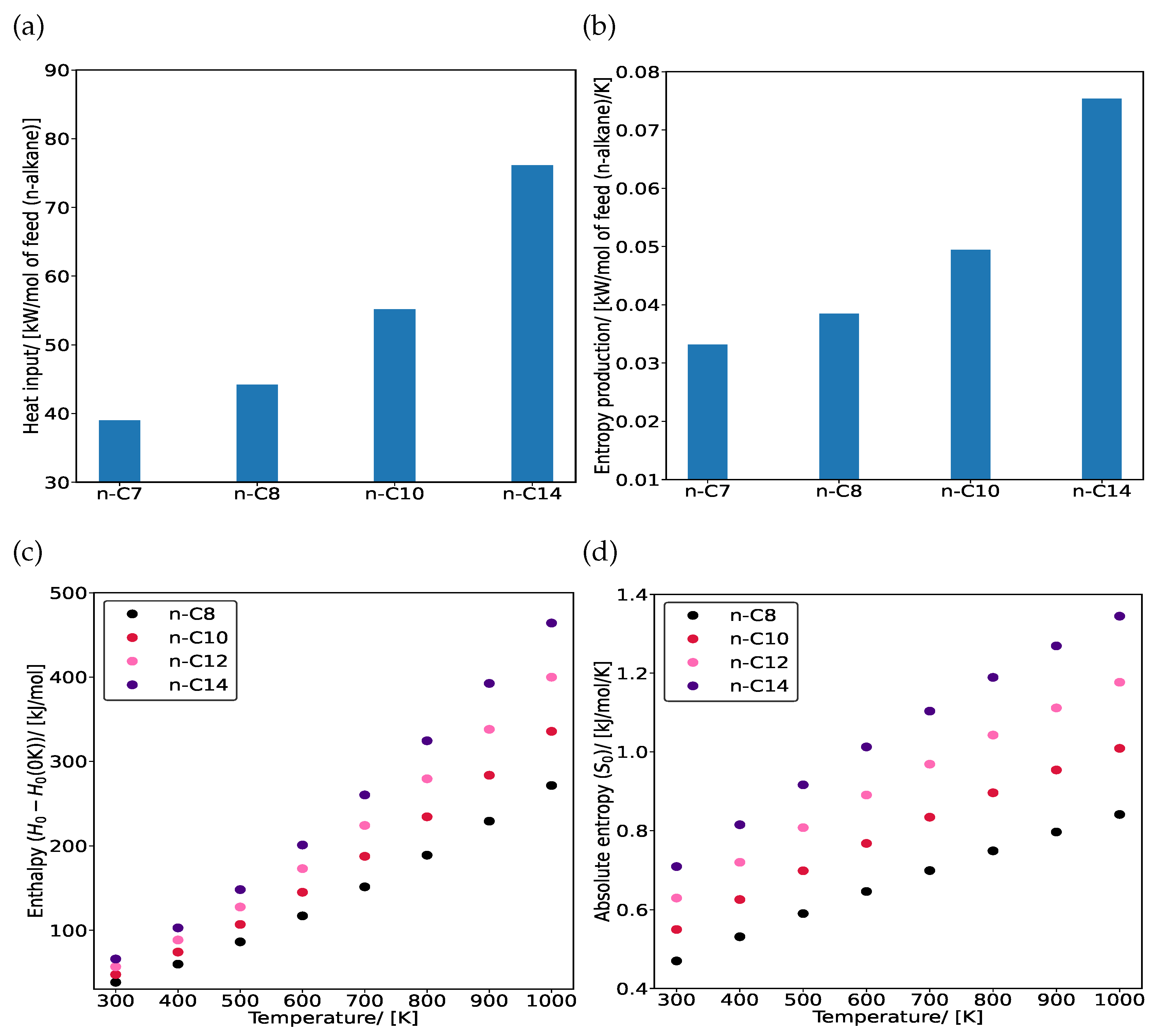
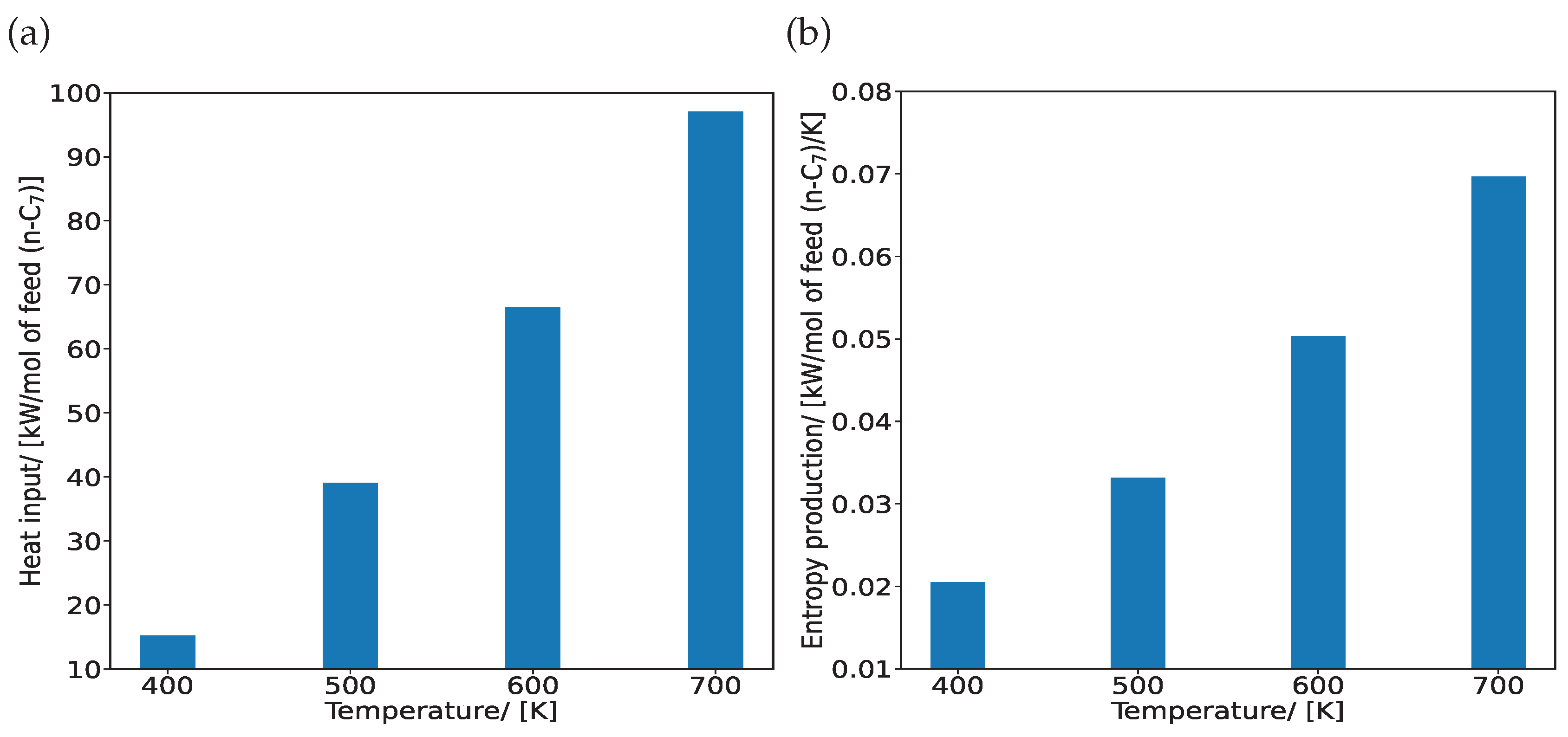
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aldosari, M.N.; Yalamanchi, K.K.; Gao, X.; Sarathy, S.M. Predicting entropy and heat capacity of hydrocarbons using machine learning. Energy AI 2021, 4, 100054. [Google Scholar] [CrossRef]
- Ruelle, P.; Kesselring, U.W. Aqueous solubility prediction of environmentally important chemicals from the mobile order thermodynamics. Chemosphere 1997, 34, 275–298. [Google Scholar] [CrossRef]
- Moran, M.J.; Shapiro, H.N.; Boettner, D.D.; Bailey, M.B. Fundamentals of engineering thermodynamics, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Calis, H.; Lüke, W.; Drescher, I.; Schütze, A. Handbook of Fuels: Energy Sources for Transportation, 2nd ed.; Wiley Online Library: Weinheim, Germany, 2021. [Google Scholar]
- Smit, B.; Maesen, T.L.M. Towards a molecular understanding of shape selectivity. Nature 2008, 451, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Smit, B.; Maesen, T.L. Molecular simulations of zeolites: Adsorption, diffusion, and shape selectivity. Chem. Rev. 2008, 108, 4125–4184. [Google Scholar] [CrossRef]
- Rigutto, M.S.; van Veen, R.; Huve, L. Zeolites in hydrocarbon processing. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; p. 855. [Google Scholar]
- Mäki-Arvela, P.; Kaka khel, T.A.; Azkaar, M.; Engblom, S.; Murzin, D.Y. Catalytic hydroisomerization of long-chain hydrocarbons for the production of fuels. Catalysts 2018, 8, 534. [Google Scholar] [CrossRef]
- Letcher, T.M. Chemical Thermodynamics for Industry, 1st ed.; Royal Society of Chemistry: Letchworth, UK, 2004. [Google Scholar]
- Kanoğlu, M.; Çengel, Y.A.; Dinçer, İ. Efficiency Evaluation of Energy Systems, 1st ed.; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Kjelstrup, S.; Bedeaux, D.; Johannessen, E.; Gross, J. Non-Equilibrium Thermodynamics for Engineers, 2nd ed.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2010. [Google Scholar]
- Scott, D.W. Chemical Thermodynamic Properties of Hydrocarbons and Related Substances: Properties of the Alkane Hydrocarbons, C1 Through C10, in the Ideal Gas State from 0 to 1500 K, 1st ed.; US Department of the Interior, Bureau of Mines: Washington, DC, USA, 1974. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. The NIST Chemistry WebBook: A chemical data resource on the internet. J. Chem. Eng. Data 2001, 46, 1059–1063. [Google Scholar] [CrossRef]
- Benson, S.W.; Cruickshank, F.; Golden, D.; Haugen, G.R.; O’neal, H.; Rodgers, A.; Shaw, R.; Walsh, R. Additivity rules for the estimation of thermochemical properties. Chem. Rev. 1969, 69, 279–324. [Google Scholar] [CrossRef]
- Constantinou, L.; Gani, R. New group contribution method for estimating properties of pure compounds. AIChE J. 1994, 40, 1697–1710. [Google Scholar] [CrossRef]
- Joback, K.G.; Reid, R.C. Estimation of pure-component properties from group-contributions. Chem. Eng. Commun. 1987, 57, 233–243. [Google Scholar] [CrossRef]
- Marrero, J.; Gani, R. Group-contribution based estimation of pure component properties. Fluid Phase Equilib. 2001, 183, 183–208. [Google Scholar] [CrossRef]
- Hukkerikar, A.S.; Meier, R.J.; Sin, G.; Gani, R. A method to estimate the enthalpy of formation of organic compounds with chemical accuracy. Fluid Phase Equilib. 2013, 348, 23–32. [Google Scholar] [CrossRef]
- Albahri, T.A.; Aljasmi, A.F. SGC method for predicting the standard enthalpy of formation of pure compounds from their molecular structures. Thermochim. Acta 2013, 568, 46–60. [Google Scholar] [CrossRef]
- Domalski, E.S.; Hearing, E.D. Estimation of the Thermodynamic Properties of Hydrocarbons at 298.15 K. J. Phys. Chem. Ref. Data 1988, 17, 1637–1678. [Google Scholar] [CrossRef]
- Yaws, C.L. Yaws’ Handbook of Thermodynamic Properties for Hydrocarbons and Chemicals, 1st ed.; Knovel: New York, NY, USA, 2009. [Google Scholar]
- Bloxham, J.C.; Redd, M.E.; Giles, N.F.; Knotts IV, T.A.; Wilding, W.V. Proper use of the DIPPR 801 database for creation of models, methods, and processes. J. Chem. Eng. Data 2020, 66, 3–10. [Google Scholar] [CrossRef]
- Hayes, M.Y.; Li, B.; Rabitz, H. Estimation of molecular properties by high-dimensional model representation. J. Phys. Chem. A 2006, 110, 264–272. [Google Scholar] [CrossRef]
- Yalamanchi, K.K.; Van Oudenhoven, V.C.; Tutino, F.; Monge-Palacios, M.; Alshehri, A.; Gao, X.; Sarathy, S.M. Machine learning to predict standard enthalpy of formation of hydrocarbons. J. Phys. Chem. A 2019, 123, 8305–8313. [Google Scholar] [CrossRef]
- Sharma, S.; Sleijfer, J.J.; op de Beek, J.; van der Zeeuw, S.; Zorzos, D.; Lasala, S.; Rigutto, M.S.; Zuidema, E.; Agarwal, U.; Baur, R.; et al. Prediction of Thermochemical Properties of Long-Chain Alkanes Using Linear Regression: Application to Hydroisomerization. J. Phys. Chem. B 2024, 128, 9619–9629. [Google Scholar] [CrossRef]
- Scott, D.W. Correlation of the chemical thermodynamic properties of alkane hydrocarbons. J. Chem. Phys. 1974, 60, 3144–3165. [Google Scholar] [CrossRef]
- Muckley, E.S.; Saal, J.E.; Meredig, B.; Roper, C.S.; Martin, J.H. Interpretable models for extrapolation in scientific machine learning. Digit. Discov. 2023, 2, 1425–1435. [Google Scholar] [CrossRef]
- Estrada-Villagrana, A.D.; De La Paz-Zavala, C. Application of chemical equilibrium for hydrocarbon isomerization analysis. Fuel 2007, 86, 1325–1330. [Google Scholar] [CrossRef]
- Gunawan, M.L.; Novita, T.H.; Aprialdi, F.; Aulia, D.; Nanda, A.S.F.; Rasrendra, C.B.; Addarojah, Z.; Mujahidin, D.; Kadja, G.T.M. Palm-oil transformation into green and clean biofuels: Recent advances in the zeolite-based catalytic technologies. Bioresour. Technol. Rep. 2023, 23, 101546. [Google Scholar] [CrossRef]
- Poursaeidesfahani, A.; de Lange, M.F.; Khodadadian, F.; Dubbeldam, D.; Rigutto, M.; Nair, N.; Vlugt, T.J.H. Product shape selectivity of MFI-type, MEL-type, and BEA-type zeolites in the catalytic hydroconversion of heptane. J. Catal. 2017, 353, 54–62. [Google Scholar] [CrossRef]
- Agarwal, U.; Rigutto, M.S.; Zuidema, E.; Jansen, A.P.J.; Poursaeidesfahani, A.; Sharma, S.; Dubbeldam, D.; Vlugt, T.J.H. Kinetics of zeolite-catalyzed heptane hydroisomerization and hydrocracking with CBMC-modeled adsorption terms: Zeolite Beta as a large pore base case. J. Catal. 2022, 415, 37–50. [Google Scholar] [CrossRef]
- Sharma, S.; Rigutto, M.S.; Zuidema, E.; Agarwal, U.; Baur, R.; Dubbeldam, D.; Vlugt, T.J.H. Understanding shape selectivity effects of hydroisomerization using a reaction equilibrium model. J. Chem. Phys. 2024, 160, 214708. [Google Scholar] [CrossRef]
- Matito-Martos, I.; Rahbari, A.; Martin-Calvo, A.; Dubbeldam, D.; Vlugt, T.J.H.; Calero, S. Adsorption equilibrium of nitrogen dioxide in porous materials. Phys. Chem. Chem. Phys. 2018, 20, 4189–4199. [Google Scholar] [CrossRef]
- Hansen, N.; Jakobtorweihen, S.; Keil, F.J. Reactive Monte Carlo and grand-canonical Monte Carlo simulations of the propene metathesis reaction system. J. Chem. Phys. 2005, 122, 164705. [Google Scholar] [CrossRef]
- Widom, B. Some topics in the theory of fluids. J. Chem. Phys. 1963, 39, 2808–2812. [Google Scholar] [CrossRef]
- Frenkel, D.; Smit, B. Understanding Molecular Simulation: From Algorithms to Applications, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Dubbeldam, D.; Torres-Knoop, A.; Walton, K.S. On the inner workings of Monte Carlo codes. Mol. Simul. 2013, 39, 1253–1292. [Google Scholar] [CrossRef]
- Dubbeldam, D.; Calero, S.; Ellis, D.E.; Snurr, R.Q. RASPA: Molecular simulation software for adsorption and diffusion in flexible nanoporous materials. Mol. Simul. 2016, 42, 81–101. [Google Scholar] [CrossRef]
- Ran, Y.A.; Sharma, S.; Balestra, S.R.G.; Li, Z.; Calero, S.; Vlugt, T.J.H.; Snurr, R.Q.; Dubbeldam, D. RASPA3: A Monte Carlo code for computing adsorption and diffusion in nanoporous materials and thermodynamics properties of fluids. J. Chem. Phys. 2024, 161, 114106. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Perrot, P. A to Z of Thermodynamics, 1st ed.; Oxford University Press: Guildford, UK, 1998. [Google Scholar]
- Delft High Performance Computing Centre (DHPC). DelftBlue Supercomputer (Phase 2). Available online: https://www.tudelft.nl/dhpc/ark:/44463/DelftBluePhase2 (accessed on 26 July 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Baur, R.; Rigutto, M.; Zuidema, E.; Agarwal, U.; Calero, S.; Dubbeldam, D.; Vlugt, T.J.H. Computing Entropy for Long-Chain Alkanes Using Linear Regression: Application to Hydroisomerization. Entropy 2024, 26, 1120. https://doi.org/10.3390/e26121120
Sharma S, Baur R, Rigutto M, Zuidema E, Agarwal U, Calero S, Dubbeldam D, Vlugt TJH. Computing Entropy for Long-Chain Alkanes Using Linear Regression: Application to Hydroisomerization. Entropy. 2024; 26(12):1120. https://doi.org/10.3390/e26121120
Chicago/Turabian StyleSharma, Shrinjay, Richard Baur, Marcello Rigutto, Erik Zuidema, Umang Agarwal, Sofia Calero, David Dubbeldam, and Thijs J. H. Vlugt. 2024. "Computing Entropy for Long-Chain Alkanes Using Linear Regression: Application to Hydroisomerization" Entropy 26, no. 12: 1120. https://doi.org/10.3390/e26121120
APA StyleSharma, S., Baur, R., Rigutto, M., Zuidema, E., Agarwal, U., Calero, S., Dubbeldam, D., & Vlugt, T. J. H. (2024). Computing Entropy for Long-Chain Alkanes Using Linear Regression: Application to Hydroisomerization. Entropy, 26(12), 1120. https://doi.org/10.3390/e26121120





