1. Introduction
Any computation performed on a physical system is subject to fundamental limitations imposed by the laws of physics. For example, the uncertainty principle implies that to perform an elementary logical operation faster than some
, at least an average amount of energy
must be consumed [
1]. This bound can be understood intuitively as a consequence of the fact that there is a fundamental limit on the maximum number of different states that a physical system can traverse per unit of time, as first demonstrated by Margolus and Levitin [
2]. Another important bound on computation, which is the main focus of this work, is set by the laws of thermodynamics. According to Landauer’s principle [
3], at least
ln 2 of heat dissipation must accompany any one-bit erasing process. Here,
is Boltzmann’s constant and
T is the ambient temperature. Equality can be achieved for quasi-static (reversible) erasure protocols. The heat released into the environment during the erasure of information assures that the total increase in the entropy of the system and bath together is a non-negative quantity. Importantly, this bound applies to any non-reversible erasing process of a memory, regardless of the physical system that was used to implement it. Therefore, Landauer’s principle demonstrates the interplay between physics and information. In recent decades, Landauer’s principle was generalized to include: a probabilistic erasure process [
4,
5]; other types of thermodynamic resources [
6]; entropically unbalanced bits [
7]; a unified view on the cost of erasing and measuring a bit [
8,
9];
N state bit [
10]; optimal erasure at finite time [
11,
12]; and others [
13].
The existence of a fundamental bound does not imply that the bound can be attained. Indeed, current computer memory devices dissipate about 6 orders of magnitude more energy than the minimum amount required by the bound. Similarly, estimations of the energy dissipated in biological computations such as DNA and gene replications show that these are performed with about an order of magnitude more dissipation than required by Landauer’s bound [
14]. Recently, however, it was demonstrated that carefully built artificial systems can actually operate very close to Landauer’s bound. This was achieved with several types of systems: a single colloidal particle in an optical [
15,
16] or feedback [
17,
18,
19] traps, nanomagnetic bits [
20,
21,
22], superconducting flux bit [
23] and even quantum systems [
24,
25]. Based on these results, it is natural to ask whether there are any biological memory-erasing processes that operate close to Landauer’s limit.
Out of many biological systems, the bacterial mechanosensitive ion channels of small and large conductance, MscS and MscL, appear to be the most tractable systems controlled by tension in the surrounding membrane [
26,
27,
28]. MscL is essentially a two-state (closed↔open) whereas MscS shows inactivating behavior (inactivated↔closed↔open), but under certain tension protocols it can be treated as a two-state channel [
29]. They function as osmolyte release valves when bacteria face changing environmental osmotic conditions, such as with drastic dilution in the rain. While the large-conductance MscL channel opens by extreme near-lytic tensions and acts as an emergency valve, the small-conductance MscS channel opens at moderate tensions and appears to be active throughout the normal bacterial lifecycle [
27,
30,
31,
32].
In this work, we present a framework for the analysis of heat dissipation in membrane channels gated by tension. We employ the patch-clamp technique applied to the native E. coli membrane to record discrete single-molecule opening and closing events in MscS under specially designed tension stimuli and extract the dissipated heat that accompanies gating transitions. The state of the ion channel, which can be either “open” or “closed”, encodes a single bit of information. Setting the experimental conditions such that the channel occupies these two states with equal probability introduces the maximum degree of randomness. Changing the biasing tension that re-distributes the channel population to one particular state is equivalent to “erasing the memory” stored in the initially randomized population. We extract the heat dissipated during the “restore to open” process imposed with different rates and show that this system dissipates substantially at high transition rates, but under slower driving protocols, MscS gating closely approaches its Landauer limit. We discuss the physiological importance of this physical trait, which predicts the activation of MscS with minimal dissipation under moderate osmotic shocks experienced by bacteria.
2. Experimental and Theoretical Setup
To measure the dissipated heat during the erasure of a single bit, Landauer suggested the use of a “restore to one” protocol [
3], which results in the bit occupying a single state—the “one” state—regardless of the initial state of the bit. He then argued that the heat dissipated in applying this protocol, averaged over the two initial states of the bit, must be at least
ln 2.
To record gating (closed↔open) transitions in MscS channels, a standard patch-clamp technique was applied to giant
E. coli spheroplasts [
33,
34,
35]. Approaching the surface of a spheroplast with a polished glass pipette with a tip diameter of ∼1.5
m and applying gentle suction forms a contact between the glass and membrane with a Giga-Ohm resistance (Giga-seal). This tight seal isolates the patch membrane under the pipette both electrically and mechanically. Excision of the patch from the spheroplast provides electrical access to both sides of the membrane, which now separates the “pipette” and “bath” aqueous solutions (
Figure 1 left). Under constant voltage of 30 mV across the patch and applying stronger suction (−60–150 mm Hg), which stretches the membrane, we can see the activation of mechanosensitive channels observed as the increase in the patch (DC) current. Tension in the membrane (
), the main activating stimulus, is related to the applied pressure (
p) through the radius of curvature of the patch (
r) according to the law of Laplace
(see the Materials and Methods for the details of tension calibration). Pressure ramps applied to multi-channel patches activate multiple (∼100) channels, and these “population currents” directly reflect the mean open probability (
) in the population when normalized to the current level at saturating pressures. The analysis of channel population responses to ramps allows us to determine the threshold, the level of saturation and the midpoint (
or
), which is the condition of equipartitioning between the closed and open states.
With a higher amplification, these molecular activation events can be monitored with pico-ampere precision, which allows us to track the distribution of channels between the open and closed states at a single-channel resolution [
36]. To achieve this resolution and discern transitions in individual channels, we switched to a special vector in which
mscS expression was controlled by a tight promotor. This allowed us to reduce the channel population to 10-20 channels per patch and observe individual molecular activation events (see, for example,
Figure 2B).
In this setting, we implemented the “restore to one” protocol on MscS ion channels, where the one-bit information is stored in the “open” and “closed” states of a single MscS ion channel (
Figure 2A). In a typical experiment, after seal formation and patch excision, a linear ramp of negative pressure (suction) from zero to the saturating level is applied to the patch with simultaneous current recording. This step determines the activation pressure midpoint (
) at which the population is equally distributed between the closed and open conformations, i.e., the state of highest uncertainty. In the following “bit erasure” protocol, the pressure is quickly ramped to
, the population is allowed to equilibrate for 3 s and then the pressure is ramped with different rates to a higher level, where all channels uniformly assume the open conformation (state of highest certainty). The recorded traces with easily discernable single-channel steps are analyzed with the “edge detection” protocol (An Edge Detector program (
http://cismm.web.unc.edu/resources/tutorials/edge-detector-1d-tutorial/, accessed on 19 May 2020, see Figure 4) was employed to detect the single channel events.) as described below.
At room temperature, the minimal dissipated heat set by Landauer’s bound,
ln 2, is extremely low—about
joules. This makes any direct measurement of the heat absorbed by the environment, e.g., by measuring its thermal expansion or temperature raising, highly challenging. Fortunately, the recent theory of stochastic thermodynamics [
37] suggests a way to measure the dissipated heat by watching the behavior of the thermal system itself, rather than measuring the environment. This method was used in measuring the saturation of Landauer’s bound in artificial systems [
15,
16,
17,
18,
19,
20,
21]. The usage of stochastic thermodynamics was already successfully demonstrated on MscS ion channels in a different context [
38].
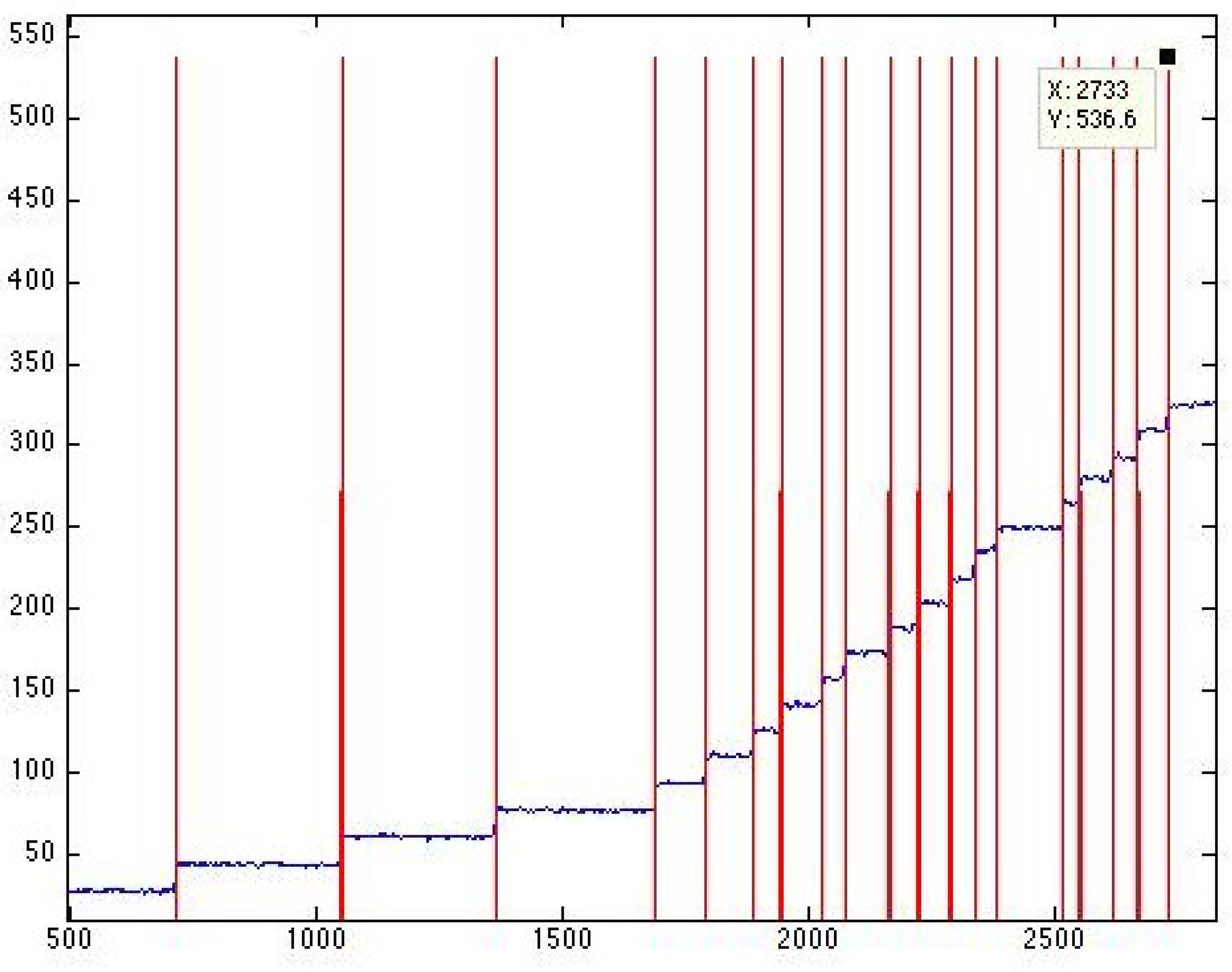
Figure 2.
(
A): Restore to open protocol. When unperturbed, the channels naturally occupy the low energy configuration, which is the closed state. In the first part of the protocol, the tension was quickly (
s) increased to the midpoint tension
nm
) [
39] at which the probability of finding a channel in the open or closed state was
. The tension was kept fixed at
for 3 s to let the channels thermalize at this specific tension value. In the final setup, the tension was increased from
to
(
nm
) in 0.25, 1, 5 and 10 s. Regardless of the initial state of the channels, at the end of the final step, all channels were forced to be in the open state. (
B): An experimental trace obtained from the restore to open protocol. In the final step, the tension was increased from
to
in 1 s. The inset shows the single-channel gating events at a higher magnification during the restore to one operation.
Figure 2.
(
A): Restore to open protocol. When unperturbed, the channels naturally occupy the low energy configuration, which is the closed state. In the first part of the protocol, the tension was quickly (
s) increased to the midpoint tension
nm
) [
39] at which the probability of finding a channel in the open or closed state was
. The tension was kept fixed at
for 3 s to let the channels thermalize at this specific tension value. In the final setup, the tension was increased from
to
(
nm
) in 0.25, 1, 5 and 10 s. Regardless of the initial state of the channels, at the end of the final step, all channels were forced to be in the open state. (
B): An experimental trace obtained from the restore to open protocol. In the final step, the tension was increased from
to
in 1 s. The inset shows the single-channel gating events at a higher magnification during the restore to one operation.
To discuss heat dissipation in the MscS ion channel, we model it as a two-state system (“open” and “closed”), and introduce a state variable,
for a closed channel and
for an open one. For a system with
N such channels, we denote with
the average over the states of the different channels. In contrast to
, which can take only 0 and 1 as its value,
can take any value between 0 and 1 with an accuracy
. Let
and
denote the energies of the closed and open states of the ion channel itself. The total energy of the ion channels and the membrane is a function of the tension
and the state variable
, and is given by [
38,
40,
41]:
The additional term
represents the decrease in the energy of the membrane that occurs when the channels open in response to the applied tension,
. Here,
is the area difference between the closed and open state of the channel. Thus, in the presence of external tension on the membrane, states with larger areas become favorable [
28,
29]. The energy and area difference between the closed and open states of a single MscS channel were already measured in previous publications [
29,
38], and are given by:
and
nm
(for more details, see
Section 5.2). In what follows, we used the standard assumption that
and
are the same for all MscS channels.
Based on the above energy in the system, the total change in energy can be expressed as follows [
42]:
The first term on the square brackets of Equation (
2) is the change in energy resulting from some external force changing the tension, which is therefore associated with work. The second term is the variation of the energy resulting from the change in the internal configuration of the system, namely due to redistribution between states. To conserve the total energy, this energetic change requires an exchange of energy with the surrounding thermal bath, and is therefore associated with heat. With these interpretations, the total heat and work associated with a realization of the experimental protocol can be written as:
where
is the protocol’s duration. In our experiments, the tension
is changed linearly with time; therefore, we can write the work integral in the following form:
which can be interpreted as
times minus the area under the
graph. The above definitions of heat and work imply the
limit, to make sense of
. The tools of stochastic thermodynamics enable us to extend these definitions to small systems with even a single channel, where
can take only discrete values of 0 or 1, and changes abruptly between them. In this case, the work in Equation (
5) can be directly used. To calculate the heat, however, we note that in this case
, shown in
Figure 1, can be approximated with the Heaviside step function:
Exploiting the relations between the Heaviside step function and the Dirac delta function, we can write the heat integral in a particular realization:
Alternatively, the heat can be expressed as the difference between the total change in energy and the work.
where we expressed
as a sum of delta functions located at the transition tensions
in this specific realization, and
and
represent the changes corresponding to the specific transition, which can be both the opening or closing of a channel. As the channels are independent, this definition also gives a heat value for each gating event. Stochastic thermodynamics assures [
37] that the average of the heat calculated by Equation (
7) over many realizations converges to the correct ensemble average of the heat dissipation. Note that Equation (
7) with a minus sign corresponds to the heat released by the system into the environment: the difference between the intrinsic transition energy in the channel molecule (which is constant) and the work that is performed on the molecule by external tension during the transition (which is proportional to applied tension) gives us the dissipated heat. By construction, the above definitions of heat and work recover both the first and second laws of thermodynamics [
38]. With the above interpretation, heat and work can be associated with every realization of the protocol. It is important to note, however, that they do not have the same value at every single realization, and they may fluctuate from one realization to the next. Therefore, averaging over many trajectories is required to obtain a reliable estimate for the dissipated heat.
3. Results
In a typical experimental setup, the MscS channels naturally reside in the closed state when no pressure is applied to the system. Therefore, in the first stage of the experiment, we increased the membrane tension by applying suction pressure on the micro-pipette, to the midpoint tension value
at which probabilities of finding the channel in the closed or open states are equal,
(this ramping is performed during
s). We then let the system thermalize at this tension value (
) by keeping the pressure fixed for 3 s. The system’s entropy at this stage can be calculated as:
(see
Figure 2A).
In the second stage of the experiment, we increased the membrane tension to nm at which . This was performed at various ramping rates. This protocol mimics Landauer’s “restore to one” operation, which deletes a single bit of information. To see why, note that the channels are restored to the open state from an initial configuration where the closed and open states are equally likely to be occupied. Since the channels are forced to the open configuration regardless of their initial status, this protocol is equivalent to the “restore to open”. The entropy of the system after this stage is given by: . Therefore, the change in the entropy of the system is . This operation corresponds to deleting a single bit of information. Formally, the system has to get back to the same tension value. However, this does not make any difference since we can always release the tension instantaneously without changing the work or heat. To compensate for the system’s entropy decrease, the heat released into the environment must be at least , otherwise, the total entropy of the system and the bath decreases, leading to a violation of the second law of thermodynamic.
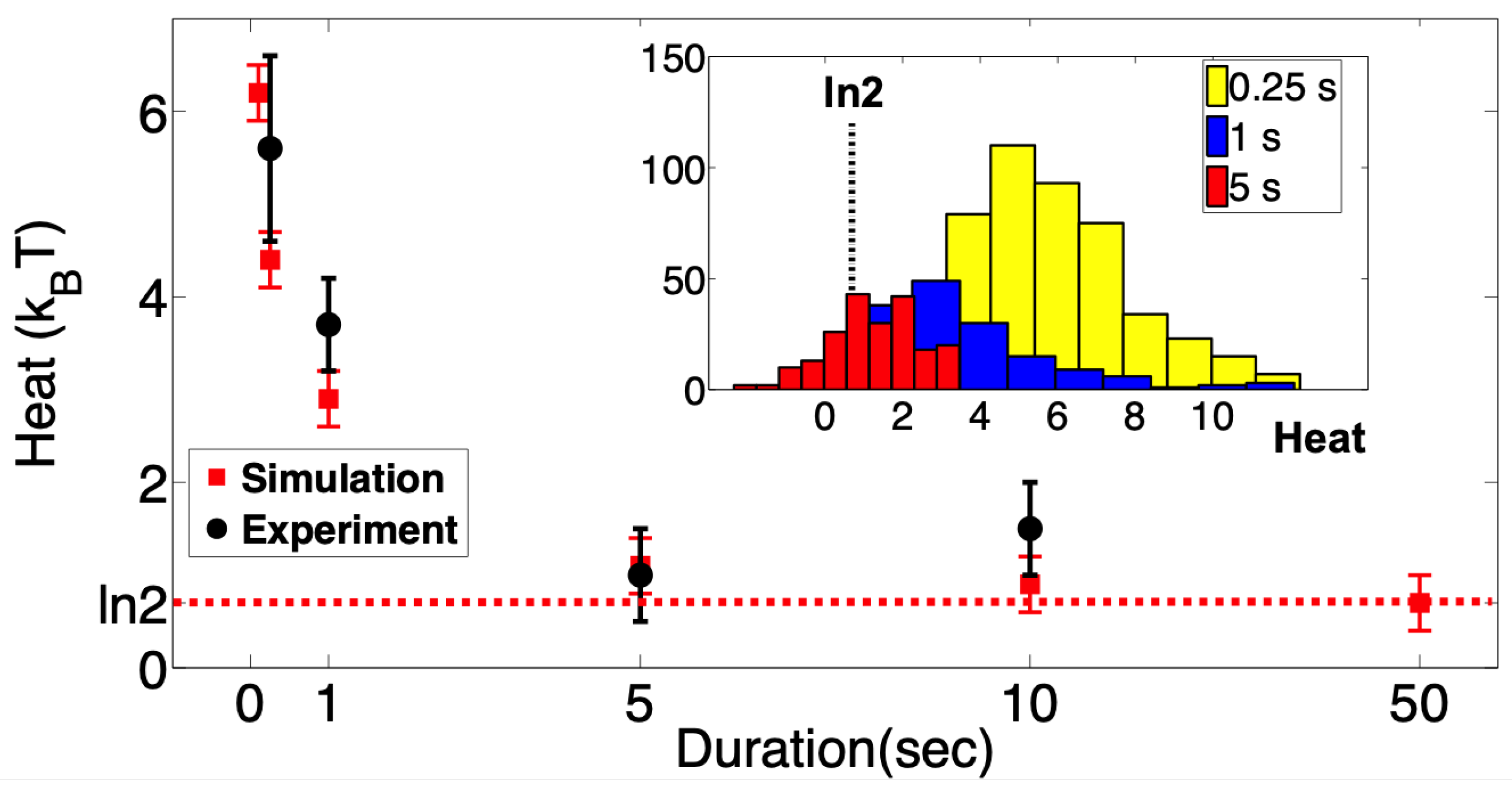
We repeated the above experiments many times and gathered ∼200 single-channel events for each erasure protocol. In each realization, we monitored the heat released into the environment using Equation (
7) and the known values of
and
. These were plotted as a function of the rate at which the tension was changed from
to
in
Figure 3. As expected, the averaged dissipated heat decreases with the protocol duration. At the slowest experimental erasure protocol achievable (see
Section 4), we reach very close to the Landauer limit of
, much closer than any other biological system reported so far.
To further verify our results, we simulated a Markovian model of MscS gating using our experimental protocol (restore to open) as the input driving force in the simulation using QUBexpress software The software is available at
https://qub.mandelics.com (accessed on 19 May 2020). The parameters used in the simulation and details of the two-state Markov model of MscS are given in
Section 5. Since in the simulations the erasure protocols can be made arbitrarily slow, we obtained the heat distribution as a function of longer erasure protocols (
Figure 2, red data points). The simulation results are in good agreement with the experimental measurements at short protocols, and for longer protocols, they in fact attain the
bound.
4. Discussion
Living systems are inherently dissipative, especially as they execute multiple steps of chemical energy conversion, pump metabolites, produce mechanical work or maintain constant temperature. The question that the researchers studying
structural information content and cellular computation try to address is not about the total energy balance and dissipation, but rather about the energy consumption by the “cellular switchboard” itself that turns the cellular processes on and off, replicating information and thus making decisions. Previous analyses based on the generalized Landauer bound [
14] have suggested that protein synthesis, which is an RNA-guided non-random polymerization of amino acids, takes about an order of magnitude more energy than the amount of information stored in the sequence requires. Synthesis of DNA on a DNA template, according to estimations [
14], consumes about two orders of magnitude more energy than the Landauer bound predicts. The problem with these systems is that the energy provided by the splitting of deoxyribonucleotide triphosphates (dNTPs) is strictly coupled with each polymer extension step, which makes this chemical energy component inseparable from the purely entropic change of information content.
In this work, we studied the mechanosensitive ion channel of small conductance (MscS) from
E. coli acting as a tension-operated membrane valve requiring no chemical energy input. MscS evolved to release excess osmolyte from cells in response to osmotic water influx that causes the cell envelope to swell and stretch. Opening the entire MS channel population during strong shock massively dissipates internal ions and osmolytes that can amount to up to 15% of cellular dry weight [
27,
43]. This undoubtedly inflicts substantial energy and metabolite loss on the cell that is trying to evade lysis at any cost. However, as our results show, the operation of MscS itself in a slow (nearly equilibrium) regime costs that minimum, exactly as the Landauer limit predicts.
Our experimental conditions allowed us to treat MscS as a two-state memory device. By applying tension to the patch membrane, we forced the population of channels to change its state occupancy, from which we measured the thermodynamic cost of deleting a single bit of information. The heat dissipated during the bit-erasing transition to the singular open state was measured as the average difference between the intrinsic transition energy in the channel molecule and the work that is performed on the molecule by external tension. The dissipated heat measured with a short “restore to open” time (0.25 s) exceeded 5 , whereas at slow ramps it approached , corresponding to the Landauer bound.
The practical requirements we had to satisfy in our experiments were as follows. (i) Because MscS channels tend to inactivate when exposed to moderate tension (
) for a prolonged period of time, the time for the state restoration protocol cannot be arbitrarily slow. In order to stay in the two-state regime, we used a short (0.5 s) pressure ramp to
, a 3-s equilibration, and a variable duration “erasure ramp” that was limited to 10 s. The non-inactivating mechanosensitive ion channel of large conductance MscL would also be a good system for dissipation analysis, but it gates at near-lytic tensions where membrane patches become unstable [
27]. For this reason, MscL was not used. (ii) The MscS expression level had to be carefully adjusted through the use of a tight-promoter expression system such that the number of channels per patch (10–20) was suitable for the edge detection analysis of individual transitions. With all these precautions, a small degree of adaptation and inactivation were still observed (expected to be around 10% for a 3 s holding time at
), which gave rise to a non-monotonic current response shown in
Figure 2.
It is important to note that for finite-time erasure processes, the Landauer bound takes the form
, where
is the erasing time and
C is a system-dependent constant [
15,
17,
44,
45]. However, depending on the intrinsic relaxation time scale of the experimental setup, it is possible to obtain effective quasi-static erasure processes, which may explain why the energetic cost of erasing the bit of information encoded in the ion channel is as low as the theoretic bound. We think that from a biological point of view, this trait seems natural. Under hyperosmotic conditions, bacteria accumulate ions and organic osmolytes to maintain a positive turgor pressure inside the cytoplasm. Moreover, bacteria maintain relatively high voltage across the cytoplasmic membrane (150–200 mV) as a part of electrochemical potential driving ATP synthesis [
46,
47]. The thermodynamic and kinetic stability of the closed state are therefore critical because thermally-driven random opening events would produce deleterious leakage and uncoupling of bacterial energetics. Thus, evolution has perfected the energy gap (∼22
) between the end states and the height of the separating barrier such that thermal energy does not produce spurious openings at rest during the lifespan of bacteria. However, in the event of a sudden osmotic down-shock such as during a rainstorm, cellular osmolytes are quickly released through mechanosensitive ion channels in order to reduce the turgor pressure. The low-threshold MscS and the high-threshold MscL channels are responsible for the bulk of osmolyte exchange in
E. coli, but each channel is specialized in handling different magnitudes of osmotic shocks. The 3−nS MscL is an emergency valve that opens abruptly at near-lytic tensions (∼3.5
nm
) and jettisons the osmolytes non-selectively. The 1−nS MscS, on the other hand, operates at moderate tensions (∼2
/nm
), and effectively counteracts small osmotic shocks. These channels evolved to defend bacteria under different osmotic conditions, e.g., emergency vs non-emergency situations, and they perform more efficiently under certain timescales [
29].
Stopped-flow experiments revealed that the characteristic time scales of bacterial swelling in response to an abrupt dilution vary from seconds at low shocks (100–300 mOsm downshifts) to 100 milliseconds at stronger (600–1000 mOsm) shocks [
27]. Such strong osmotic down-shock experiments yield the typical timescales at which an emergency valve operates in nature. MscS populations residing in the cytoplasmic membrane of a bacterium are usually able to meet the kinetic requirement, i.e., opening and helping to reduce the internal turgor pressure by quickly releasing the excessive osmotic gradient before water influx rips open the cell. However, MscS, not being a true emergency valve, is somewhat inefficient when it is forced to open under timescales of 30–50 ms that correspond to super-threshold tensions in the cytoplasmic membrane generated by fast dilution in vivo. This dissipation at higher tensions (and rates) is a “tax” imposed by a relatively high transition barrier providing a “safety curb” that precludes spurious openings at low tensions. However, under moderate osmotic shock conditions, when tension buildup in the cytoplasmic membrane occurs within a time span of a few seconds, MscS performs a smooth action in a non-dissipative manner, which seems to be consistent with the in vivo role of MscS in the overall osmotic fitness of
E. coli.