Estimation of Entropy Generation in a SCR-DeNOx System with AdBlue Spray Dynamic Using Large Eddy Simulation
Abstract
1. Introduction
2. Numerical Methodology
2.1. Lagrangian Droplet Tracking
2.2. Thermal Decomposition
2.3. Large-Eddy Simulation
2.4. Turbulence-Chemistry Interaction: Eulerian Stochastic Field (ESF)
2.5. Estimation of Entropy Production Rates
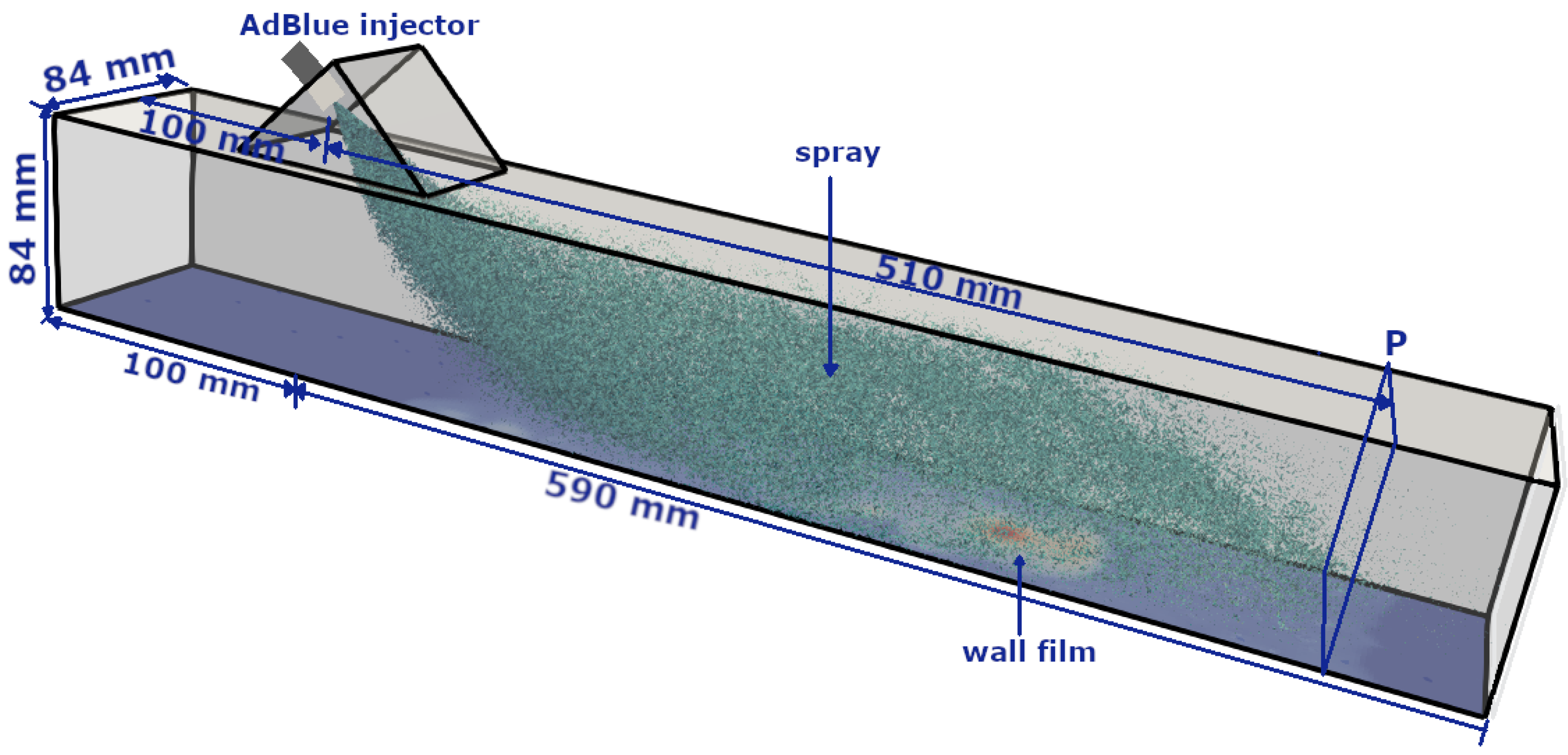
3. Numerical Configuration: A Generic SCR System
4. Results and Discussion
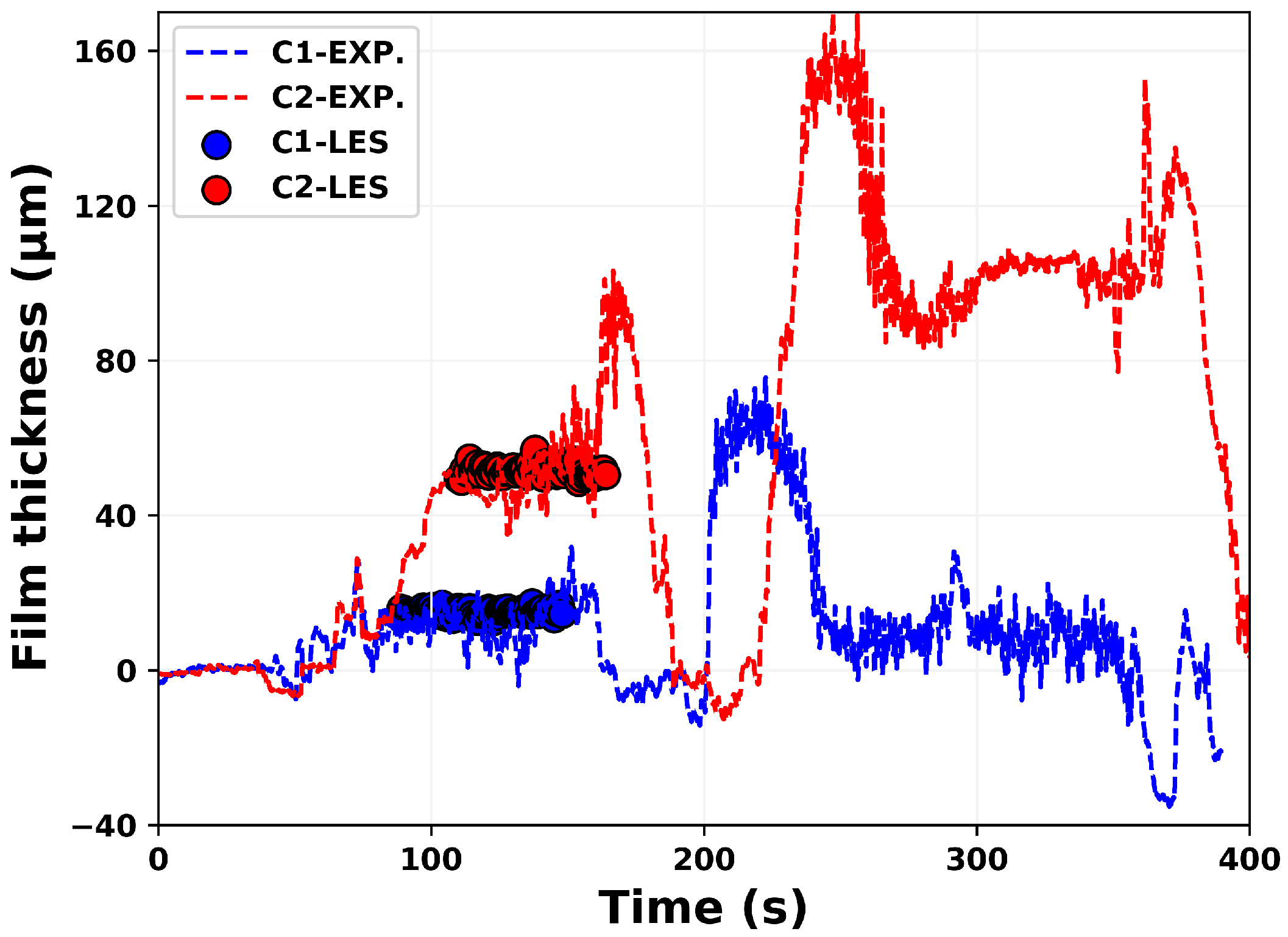
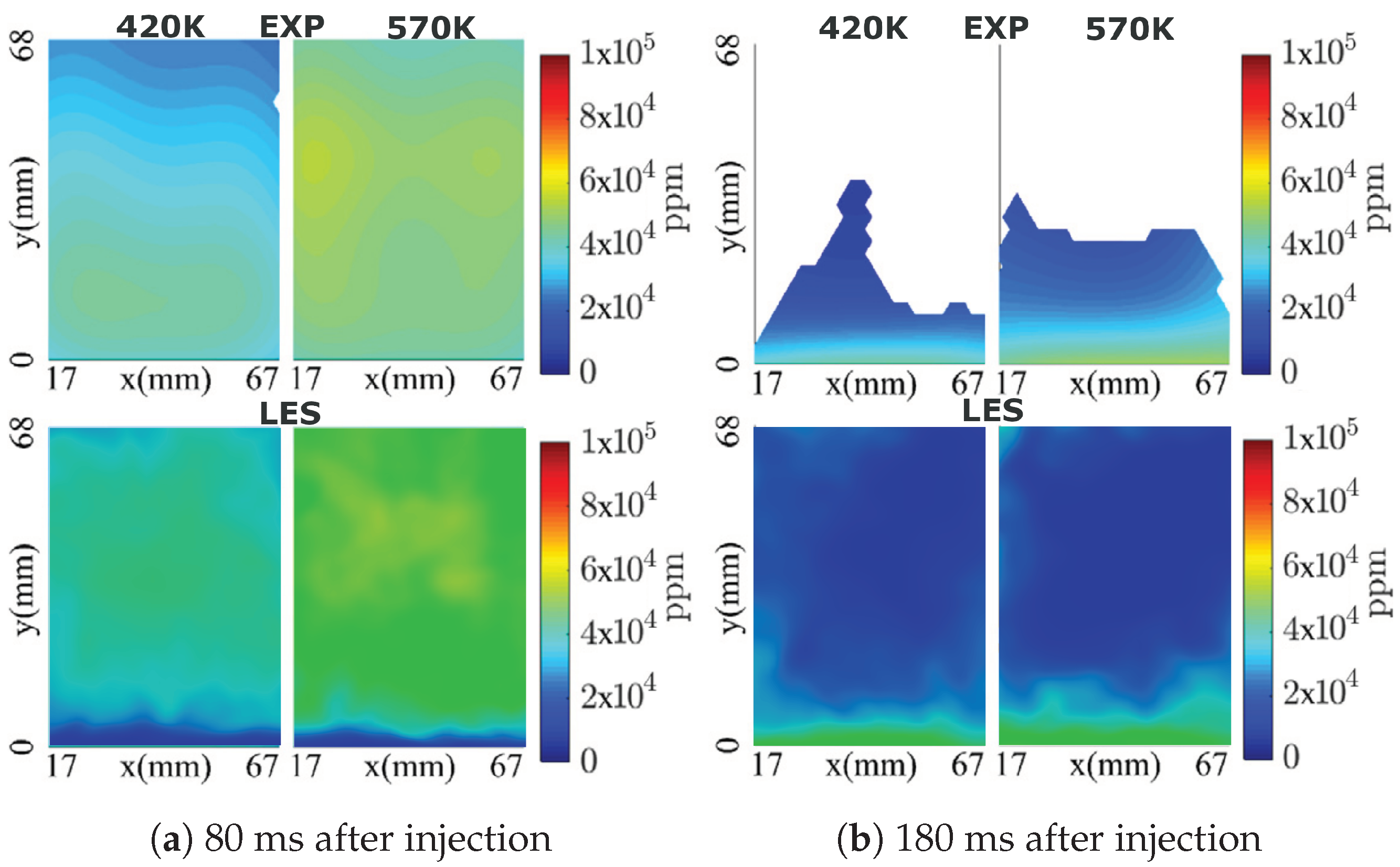
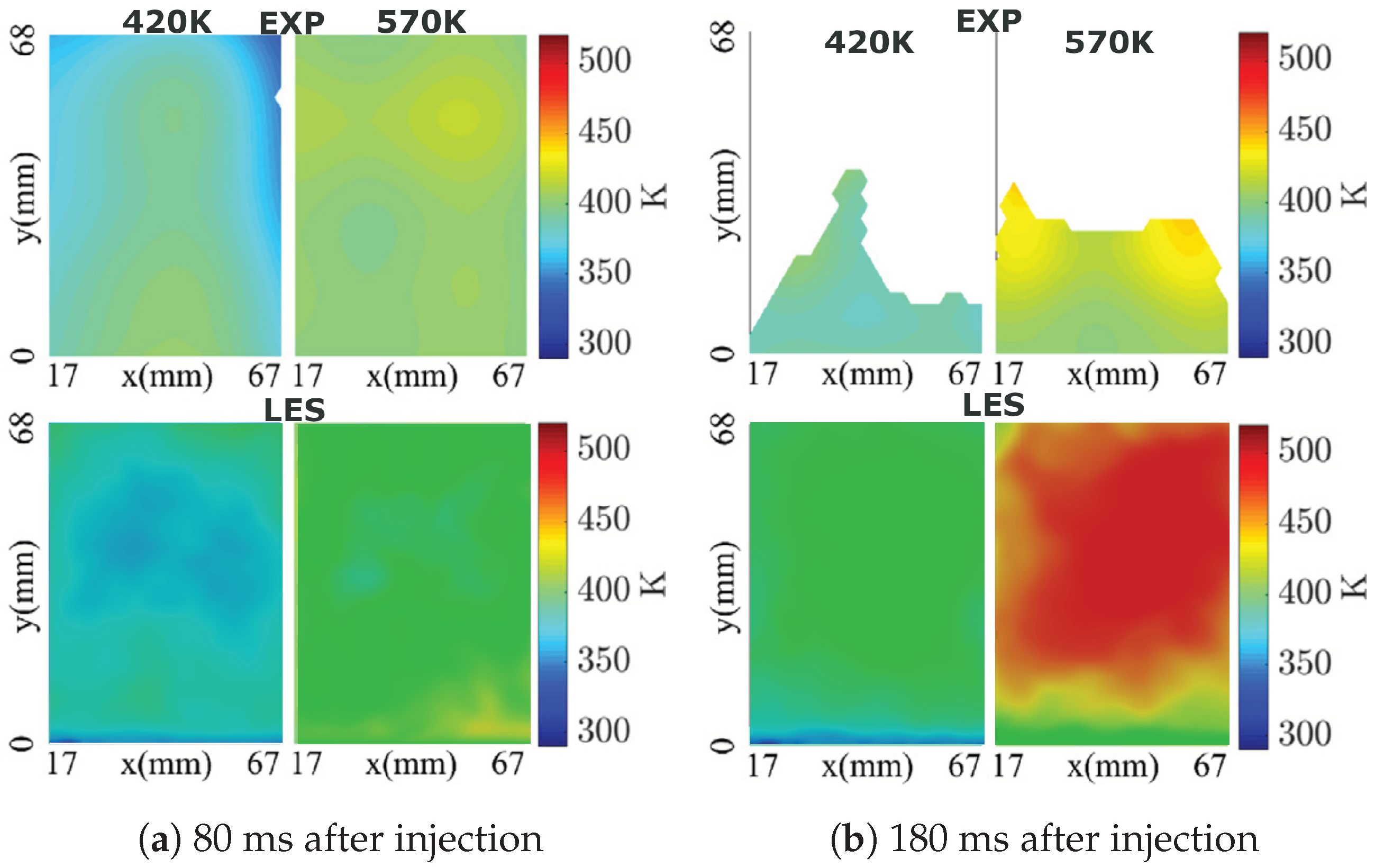
4.1. Model Validation
4.2. AdBlue Injection and SCR Flow Dynamics
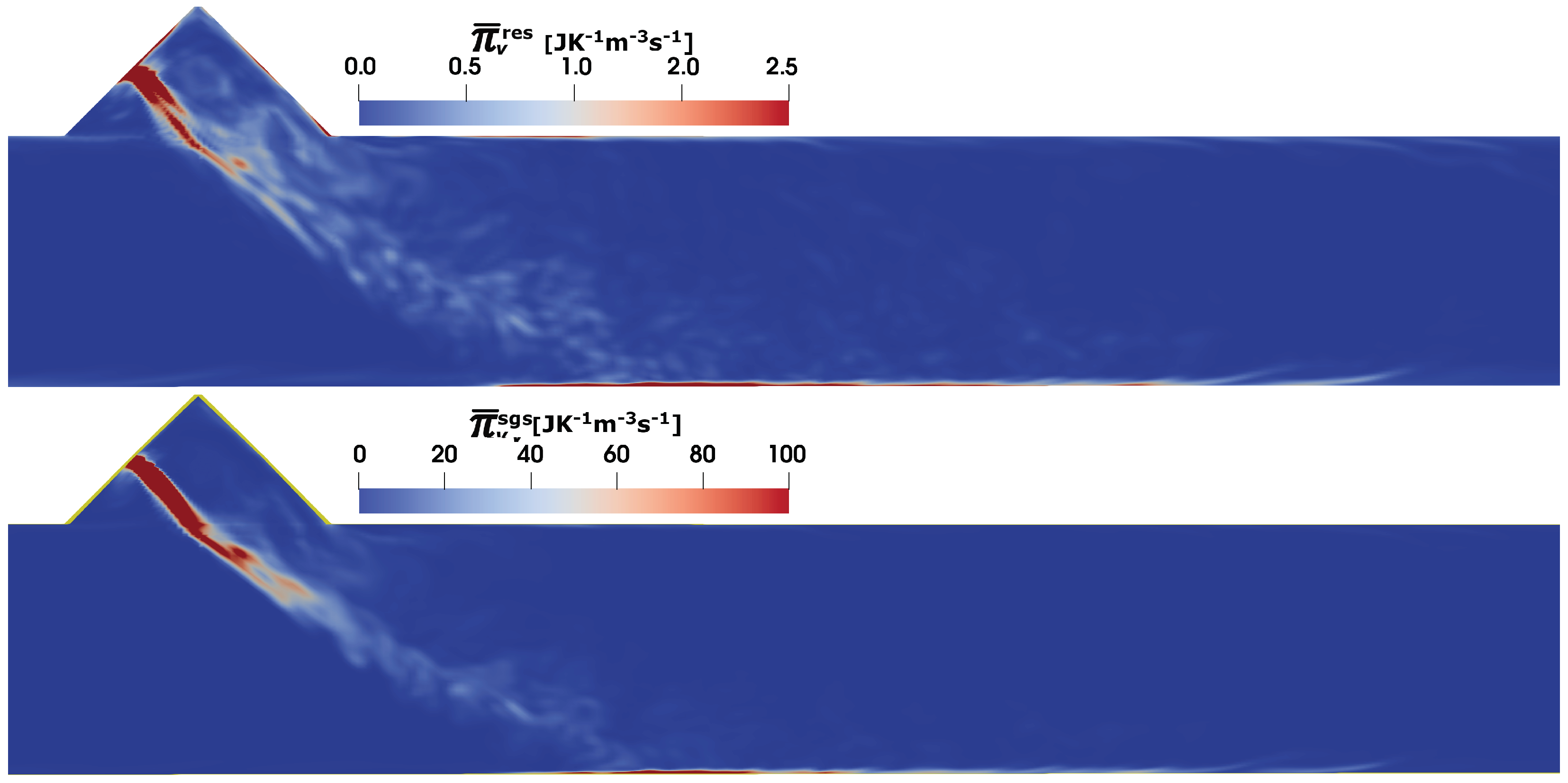
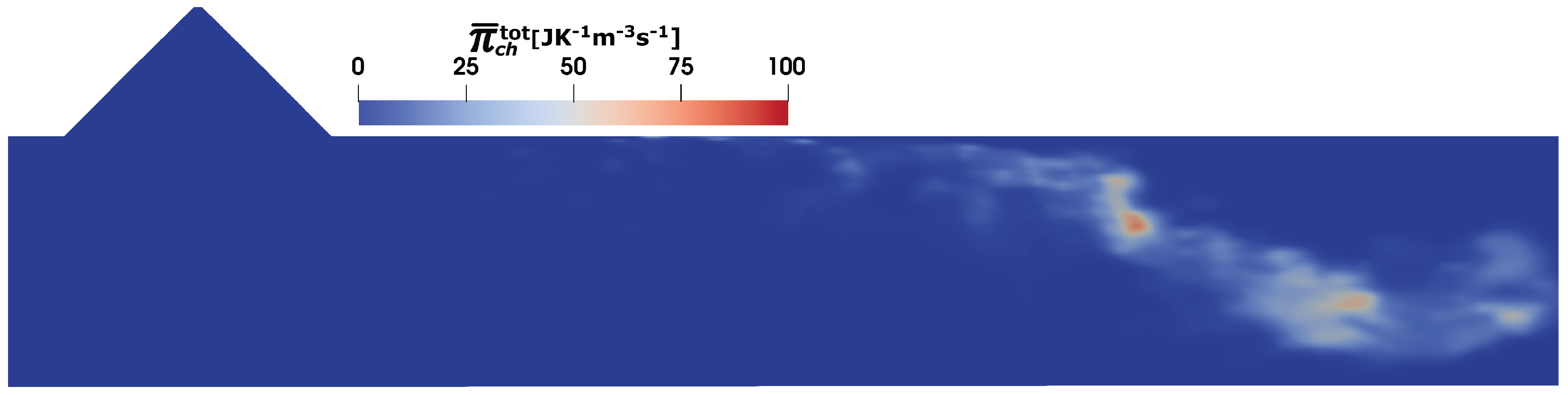
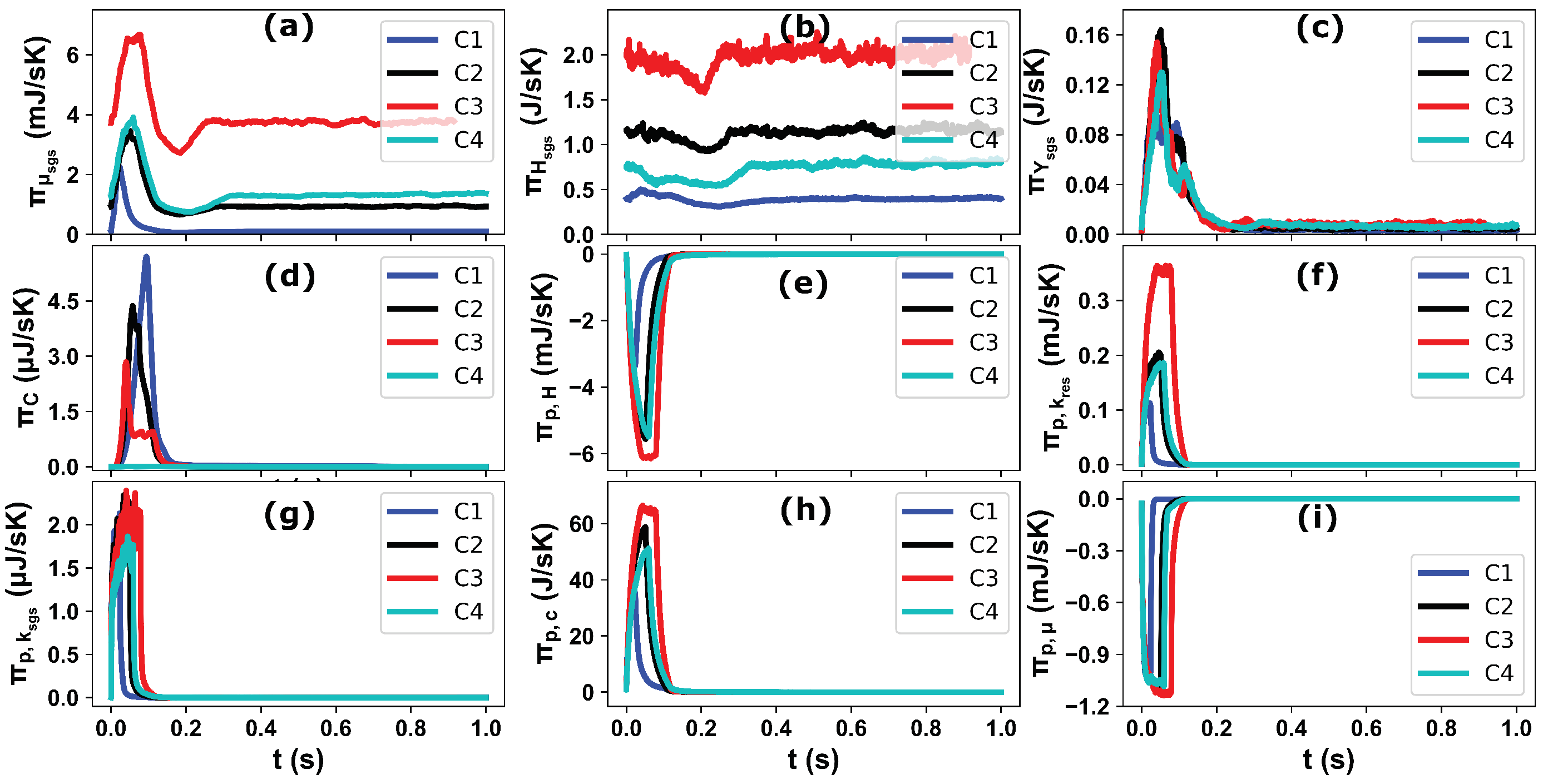
4.3. Predictions of Entropy Production Rates
5. Conclusions
- The entropy production is essentially a sub-grid-scale process as proven by the results of the viscous dissipation, heat dissipation and mass diffusion contributions.
- The impact of the spray dynamics is significant near the injection region through the modified thermal flow field and their gradient.
- Apart from the modified thermal profile in injection region, the higher entropy production due to heat dissipation is also associated with the near wall phenomena such as wall–film dynamics and spray transport close to wall region after spray–wall interaction.
- The total entropy production due to the chemical reaction is relatively low caused by the incomplete urea decomposition and NH3 conversion.
- The entropy contribution associated with the kinetic energy of evaporated mass features the lowest values because of the overall reduced evaporated mass. However, the negative entropy production rates (entropy destruction) are observed due to the slip velocity between the dispersed phase and the utilization of the gas phase sensible heat during the spray droplet evaporation . The entropy production due to the chemical potential of evaporated mass is the most significant compared to other processes related to the spray dynamics.
- Depending upon the respective AdBlue injection duration and injected mass the effects of the operating parameters (, ) on the entropy production is closely linked through the evolved flow, thermal, phase change, mixing and chemical reaction processes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EGAS | exhaust-gas-after-treatment system |
| BEV | Battery Electric Vehicle |
| EGM | Entropy Generation Minimization |
| LES | Large-Eddy Simulation |
| RANS | Reynolds-averaged Navier–Stokes |
| SCR | Selective Catalytic Reduction |
| CFD | Computation Fluid Dynamics |
| ESF | Eulerian Stochastic Field |
| TCI | Turbulence–chemistry interaction |
References
- Demirbas, A. Future Fuels for Internal Combustion Engines. Energy Sources Part A Recovery Util. Environ. Effects 2010, 32, 1273–1281. [Google Scholar] [CrossRef]
- Wallner, T.; Miers, S.A. Internal Combustion Engines, Alternative Fuels for. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2012; pp. 5461–5499. [Google Scholar] [CrossRef]
- Bae, C.; Kim, J. Alternative fuels for internal combustion engines. Proc. Combust. Inst. 2017, 36, 3389–3413. [Google Scholar] [CrossRef]
- Martins, J.; Brito, F.P. Alternative Fuels for Internal Combustion Engines. Energies 2020, 13, 4086. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, K.; Hao, H.; Liu, Z. Challenges, Potential and Opportunities for Internal Combustion Engines in China. Sustainability 2020, 12, 4955. [Google Scholar] [CrossRef]
- Habchi, C.; Nicolle, A.; Gillet, N. Numerical Study of Deposits Formation in SCR Systems Using Urea-Water Solution Injection. J. Mater. Sci. Nanotechnol. 2018, 6, 201. [Google Scholar] [CrossRef]
- Brack, W.; Heine, B.; Birkhold, F.; Kruse, M.; Deutschmann, O. Formation of Urea-Based Deposits in an Exhaust System: Numerical Predictions and Experimental Observations on a Hot Gas Test Bench. Emiss. Control Sci. Technol. 2016, 2, 115–123. [Google Scholar] [CrossRef]
- Payri, R.; Bracho, G.; Martí-Aldaraví, P.; Marco-Gimeno, J. Numerical Analysis of Urea to Ammonia Conversion in Automotive Selective Catalytic Reduction Realistic Conditions. Ind. Eng. Chem. Res. 2021, 60, 14329–14340. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ryu, S.H.; Ha, J.S. Numerical Prediction on the Characteristics of Spray-Induced Mixing and Thermal Decomposition of Urea Solution in SCR System. In Proceedings of the ASME 2004 Internal Combustion Engine Division Fall Technical Conference. ASMEDC, Long Beach, CA, USA, 24–27 October 2004; pp. 165–170. [Google Scholar] [CrossRef]
- Nishad, K.; Ries, F.; Janicka, J.; Sadiki, A. Analysis of spray dynamics of urea–water-solution jets in a SCR-DeNOx system: An LES based study. Int. J. Heat Fluid Flow 2018, 70, 247–258. [Google Scholar] [CrossRef]
- Nishad, K.; Sadiki, A.; Janicka, J. Numerical Investigation of AdBlue Droplet Evaporation and Thermal Decomposition in the Context of NOx-SCR Using a Multi-Component Evaporation Model. Energies 2018, 11, 222. [Google Scholar] [CrossRef]
- Nishad, K.; Stein, M.; Ries, F.; Bykov, V.; Maas, U.; Deutschmann, O.; Janicka, J.; Sadiki, A. Thermal Decomposition of a Single AdBlue® Droplet Including Wall-Film Formation in Turbulent Cross-Flow in an SCR System. Energies 2019, 12, 2600. [Google Scholar] [CrossRef]
- Kaario, O.T.; Vuorinen, V.; Zhu, L.; Larmi, M.; Liu, R. Mixing and evaporation analysis of a high-pressure SCR system using a hybrid LES-RANS approach. Energy 2017, 120, 827–841. [Google Scholar] [CrossRef]
- Bejan, A. Second law analysis in heat transfer. Energy 1980, 5, 720–732. [Google Scholar] [CrossRef]
- Bejan, A. Method of entropy generation minimization, or modeling and optimization based on combined heat transfer and thermodynamics. Revue Générale de Thermique 1996, 35, 637–646. [Google Scholar] [CrossRef]
- Ries, F.; Li, Y.; Rißmann, M.; Klingenberg, D.; Nishad, K.; Böhm, B.; Dreizler, A.; Janicka, J.; Sadiki, A. Database of Near-Wall Turbulent Flow Properties of a Jet Impinging on a Solid Surface under Different Inclination Angles. Fluids 2018, 3, 5. [Google Scholar] [CrossRef]
- Ries, F.; Kütemeier, D.; Li, Y.; Nishad, K.; Sadiki, A. Effect Chain Analysis of Supercritical Fuel Disintegration Processes Using an LES-based Entropy Generation Analysis. Combust. Sci. Technol. 2020, 192, 2171–2188. [Google Scholar] [CrossRef]
- Keenan, J.H. Availability and irreversibility in thermodynamics. Br. J. Appl. Phys. 1951, 2, 183–192. [Google Scholar] [CrossRef]
- Afridi, M.I.; Qasim, M.; Makinde, O.D. Entropy Generation Due to Heat and Mass Transfer in a Flow of Dissipative Elastic Fluid Through a Porous Medium. J. Heat Transf. 2018, 141, 022002. [Google Scholar] [CrossRef]
- Ries, F.; Li, Y.; Klingenberg, D.; Nishad, K.; Janicka, J.; Sadiki, A. Near-Wall Thermal Processes in an Inclined Impinging Jet: Analysis of Heat Transport and Entropy Generation Mechanisms. Energies 2018, 11, 1354. [Google Scholar] [CrossRef]
- Ries, F.; Li, Y.; Nishad, K.; Janicka, J.; Sadiki, A. Entropy Generation Analysis and Thermodynamic Optimization of Jet Impingement Cooling Using Large Eddy Simulation. Entropy 2019, 21, 129. [Google Scholar] [CrossRef]
- Afridi, M.I.; Qasim, M. Entropy Generation in Three Dimensional Flow of Dissipative Fluid. Int. J. Appl. Comput. Math. 2017, 4, 117–128. [Google Scholar] [CrossRef]
- Farooq, U.; Afridi, M.; Qasim, M.; Lu, D. Transpiration and Viscous Dissipation Effects on Entropy Generation in Hybrid Nanofluid Flow over a Nonlinear Radially Stretching Disk. Entropy 2018, 20, 668. [Google Scholar] [CrossRef]
- Reddy, G.J.; Kumar, M.; Kethireddy, B.; Chamkha, A.J. Colloidal study of unsteady magnetohydrodynamic couple stress fluid flow over an isothermal vertical flat plate with entropy heat generation. J. Mol. Liq. 2018, 252, 169–179. [Google Scholar] [CrossRef]
- Afridi, M.; Qasim, M.; Hussanan, A. Second Law Analysis of Dissipative Flow over a Riga Plate with Non-Linear Rosseland Thermal Radiation and Variable Transport Properties. Entropy 2018, 20, 615. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; ul Karim, F.; Khan, I.; Ali, F.; Khan, D. Irreversibility analysis in unsteady flow over a vertical plate with arbitrary wall shear stress and ramped wall temperature. Results Phys. 2018, 8, 1283–1290. [Google Scholar] [CrossRef]
- Adesanya, S.O.; Makinde, O.D. Effects of couple stresses on entropy generation rate in a porous channel with convective heating. Comput. Appl. Math. 2014, 34, 293–307. [Google Scholar] [CrossRef]
- Makinde, O. Entropy analysis for MHD boundary layer flow and heat transfer over a flat plate with a convective surface boundary condition. Int. J. Exergy 2012, 10, 142. [Google Scholar] [CrossRef]
- Rashidi, M.; Mohammadi, F.; Abbasbandy, S.; Alhuthali, M.S. Entropy Generation Analysis for Stagnation Point Flow in a Porous Medium over a Permeable Stretching Surface. J. Appl. Fluid Mech. 2015, 8, 753–765. [Google Scholar] [CrossRef]
- Ziefuss, M.; Karimi, N.; Ries, F.; Sadiki, A.; Mehdizadeh, A. Entropy Generation Assessment for Wall-Bounded Turbulent Shear Flows Based on Reynolds Analogy Assumptions. Entropy 2019, 21, 1157. [Google Scholar] [CrossRef]
- Li, Y.; Ries, F.; Nishad, K.; Sadiki, A. Predictions of Conjugate Heat Transfer in Turbulent Channel Flow Using Advanced Wall-Modeled Large Eddy Simulation Techniques. Entropy 2021, 23, 725. [Google Scholar] [CrossRef]
- Agrebi, S.; Dreßler, L.; Nicolai, H.; Ries, F.; Nishad, K.; Sadiki, A. Analysis of Local Exergy Losses in Combustion Systems Using a Hybrid Filtered Eulerian Stochastic Field Coupled with Detailed Chemistry Tabulation: Cases of Flames D and E. Energies 2021, 14, 6315. [Google Scholar] [CrossRef]
- Agrebi, S.; Dreßler, L.; Nishad, K. The Exergy Losses Analysis in Adiabatic Combustion Systems including the Exhaust Gas Exergy. Entropy 2022, 24, 564. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rico Cortes, L.F.; Hamel, H.; Nishad, K.; Biondo, L.; Ries, F. Prediction of Heat Transfer and Fluid Flow Effects on Entropy Generation in a Monolithic Catalytic Converter Using Large-Eddy Simulation. Entropy 2022, 24, 602. [Google Scholar] [CrossRef] [PubMed]
- Sadiki, A.; Agrebi, S.; Ries, F. Entropy Generation Analysis in Turbulent Reacting Flows and Near Wall: A Review. Entropy 2022, 24, 1099. [Google Scholar] [CrossRef] [PubMed]
- OpenCFD Ltd. OpenFOAM—The Open Source CFD Toolbox, 1612+ ed.; OpenCFD Ltd.: London, UK, 2016. [Google Scholar]
- Yoshizawa, A.; Horiuti, K. A Statistically-Derived Subgrid-Scale Kinetic Energy Model for the Large-Eddy Simulation of Turbulent Flows. J. Phys. Soc. Jpn. 1985, 54, 2834–2839. [Google Scholar] [CrossRef]
- Birkhold, F. Selektive katalytische Reduktion von Stickoxiden in Kraftfahrzeugen: Untersuchung der Einspritzung von Harnstoffwasserlösung. Ph.D. Thesis, Karlsruher Institut für Technologie, Karlsruhe, Germany, 2007. [Google Scholar]
- Pope, S.B. A Monte Carlo Method for the PDF Equations of Turbulent Reactive Flow. Combust. Sci. Technol. 1981, 25, 159–174. [Google Scholar] [CrossRef]
- Jones, W.; Marquis, A.; Vogiatzaki, K. Large-eddy simulation of spray combustion in a gas turbine combustor. Combust. Flame 2014, 161, 222–239. [Google Scholar] [CrossRef]
- Dressler, L.; Sacomano Filho, F.L.; Ries, F.; Nicolai, H.; Janicka, J.; Sadiki, A. Numerical Prediction of Turbulent Spray Flame Characteristics Using the Filtered Eulerian Stochastic Field Approach Coupled to Tabulated Chemistry. Fluids 2021, 6, 50. [Google Scholar] [CrossRef]
- Kuhnke, D. Spray Wall Interaction Modelling by Dimensionless Data Analysis; Berichte aus der Strömungstechnik, Shaker: Aachen, Germany, 2004. [Google Scholar]
- Bai, C.X.; Rusche, H.; Gosman, A.D. Modeling of gasoline spray impingement. At. Sprays 2002, 12, 1–28. [Google Scholar] [CrossRef]
- Börnhorst, M.; Deutschmann, O. Single droplet impingement of urea water solution on a heated substrate. Int. J. Heat Fluid Flow 2018, 69, 55–61. [Google Scholar] [CrossRef]
- Miller, R.S.; Harstad, K.; Bellan, J. Evaluation of equilibrium and non-equilibrium evaporation models for many-droplet gas-liquid flow simulations. Int. J. Multiph. Flow 1998, 24, 1025–1055. [Google Scholar] [CrossRef]
- Abramzon, B.; Sirignano, W. Droplet vaporization model for spray combustion calculations. Int. J. Heat Mass Transf. 1989, 32, 1605–1618. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Madia, G. Reaction pathways in the selective catalytic reduction process with NO and NO2 at low temperatures. Ind. Eng. Chem. Res. 2001, 40, 52–59. [Google Scholar] [CrossRef]
- Li, Y.; Ries, F.; Leudesdorff, W.; Nishad, K.; Pati, A.; Hasse, C.; Janicka, J.; Jakirlić, S.; Sadiki, A. Non-equilibrium wall functions for large Eddy simulations of complex turbulent flows and heat transfer. Int. J. Heat Fluid Flow 2021, 88, 108758. [Google Scholar] [CrossRef]
- Ries, F.; Obando, P.; Shevchuck, I.; Janicka, J.; Sadiki, A. Numerical analysis of turbulent flow dynamics and heat transport in a round jet at supercritical conditions. Int. J. Heat Fluid Flow 2017, 66, 172–184. [Google Scholar] [CrossRef]
- Ries, F. Numerical Modeling and Prediction of Irreversibilities in Sub- and Supercritical Near-Wall Flows. Ph.D. Thesis, Institute of Energy and Power Plant Technology, Technische Universität Darmstadt, Darmstadt, Germany, 2018. [Google Scholar]
- Valino, L. A Field Monte Carlo Formulation for Calculating the Probability Density Function of a Single Scalar in a Turbulent Flow. Flow Turbul. Combust. 1998, 60, 157–172. [Google Scholar] [CrossRef]
- Pope, S. PDF methods for turbulent reactive flows. Prog. Energy Combust. Sci. 1985, 11, 119–192. [Google Scholar] [CrossRef]
- Valino, L.; Mustata, R.; Letaief, K.B. Consistent Behavior of Eulerian Monte Carlo fields at Low Reynolds Numbers. Flow Turbul. Combust. 2016, 96, 503–512. [Google Scholar] [CrossRef]
- Nishad, K.; Dressler, L.; Hasse, C.; Sadiki, A. Numerical investigation of AdBlue film formation and NH3 conversion in generic SCR system using Eulerian stochastic fields method. Int. J. Heat Fluid Flow 2023, 99, 109096. [Google Scholar] [CrossRef]
- Safari, M.; Hadi, F.; Sheikhi, M.R.H. Progress in the Prediction of Entropy Generation in Turbulent Reacting Flows Using Large Eddy Simulation. Entropy 2014, 16, 5159–5177. [Google Scholar] [CrossRef]
- Corrsin, S. On the Spectrum of Isotropic Temperature Fluctuations in an Isotropic Turbulence. J. Appl. Phys. 1951, 22, 469–473. [Google Scholar] [CrossRef]
- Smagorinsky, J. General circulation experiments with the primitive equations. Mon. Weather Rev. 1963, 91, 99–164. [Google Scholar] [CrossRef]
- Okong’O, N.A.; Bellan, J. Consistent large-eddy simulation of a temporal mixing layer laden with evaporating drops. Part 1. Direct numerical simulation, formulation and a priori analysis. J. Fluid Mech. 2004, 499, 1–47. [Google Scholar] [CrossRef]
- Leboissetier, A.; Okong’O, N.A.; Bellan, J. Consistent large-eddy simulation of a temporal mixing layer laden with evaporating drops. Part 2. A posteriori modelling. J. Fluid Mech. 2005, 523, 37–78. [Google Scholar] [CrossRef]
- Schmidt, A.; Bonarens, M.; Roisman, I.V.; Nishad, K.; Sadiki, A.; Dreizler, A.; Hussong, J.; Wagner, S. Experimental Investigation of AdBlue Film Formation in a Generic SCR Test Bench and Numerical Analysis Using LES. Appl. Sci. 2021, 11, 6907. [Google Scholar] [CrossRef]
- Schmidt, A.; van der Kley, S.; Wagner, S. Optically accessible generic exhaust gas test bench for the investigation of fundamental SCR-relevant processes. Appl. Opt. 2020, 59, 6953–6958. [Google Scholar] [CrossRef]
- Van der Kley, S.; Emmert, J.; Schmidt, A.; Dreizler, A.; Wagner, S. Tomographic spectrometer for the temporally-resolved 2D reconstruction of gas phase parameters within a generic SCR test rig. Proc. Combust. Inst. 2021, 38, 1703–1710. [Google Scholar] [CrossRef]
- Klein, M.; Sadiki, A.; Janicka, J. A digital filter based generation of inflow data for spatially developing direct numerical or large eddy simulations. J. Comput. Phys. 2003, 186, 652–665. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishad, K.; Agrebi, S. Estimation of Entropy Generation in a SCR-DeNOx System with AdBlue Spray Dynamic Using Large Eddy Simulation. Entropy 2023, 25, 475. https://doi.org/10.3390/e25030475
Nishad K, Agrebi S. Estimation of Entropy Generation in a SCR-DeNOx System with AdBlue Spray Dynamic Using Large Eddy Simulation. Entropy. 2023; 25(3):475. https://doi.org/10.3390/e25030475
Chicago/Turabian StyleNishad, Kaushal, and Senda Agrebi. 2023. "Estimation of Entropy Generation in a SCR-DeNOx System with AdBlue Spray Dynamic Using Large Eddy Simulation" Entropy 25, no. 3: 475. https://doi.org/10.3390/e25030475
APA StyleNishad, K., & Agrebi, S. (2023). Estimation of Entropy Generation in a SCR-DeNOx System with AdBlue Spray Dynamic Using Large Eddy Simulation. Entropy, 25(3), 475. https://doi.org/10.3390/e25030475






