Cardio-Hypothalamic-Pituitary Coupling during Rest and in Response to Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
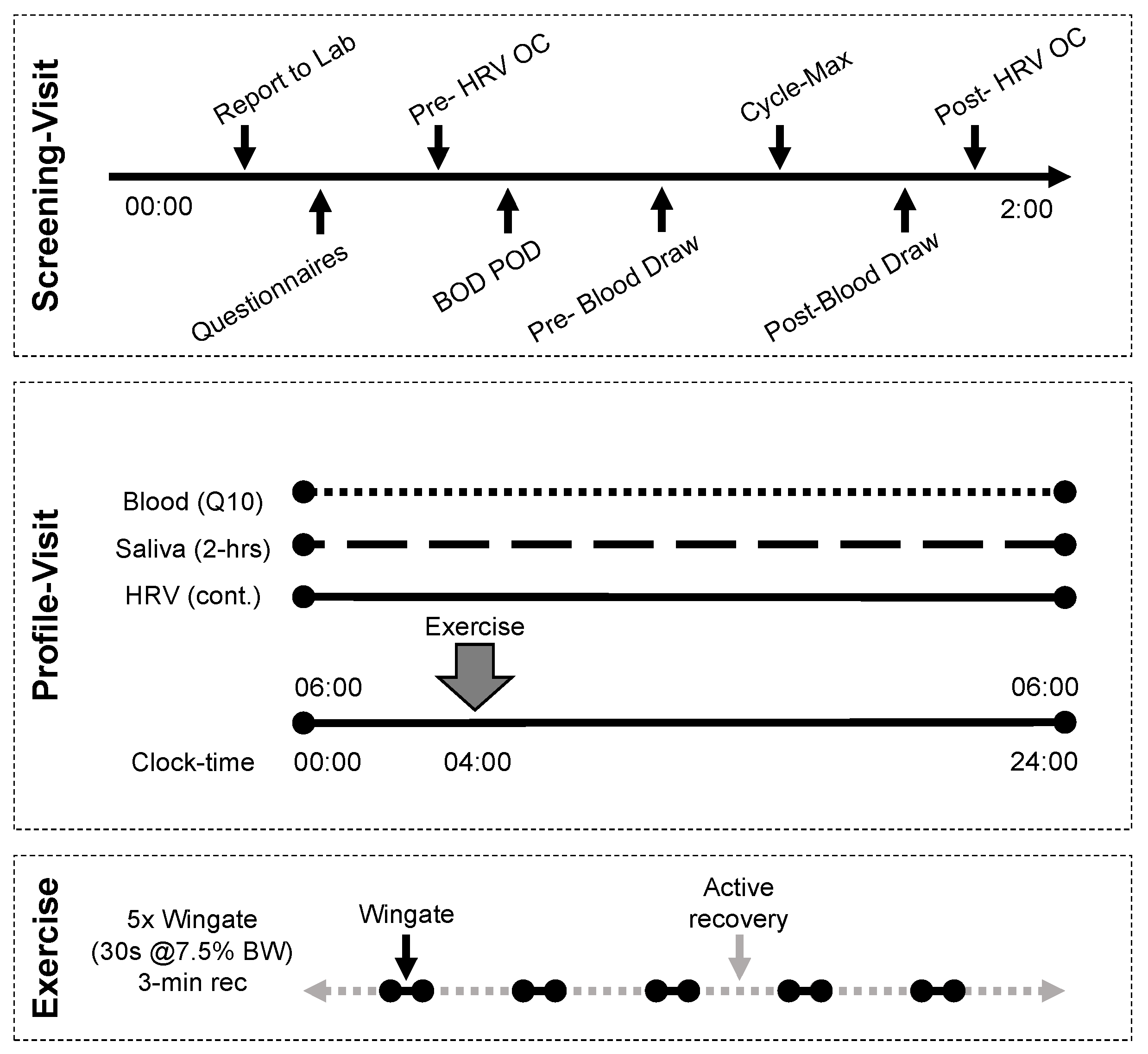
2.2. Screening Visit
2.3. Profile Visit
2.4. Exercise Protocol
2.5. Biological Sample Collection and Analysis
2.6. RR-Recordings
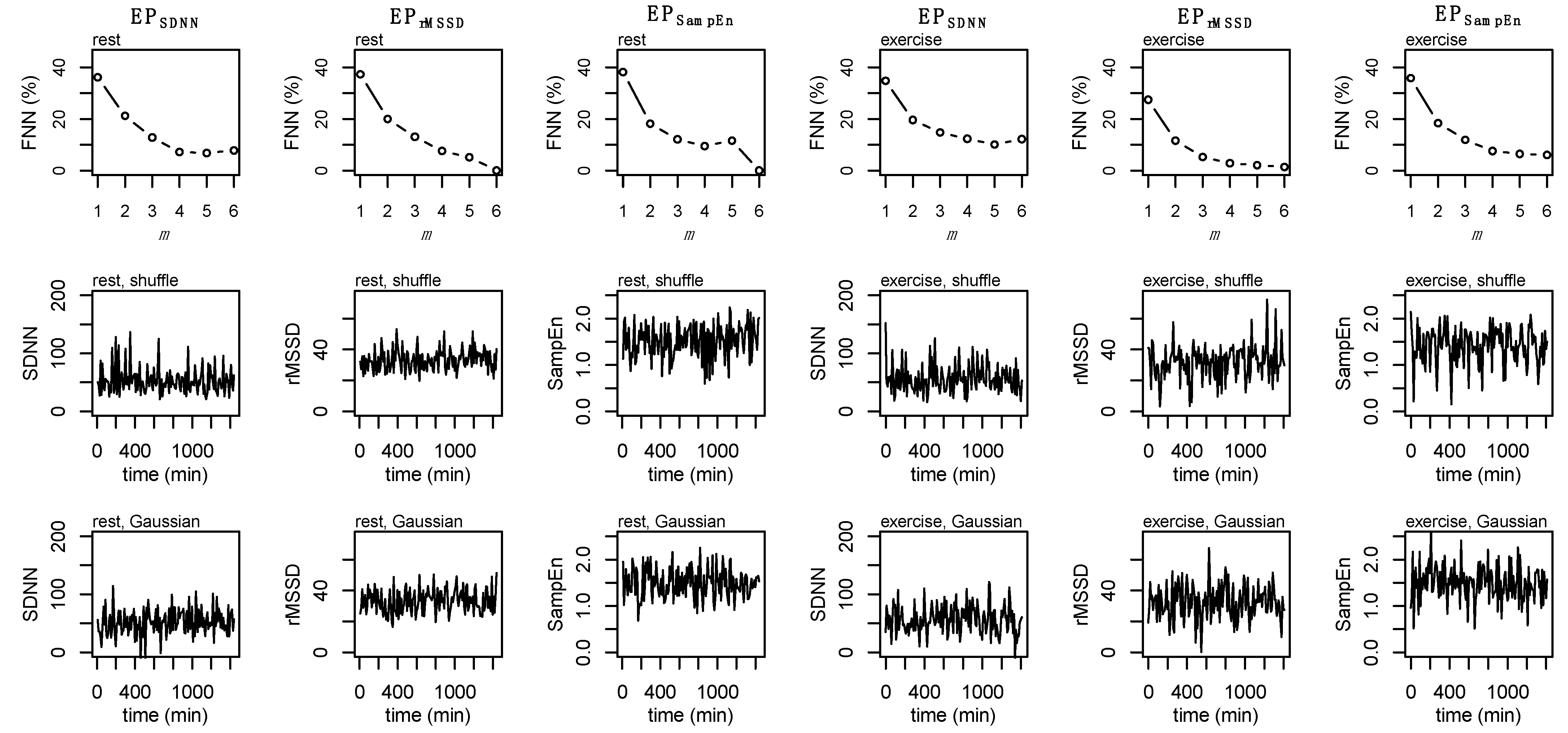
2.7. Epoched RR-Recordings
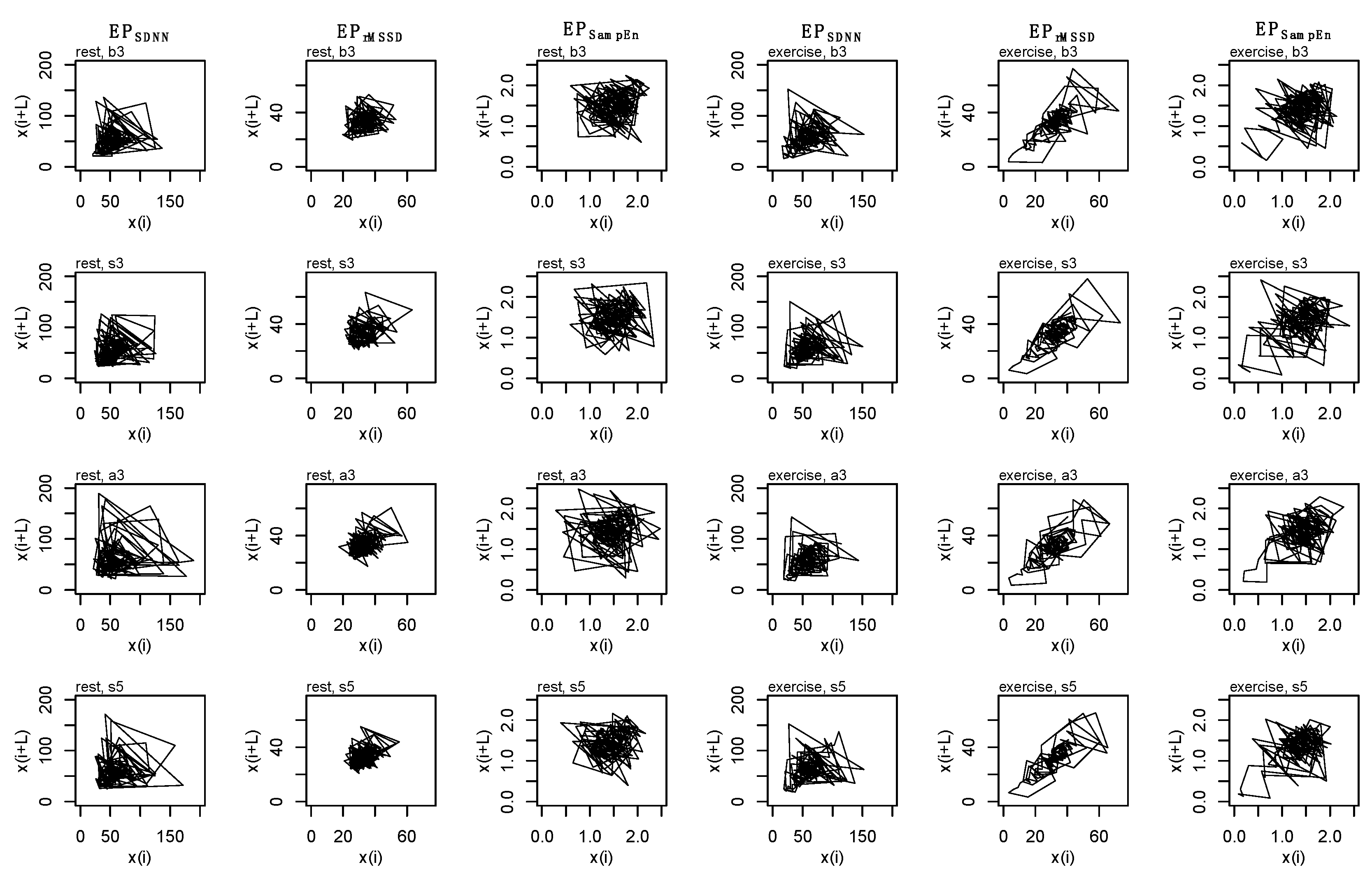
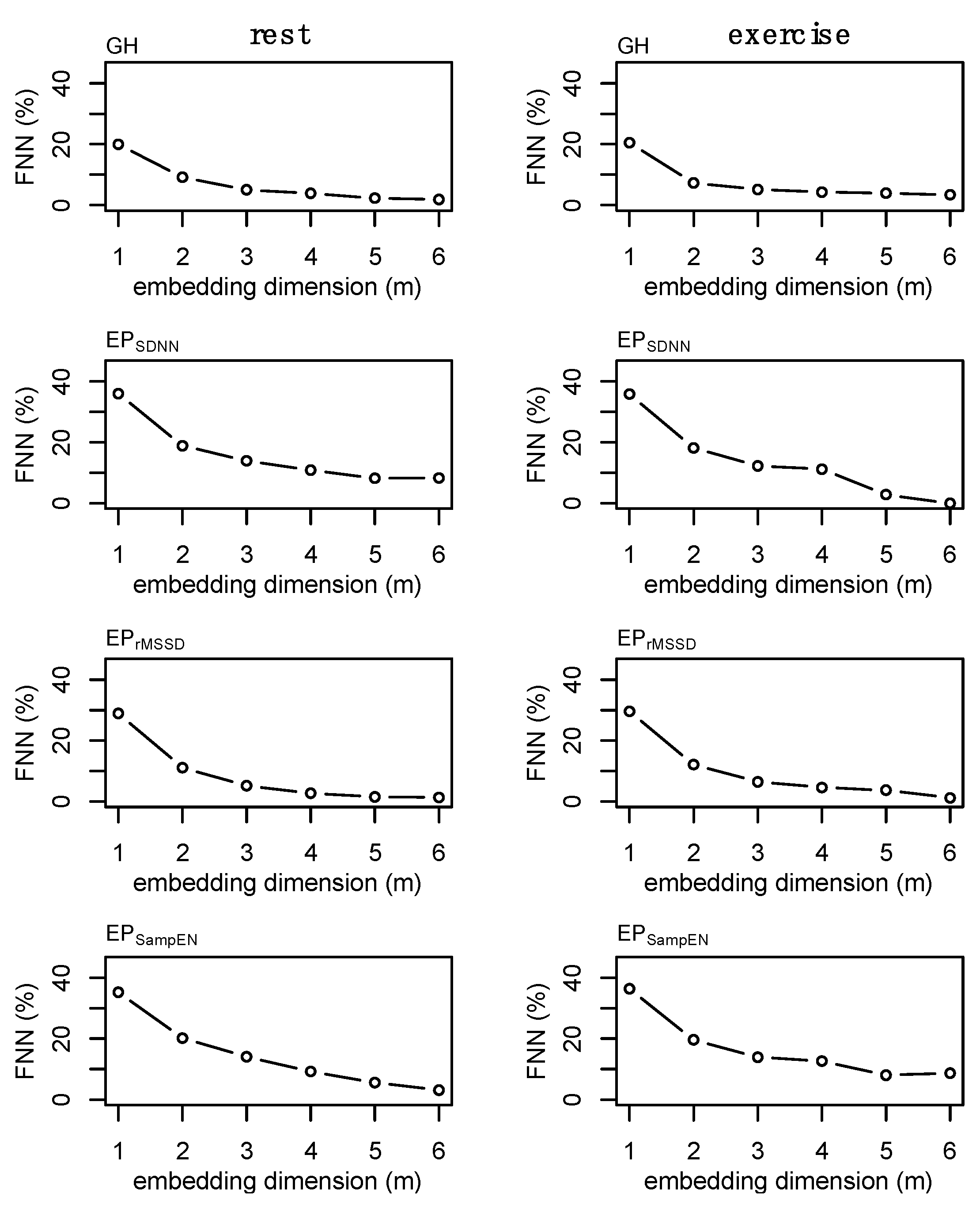
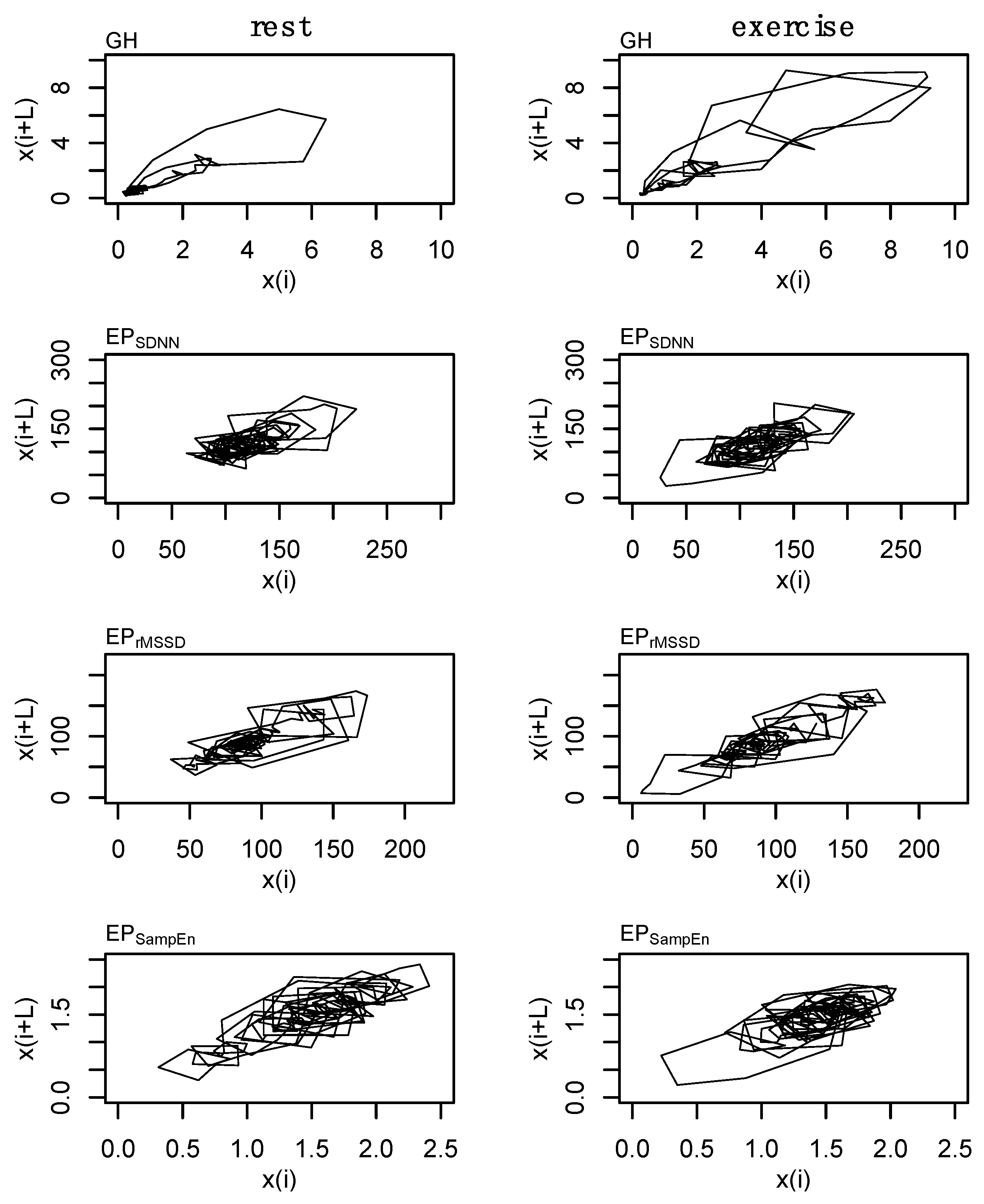
2.8. State-Space Reconstruction
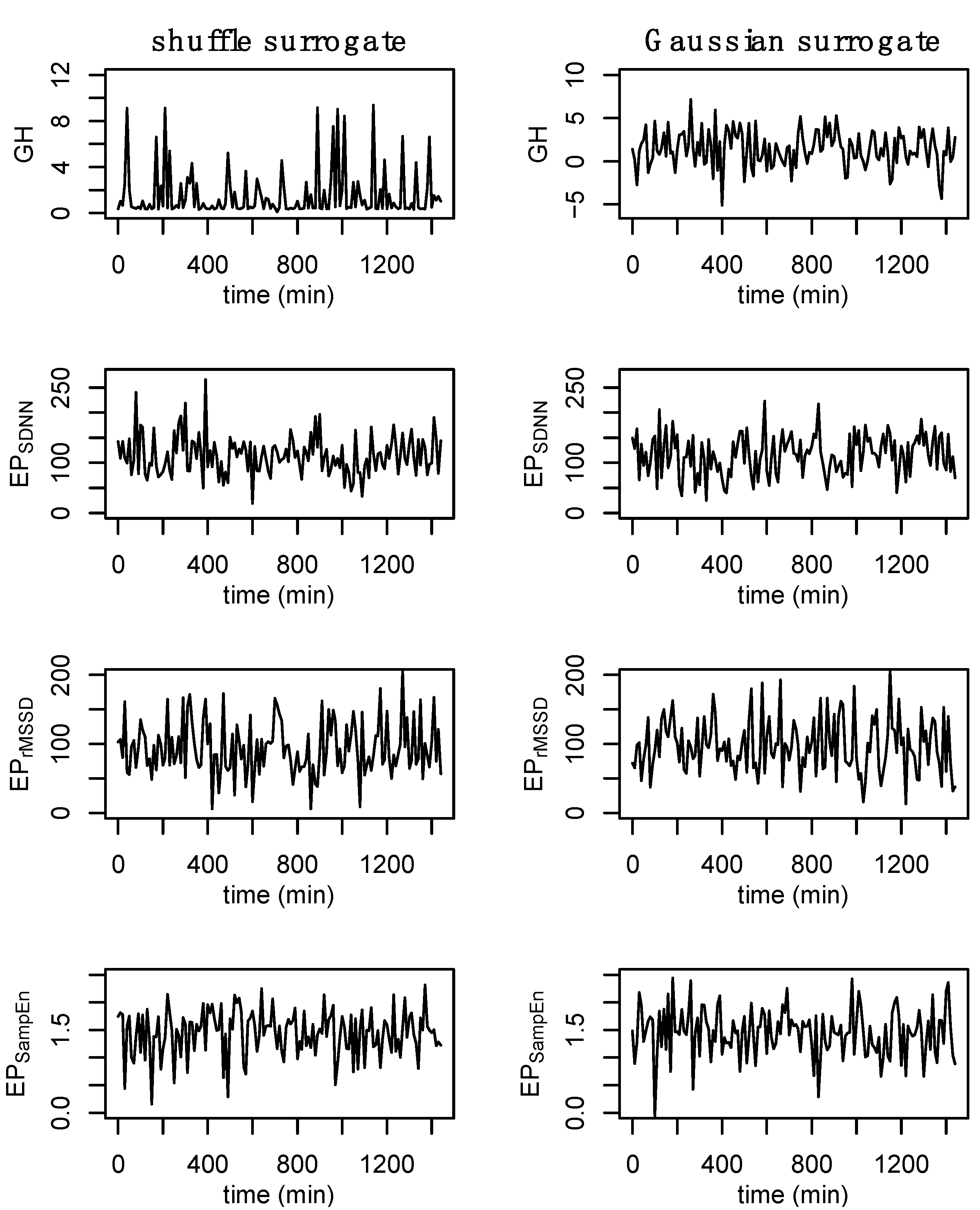
2.9. Surrogate Data
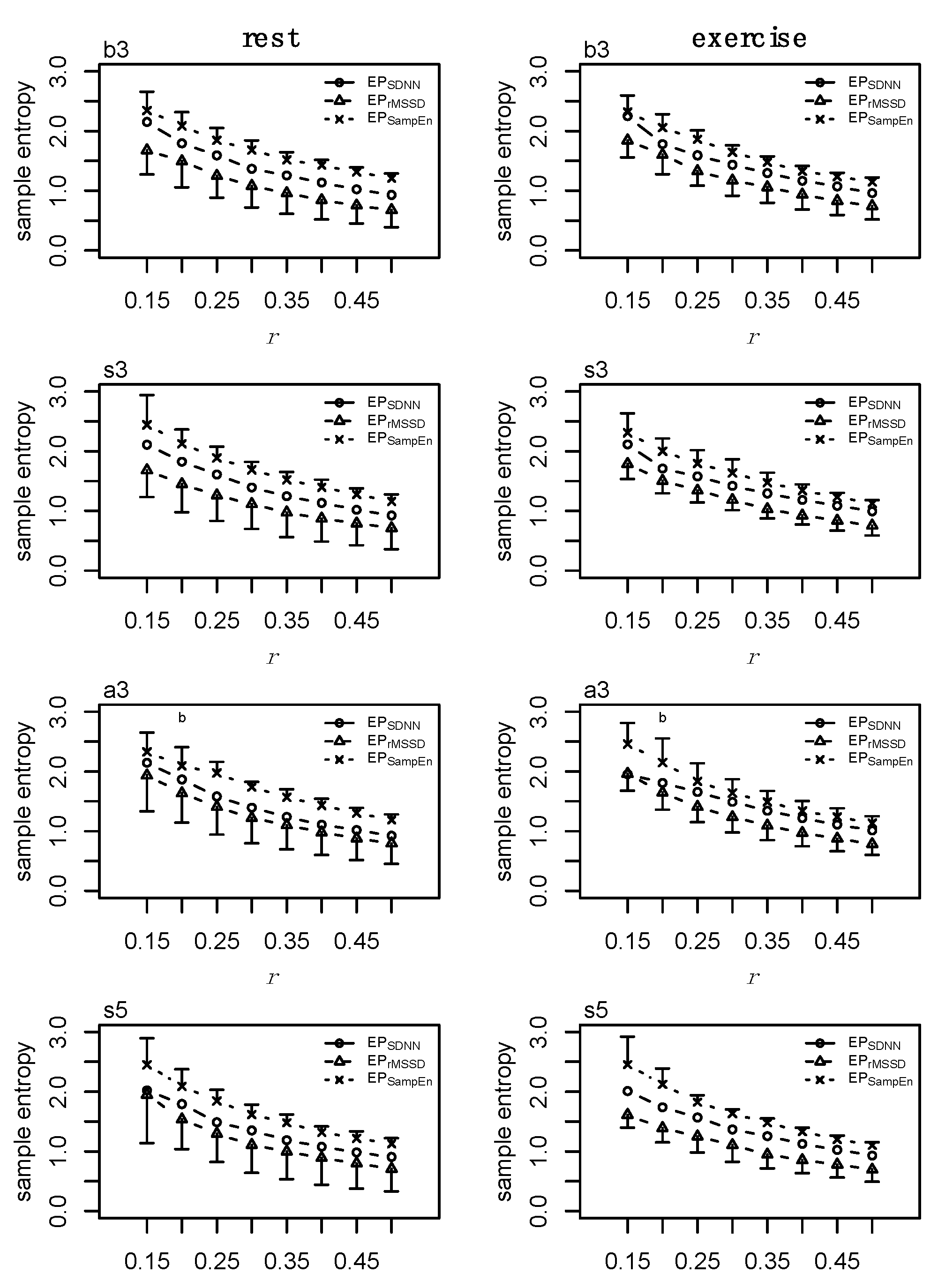
2.10. Individual Dynamics
2.11. Coupling
2.12. Statistics
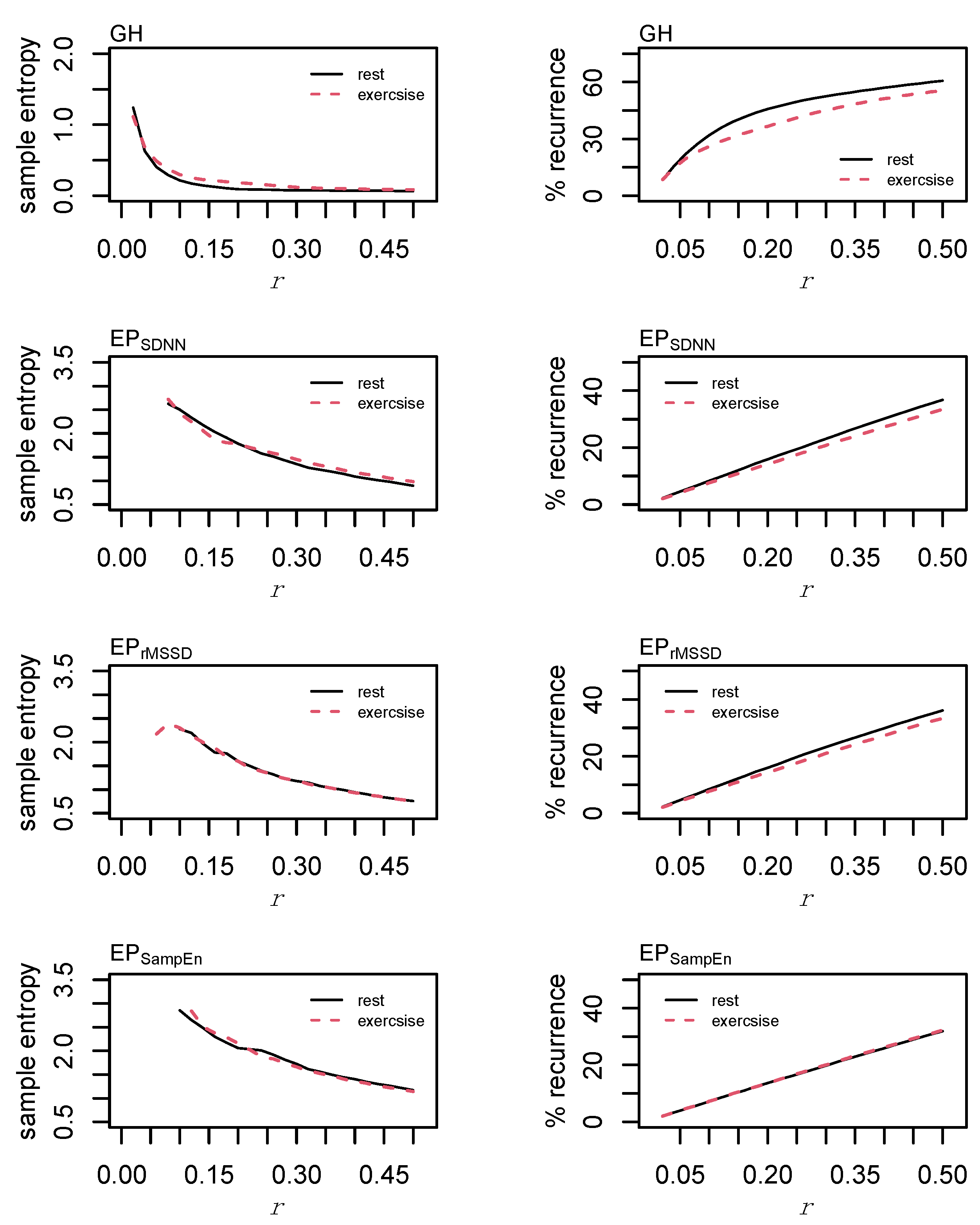
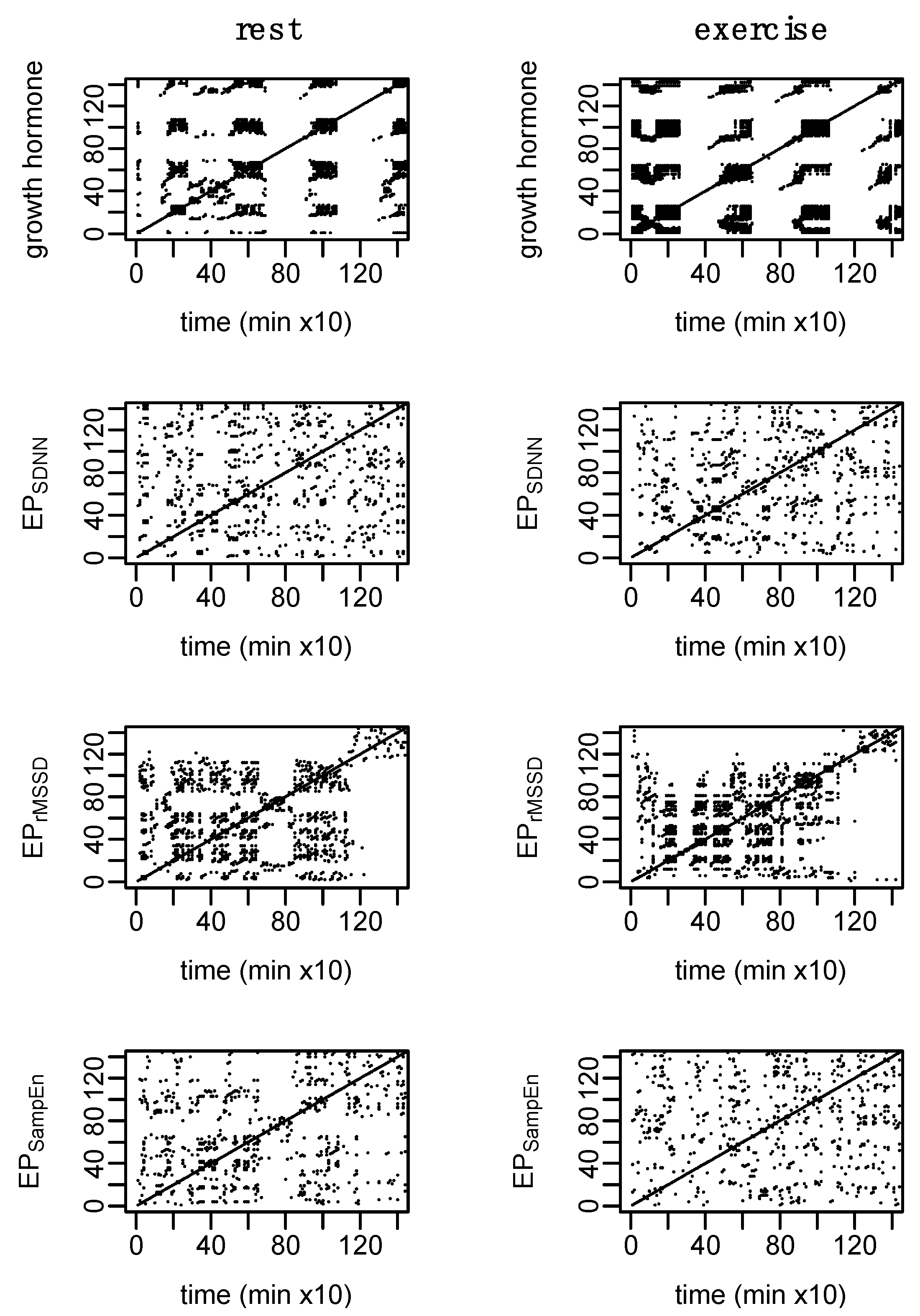
3. Results
4. Discussion
4.1. Heart Rate Variability
4.2. Cardio-Hypothalamic-Pituitary Coupling
4.3. Limitations
5. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Peng, C.K.; Havlin, S.; Stanley, H.E.; Goldberger, A.L. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 1995, 5, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.K.; Mietus, J.; Hausdorff, J.M.; Havlin, S.; Stanley, H.E.; Goldberger, A.L. Long-range anticorrelations and non-Gaussian behavior of the heartbeat. Phys. Rev. Lett. 1993, 70, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Berry, N.T.; Bechke, E.; Shriver, L.H.; Calkins, S.D.; Keane, S.P.; Shanahan, L.; Wideman, L. Heart Rate Dynamics During Acute Recovery From Maximal Aerobic Exercise in Young Adults. Front. Physiol. 2021, 12, 627320. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.; Huang, J.W.; Lin, L.Y.; Chang, C.H.; Chu, F.Y.; Lin, Y.H.; Wu, C.K.; Lee, J.K.; Hwang, J.J.; Lin, J.L.; et al. Detrended Fluctuation Analysis of Heart Rate Dynamics Is an Important Prognostic Factor in Patients with End-Stage Renal Disease Receiving Peritoneal Dialysis. PLoS ONE 2016, 11, e0147282. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.; Hausdorff, J.M.; Ivanov, P.; Peng, C.K.; Stanley, H.E. Fractal dynamics in physiology: Alterations with disease and aging. Proc. Natl. Acad. Sci. USA 2002, 99 (Suppl. 1), 2466–2472. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Frohlinde, V.; Ashkenazy, Y.; Goldberger, A.L.; Ivanov, P.; Costa, M.; Morley-Davies, A.; Stanley, H.E.; Glass, L. Complex patterns of abnormal heartbeats. Phys. Rev. E Stat. Nonlinear Biol. Soft Matter Phys. 2002, 66, 031901. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M.; Peng, C.K.; Ladin, Z.; Wei, J.Y.; Goldberger, A.L. Is walking a random walk? Evidence for long-range correlations in stride interval of human gait. J. Appl. Physiol. 1995, 78, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M.; Edelberg, H.K.; Mitchell, S.L.; Goldberger, A.L.; Wei, J.Y. Increased gait unsteadiness in community-dwelling elderly fallers. Arch. Phys. Med. Rehabil. 1997, 78, 278–283. [Google Scholar] [CrossRef]
- Hausdorff, J.M. Gait dynamics, fractals and falls: Finding meaning in the stride-to-stride fluctuations of human walking. Hum. Mov. Sci. 2007, 26, 555–589. [Google Scholar] [CrossRef]
- Rhea, C.K.; Kiefer, A.W. Patterned variability in gait behavior: How can it be measured and what does it mean? In Gait Biometrics: Basic Patterns, Role of Neurological Disorders and Effects of Physical Activity; Nova Science: Hauppauge, NY, USA, 2014. [Google Scholar]
- Rhea, C.K.; Kiefer, A.W.; Wittstein, M.W.; Leonard, K.B.; MacPherson, R.P.; Wright, W.G.; Haran, F.J. Fractal gait patterns are retained after entrainment to a fractal stimulus. PLoS ONE 2014, 9, e106755. [Google Scholar] [CrossRef]
- Stergiou, N.; Decker, L.M. Human movement variability, nonlinear dynamics, and pathology: Is there a connection? Hum. Mov. Sci. 2011, 30, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.H. Dynamic Walking Principles Applpied to Human Gait; The University of Michigan: Ann Arbor, MI, USA, 2008. [Google Scholar]
- Manor, B.; Costa, M.D.; Hu, K.; Newton, E.; Starobinets, O.; Kang, H.G.; Peng, C.K.; Novak, V.; Lipsitz, L.A. Physiological complexity and system adaptability: Evidence from postural control dynamics of older adults. J. Appl. Physiol. 2010, 109, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Rhea, C.K.; Silver, T.A.; Hong, S.L.; Ryu, J.H.; Studenka, B.E.; Hughes, C.M.; Haddad, J.M. Noise and complexity in human postural control: Interpreting the different estimations of entropy. PLoS ONE 2011, 6, e17696. [Google Scholar] [CrossRef]
- Kuznetsov, N.A.; Riley, M.A. The role of task constraints in relating laboratory and clinical measures of balance. Gait Posture 2015, 42, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.J.; De Luca, C.J. Upright, correlated random walks: A statistical-biomechanics approach to the human postural control system. Chaos 1994, 5, 57–63. [Google Scholar] [CrossRef]
- Collins, J.J.; De Luca, C.J.; Pavlik, A.E.; Roy, S.H.; Emley, M.S. The effects of spaceflight on open-loop and closed-loop postural control mechanisms: Human neurovestibular studies on SLS-2. Exp. Brain Res. 1995, 107, 145–150. [Google Scholar] [CrossRef]
- West, B.J.; Goldberger, A.L. Physiology in Fractal Dimensions. Am. Sci. 1987, 75, 354–365. [Google Scholar]
- Lipsitz, L.A.; Goldberger, A.L. Loss of complexity and aging. Potential applications of fractals and chaos theory to senescence. J. Am. Med. Assoc. 1992, 267, 1806–1809. [Google Scholar] [CrossRef]
- Lipsitz, L.A. Dynamics of Stability: The Physiologic Basis of Functional Health and Frailty. J. Gerontol. 2002, 57, B115–B125. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Peng, C.K.; Lipsitz, L.A. What is physiologic complexity and how does it change with aging and disease? Neurobiol. Aging 2002, 23, 23–26. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Rigney, D.R.; West, B.J. Chaos and fractals in human physiology. Sci. Am. 1990, 262, 42–49. [Google Scholar] [CrossRef]
- Task-Force. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.K.; Reddy, A. Nonlinear heart rate variability and risk stratification in cardiovascular disease. Indian Pacing Electrophysiol. 2005, 5, 210–220. [Google Scholar]
- Godin, P.J.; Buchman, T.G. Uncoupling of biological oscillators: A complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit. Care Med. 1996, 24, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Seely, A.J.; Christou, N.V. Multiple organ dysfunction syndrome: Exploring the paradigm of complex nonlinear systems. Crit. Care Med. 2000, 28, 2193–2200. [Google Scholar] [CrossRef]
- Novak, V.; Hu, K.; Vyas, M.; Lipsitz, L.A. Cardiolocomotor coupling in yound and elderly people. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 86–92. [Google Scholar] [CrossRef]
- Ferguson, A.V.; Latchford, K.J.; Samson, W.K. The paraventricular nucleus of the hypothalamus—A potential target for integrative treatment of autonomic dysfunction. Expert Opin. Target. 2008, 12, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Veldhuis, J. Pathophysiology of the Neuroregulation of Growth Hormone Secretion in Experimental Animals and the Human. Endocr. Rev. 1998, 19, 717–797. [Google Scholar] [PubMed]
- Wideman, L.; Consitt, L.; Patrie, J.; Swearingin, B.; Bloomer, R.; Davis, P.; Weltman, A. The impact of sex and exercise duration on growth hormone secretion. J. Appl. Physiol. 2006, 101, 1641–1647. [Google Scholar] [CrossRef]
- Hartman, M.L.; Veldhuis, J.D.; Thorner, M.O. Normal control of growth hormone secretion. Horm. Res. 1993, 40, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.K.; Kleiger, R.E.; Rottman, J.N. Differing Effects of Age on Heart Rate Variability in Men and Women. Am. J. Cardiol. 1997, 80, 302–305. [Google Scholar] [CrossRef]
- Agorastos, A.; Heinig, A.; Stiedl, O.; Hager, T.; Sommer, A.; Müller, J.C.; Schruers, K.R.; Wiedemann, K.; Demiralay, C. Vagal effects of endocrine HPA axis challenges on resting autonomic activity assessed by heart rate variability measures in healthy humans. Psychoneuroendocrinology 2019, 102, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Pulopulos, M.M.; Vanderhasselt, M.A.; De Raedt, R. Association between changes in heart rate variability during the anticipation of a stressful situation and the stress-induced cortisol response. Psychoneuroendocrinology 2018, 94, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Adlan, A.M.; Veldhuijzen van Zanten, J.; Lip, G.Y.H.; Paton, J.F.R.; Kitas, G.D.; Fisher, J.P. Acute hydrocortisone administration reduces cardiovagal baroreflex sensitivity and heart rate variability in young men. J. Physiol. 2018, 596, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Nonell, A.; Bodenseh, S.; Lederbogen, F.; Kopf, D.; Hamann, B.; Gilles, M.; Deuschle, M. Chronic but not acute hydrocortisone treatment shifts the response to an orthostatic challenge towards parasympathetic activity. Neuroendocrinology 2005, 81, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Liñares, L.; Vila, X.A.; Méndez, A.J.; Lado, M.J.; Olivieri, D. R-HRV: An R-based software package for Heart Rate Variability analysis of ECG recordings. In Proceedings of the 3rd Iberian Conference in Systems and Information Technologies, Vigo, Spain, 19–21 June 2008; pp. 565–574. [Google Scholar]
- Yentes, J.M.; Hunt, N.; Schmid, K.K.; Kaipust, J.P.; McGrath, D.; Stergiou, N. The appropriate use of approximate entropy and sample entropy with short data sets. Ann. Biomed. Eng. 2013, 41, 349–365. [Google Scholar] [CrossRef]
- Pincus, S. A regularity statistic for medical data analysis. J. Clin. Monit. 1991, 7, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Sassi, R.; Cerutti, S.; Lombardi, F.; Malik, M.; Huikuri, H.V.; Peng, C.K.; Schmidt, G.; Yamamoto, Y. Advances in heart rate variability signal analysis: Joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace 2015, 17, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002, 89, 068102. [Google Scholar] [CrossRef]
- Heffernan, K.S.; Fahs, C.A.; Shinsako, K.K.; Jae, S.Y.; Fernhall, B. Heart rate recovery and heart rate complexity following resistance exercise training and detraining in young men. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3180–H3186. [Google Scholar] [CrossRef]
- Kennel, M.B.; Brown, R.; Abarbanel, H.D. Determining embedding dimension for phase-space reconstruction using a geometrical construction. Phys. Rev. A 1992, 45, 3403–3411. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.; Morari, M. The false nearest neighbors algorithm: An overview. Comput. Chem. Eng. 1997, 21, 1149–1154. [Google Scholar] [CrossRef]
- Hussain, V.S.; Spano, M.L.; Lockhart, T.E. Effect of data length on time delay and embedding dimension for calculating the Lyapunov exponent in walking. J. R. Soc. Interface 2020, 17, 20200311. [Google Scholar] [CrossRef] [PubMed]
- Theiler, J.; Eubank, S.; Longtin, A.; Galdrikian, B.; Farmer, J.D. Testing for nonlinearity in time series: The method of surrogate data. Phys. D 1992, 58, 77–94. [Google Scholar] [CrossRef]
- Hurst, H.E. The Problem of Long-Term Storage in Reservoirs. Int. Assoc. Sci. Hydrology. Bull. 1956, 1, 13–27. [Google Scholar] [CrossRef]
- Weron, R. Estimating long-range dependence: Finite sample properties and confidence intervals. Phys. A 2002, 312, 285–299. [Google Scholar] [CrossRef]
- Webber, C.L.; Zbilut, J.P. Recurrence quantification analysis of nonlinear dynamical systems. Tutor. Contemp. Nonlinear Methods Behav. Sci. 2005, 94, 26–94. [Google Scholar]
- Coco, M.I.; Dale, R. Cross-recurrence quantification analysis of categorical and continuous time series: An R package. Front. Psychol. 2014, 5, 510. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed]
- Zbilut, J.P.; Giuliani, A.; Webber, C.L. Detecting deterministic signals in exceptionally noisy environments using cross-recurrence quantification. Phys. Lett. A 1998, 246, 122–128. [Google Scholar] [CrossRef]
- Shockley, K.; Butwill, M.; Zbilut, J.P.; Webber, C.L. Cross recurrence quantification of coupled oscillators. Phys. Lett. A 2002, 305, 59–62. [Google Scholar] [CrossRef]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Kanaley, J.A.; Weltman, J.Y.; Veldhuis, J.D.; Rogol, A.D.; Hartman, M.L.; Weltman, A. Human growth hormone response to repeated bouts of aerobic exercise. J. Appl. Physiol. 1997, 83, 1756–1761. [Google Scholar] [CrossRef] [PubMed]
- Wideman, L.; Weltman, J.Y.; Hartman, M.L.; Veldhuis, J.D.; Weltman, A. Growth hormone release during acute and chronic aerobic and resistance exercise. Sports Med. 2002, 32, 987–1004. [Google Scholar] [CrossRef] [PubMed]
- Weltman, A.; Weltman, J.Y.; Watson Winfield, D.D.; Frick, K.; Patrie, J.; Kok, P.; Keenan, D.M.; Gaesser, G.A.; Veldhuis, J.D. Effects of continuous versus intermittent exercise, obesity, and gender on growth hormone secretion. J. Clin. Endocrinol. Metab. 2008, 93, 4711–4720. [Google Scholar] [CrossRef] [PubMed]
- Buchman, T.G. Multiple organ dysfunction syndrome. In Surgery; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Berry, N.T.; Wideman, L.; Rhea, C.K. Variability and Complexity of Non-stationary Functions: Methods for Post-exercise HRV. Nonlinear Dyn. Psychol. Life Sci. 2020, 24, 367–387. [Google Scholar]
- Bonnemeier, H.; Weigand, U.K.H.; Brandes, A.; Kluge, N.; Katus, H.A.; Richardt, G.; Potratz, J. Circadian Profile of Cardiac Autonomic Nervous Modulation in Healthy Subjects: Differing Effects of Aging and Gender on Heart Rate Variability. J. Cardiovasc. Electrophysiol. 2003, 14, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Berry, N.T.; Wideman, L.; Rhea, C.K.; Labban, J.; Chon, K.H.; Shykoff, B.E.; Haran, F.J.; Florian, J.P. Effects of prolonged and repeated immersions of heart rate variability and complexity in military divers. Undersea Hyperb. Med. 2017, 44, 589–600. [Google Scholar] [CrossRef]
- Weltman, A.; Weltman, J.Y.; Hartman, M.L.; Abbott, R.D.; Rogol, A.D.; Evans, W.S.; Veldhuis, J.D. Relationship between age, percentage body fat, fitness, and 24-hour growth hormone release in healthy young adults: Effects of gender. J. Clin. Endocrinol. Metab. 1994, 78, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Kanaley, J.A.; Weatherup-Dentes, M.M.; Jaynes, E.B.; Hartman, M.L. Obesity attenuates the growth hormone response to exercise. J. Clin. Endocrinol. Metab. 1999, 84, 3156–3161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frystyk, J. Exercise and the growth hormone-insulin-like growth factor axis. Med. Sci. Sports Exerc. 2010, 42, 58–66. [Google Scholar] [CrossRef]
- Nindl, B.C.; Pierce, J.R.; Rarick, K.R.; Tuckow, A.P.; Alemany, J.A.; Sharp, M.A.; Kellogg, M.D.; Patton, J.F. Twenty-hour growth hormone secretory profiles after aerobic and resistance exercise. Med. Sci. Sports Exerc. 2014, 46, 1917–1927. [Google Scholar] [CrossRef]
- Hirako, S.; Wada, N.; Kageyama, H.; Takenoya, F.; Izumida, Y.; Kim, H.; Iizuka, Y.; Matsumoto, A.; Okabe, M.; Kimura, A.; et al. Autonomic nervous system-mediated effects of galanin-like peptide on lipid metabolism in liver and adipose tissue. Sci. Rep. 2016, 6, 21481. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; He, B.; Shi, M.; Zhu, Y.; Bo, P.; Zhang, Z. Crosstalk between exercise and galanin system alleviates insulin resistance. Neurosci. Biobehav. Rev. 2015, 59, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, C.; Neri, G.; Nussdorfer, G.G. Galanin in the regulation of the HPA (review). Int. J. Mollecular Med. 2007, 19, 639–647. [Google Scholar]
- Giustina, A.; Licini, M.; Schettino, M.; Doga, M.; Pizzocolo, G.; Negro-Vilar, A. Physiological role of galanin in the regulation of anterior pituitary function in humans. Am. J. Physiol. Endocrinol. Metab. 1994, 266, E57–E61. [Google Scholar] [CrossRef]
- Sandoval-Alzate, H.F.; Agudelo-Zapata, Y.; Gonzalez-Clavijo, A.M.; Poveda, N.E.; Espinel-Pachon, C.F.; Escamilla-Castro, J.A.; Marquez-Julio, H.L.; Alvarado-Quintero, H.; Rojas-Rodriguez, F.G.; Arteaga-Diaz, J.M.; et al. Serum Galanin Levels in Young Healthy Lean and Obese Non-Diabetic Men during an Oral Glucose Tolerance Test. Sci. Rep. 2016, 6, 31661. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Shi, M.; Zhu, Y.; Bo, P.; Zhang, Z. Type 2 diabetes mellitus as a disorder of galanin resistance. Exp. Gerontol. 2016, 73, 72–77. [Google Scholar] [CrossRef]
- Mogharnasi, M.; TaheriChadorneshin, H.; Papoli-Baravati, S.A.; Teymuri, A. Effects of upper-body resistance exercise training on serum nesfatin-1 level, insulin resistance, and body composition in obese paraplegic men. Disabil. Health J. 2019, 12, 29–34. [Google Scholar] [CrossRef]
- Li, Q.C.; Wang, H.Y.; Chen, X.; Guan, H.Z.; Jiang, Z.Y. Fasting plasma levels of nesfatin-1 in patients with type 1 and type 2 diabetes mellitus and the nutrient-related fluctuation of nesfatin-1 level in normal humans. Regul. Pept. 2010, 159, 72–77. [Google Scholar] [CrossRef]
- Dore, R.; Levata, L.; Lehnert, H.; Schulz, C. Nesfatin-1: Functions and physiology of a novel regulatory peptide. J. Endocrinol. 2017, 232, R45–R65. [Google Scholar] [CrossRef]
- Scotece, M.; Conde, J.; Abella, V.; Lopez, V.; Lago, F.; Pino, J.; Gomez-Reino, J.J.; Gualillo, O. NUCB2/nesfatin-1: A new adipokine expressed in human and murine chondrocytes with pro-inflammatory properties, an in vitro study. J. Orthop. Res. 2014, 32, 653–660. [Google Scholar] [CrossRef]
- Tanida, M.; Gotoh, H.; Yamamoto, N.; Wang, M.; Kuda, Y.; Kurata, Y.; Mori, M.; Shibamoto, T. Hypothalamic Nesfatin-1 Stimulates Sympathetic Nerve Activity via Hypothalamic ERK Signaling. Diabetes 2015, 64, 3725–3736. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.S.; Shimizu, H.; Satoh, T.; Okada, S.; Adachi, S.; Inoue, K.; Eguchi, H.; Yamamoto, M.; Imaki, T.; Hashimoto, K.; et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 2006, 443, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Konczol, K.; Bodnar, I.; Zelena, D.; Pinter, O.; Papp, R.S.; Palkovits, M.; Nagy, G.M.; Toth, Z.E. Nesfatin-1/NUCB2 may participate in the activation of the hypothalamic-pituitary-adrenal axis in rats. Neurochem. Int. 2010, 57, 189–197. [Google Scholar] [CrossRef]


| Rest | Exercise | ||
|---|---|---|---|
| Age (years) | 25.4 (±2.6) | - | |
| Height (cm) | 174.7 (±7.8) | - | |
| Body mass (kg) | 72.5 (±13.7) | 74.3 (±13.2) | |
| Body fat (%) | 9.46 (±2.88) | 10.59 (±3.8) | |
| Fat mass (kg) | 6.98 (±2.7) | 8.05 (±3.8) | |
| VO2max (mL/kg/min) | 66.9 (±8.7) | 70.1 (±10.8) | |
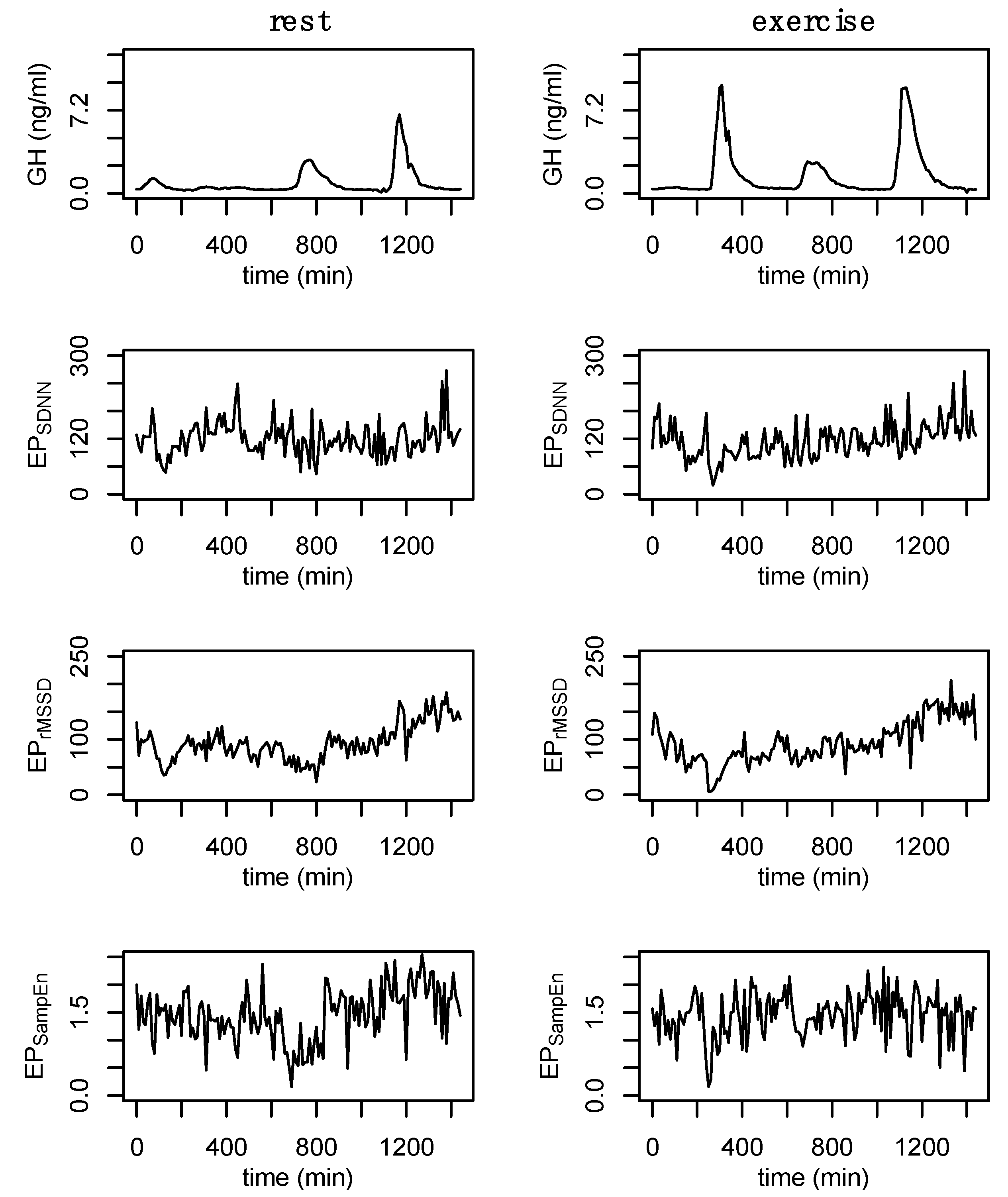
| GH | Total (24 h) (ng) | 1083.3 (±152.5) | 1596.7 (±276.9) |
| Daytime (ng) | 307.0 (±77.5) * | 735.1 (±108.1) | |
| Nighttime (ng) | 679.4 (±85.8) | 785.9 (±166.9) | |
| Exercise (ng) | 73.1 (±39.4) * | 458.1 (±91.2) | |
| Nighttime peak (ng/mL) | 5.5 (±0.9) | 5.5 (±1.4) | |
| Exercise peak (ng/mL) | 0.8 (±0.4) * | 7.8 (±1.6) | |
| Nadir (ng/mL) | 0.1 (±0.03) | 0.1 (±0.03) | |
| 24 h HRV | SDNN | 181.5 (±49.4) * | 210.9 (±42.6) |
| rMSSD | 76.2 (±35.3) | 75.9 (±36.8) | |
| SampEn | 1.61 (±0.22) * | 0.75 (±0.07) |
| Rest | Exercise | ||||||
|---|---|---|---|---|---|---|---|
| Method | Observed | Shuffle | Gaussian | Observed | Shuffle | Gaussian | |
| GH | - | 0.73 (±0.03) | 0.50 (±0.05) | 0.51 (±0.06) | 0.68 (±0.03) | 0.53 (±0.05) | 0.53 (±0.04) |
| EPSDNN | b3 | 0.68 (±0.08) | 0.52 (±0.05) | 0.53 (±0.06) | 0.68 (±0.06) | 0.48 (±0.03) | 0.53 (±0.06) |
| s3 | 0.65 (±0.09) | 0.52 (±0.04) | 0.53 (±0.04) | 0.65 (±0.07) | 0.52 (±0.05) | 0.52 (±0.06) | |
| a3 | 0.66 (±0.07) | 0.53 (±0.06) | 0.51 (±0.04) | 0.65 (±0.06) | 0.52 (±0.05) | 0.52 (±0.07) | |
| s5 | 0.67 (±0.09) | 0.52 (±0.04) | 0.52 (±0.05) | 0.67 (±0.06) | 0.54 (±0.04) | 0.51 (±0.04) | |
| EPrMSSD | b3 | 0.71 (±0.09) | 0.52 (±0.04) | 0.51 (±0.05) | 0.74 (±0.05) | 0.52 (±0.04) | 0.53 (±0.03) |
| s3 | 0.71 (±0.1) | 0.52 (±0.05) | 0.52 (±0.06) | 0.73 (±0.05) | 0.49 (±0.04) | 0.53 (±0.03) | |
| a3 | 0.71 (±0.09) | 0.52 (±0.06) | 0.53 (±0.06) | 0.73 (±0.04) | 0.51 (±0.04) | 0.51 (±0.05) | |
| s5 | 0.72 (±0.09) | 0.49 (±0.04) | 0.55 (±0.04) | 0.74 (±0.05) | 0.52 (±0.05) | 0.53 (±0.06) | |
| EPSampEn | b3 | 0.60 (±0.06) | 0.57 (±0.02) | 0.51 (±0.05) | 0.64 (±0.05) | 0.54 (±0.05) | 0.52 (±0.03) |
| s3 | 0.59 (±0.08) | 0.50 (±0.04) | 0.52 (±0.04) | 0.62 (±0.05) | 0.54 (±0.03) | 0.53 (±0.05) | |
| a3 | 0.61 (±0.08) | 0.54 (±0.03) | 0.52 (±0.04) | 0.63 (±0.05) | 0.53 (±0.04) | 0.51 (±0.05) | |
| s5 | 0.60 (±0.08) | 0.53 (±0.05) | 0.48 (±0.05) | 0.64 (±0.03) | 0.52 (±0.06) | 0.50 (±0.06) |
| Rest | Exercise | ||
|---|---|---|---|
| GH | SampEn | 0.10 (±0.03) * | 0.18 (±0.09) |
| %REC | 15.8 (±5.0) | 14.9 (±5.5) | |
| %DET | 64.1 (±8.7) | 65.8 (±21.7) | |
| NRLINE | 715.3 (±292.3) | 554.7 (±236.6) | |
| LL | 3.0 (±0.2) | 3.9 (±1.2) | |
| LAM (%) | 73.1 (±7.1) | 70.7 (±22.6) | |
| TT | 3.3 (±0.5) | 4.6 (±2.2) | |
| ENTR | 1.22 (±0.18) | 1.44 (±0.70) | |
| EPSDNN | SampEn b | 1.78 (±0.20) | 1.78 (±0.18) |
| %REC | 15.9 (±2.3) * | 14.2 (±1.1) | |
| %DET a | 35.2 (±3.0) | 33.2 (±2.1) | |
| NRLINE b | 468.4 (±101.6) | 382.1 (±58.6) | |
| LL a | 2.5 (±0.1) | 2.6 (±0.1) | |
| LAM (%) b | 44.9 (±4.1) | 39.7 (±4.8) | |
| TT | 2.5 (±0.1) | 2.4 (±0.1) | |
| ENTR a | 0.57 (±0.08) | 0.59 (±0.04) | |
| EPrMSSD | SampEn c | 1.60 (±0.52) | 1.60 (±0.26) |
| %REC c | 16.0 (±2.8) * | 14.3 (±0.7) | |
| %DET a,c | 41.5 (±13.8) | 40.0 (±6.5) | |
| NRLINE c | 536.4 (±240.9) | 456.4 (±82.2) | |
| LL a | 2.7 (±0.2) | 2.7 (±0.1) | |
| LAM (%) c | 47.9 (±17.9) | 49.6 (±6.9) | |
| TT c | 2.6 (±0.5) | 2.5 (±0.2) | |
| ENTR a,c | 0.74 (±0.31) | 0.73 (±0.14) | |
| EPSampEn | SampEn b,c | 2.06 (±0.34) | 2.16 (±0.43) |
| %REC c | 13.6 (±0.4) | 13.6 (±0.5) | |
| %DET c | 28.9 (±1.9) | 29.5 (±3.2) | |
| NRLINE b,c | 317.3 (±27.3) | 325.3 (±49.5) | |
| LL | 2.6 (±0.1) | 2.61 (±0.1) | |
| LAM (%) b,c | 32.7 (±6.6) | 33.6 (±5.0) | |
| TT c | 2.3 (±0.1) | 2.3 (±0.1) | |
| ENTR c | 0.46 (±0.08) | 0.48 (±0.11) |
| Rest | Exercise | ||
|---|---|---|---|
| GH-EPSDNN | Cross-SampEn | 1.80 (±0.25) † | 1.61 (±0.22) a |
| %REC | 4.3 (0.8) b | 4.6 (0.7) a | |
| %DET | 43.4 (6.0) | 48.4 (6.3) a | |
| NRLINE | 169.6 (46.5) b | 196.6 (43.5) a | |
| LLMax a | 4.9 (0.9) | 5.3 (1) | |
| LL a | 2.3 (0.1) | 2.4 (0.1) | |
| LAM a | 35.5 (7.6) | 40.7 (10.7) | |
| TT a | 2.3 (0.2) | 2.4 (0.2) | |
| ENTR a | 0.66 (0.15) | 0.79 (0.15) | |
| GH-EPrMSSD | Cross-SampEn | 1.67 (±0.27) * | 1.23 (±0.25) a,c |
| %REC | 4.5 (1.0) c,* | 6.8 (1.5) a | |
| %DET | 47.8 (7.5) * | 59.7 (9.4) a,c | |
| NRLINE | 180.7 (55.7) c,* | 316.3 (82.4) a,c | |
| LLMax a | 6.1 (0.7) * | 8.0 (1.6) | |
| LL a,c | 2.5 (0.1) | 2.7 (0.3) | |
| LAM a | 39.5 (11.2) * | 55.5 (10.1) | |
| TT a,c | 2.5 (0.1) | 2.8 (0.4) | |
| ENTR a,c | 0.91 (0.15) | 1.05 (0.25) | |
| GH-EPSampEn | Cross-SampEn | 1.58 (±0.20) | 1.52 (±0.13) c |
| %REC | 6.3 (1.3) | 5.9 (0.5) | |
| %DET | 47.9 (6.6) | 47.9 (5.5) c | |
| NRLINE | 269.6 (88.5) b,c | 239.4 (40.2) c | |
| LLmax | 6 (1.5) | 6.3 (1) | |
| LL c | 2.4 (0.1) | 2.4 (0.1) | |
| LAM | 38.3 (12.5) | 40.8 (8.6) | |
| TT c | 2.3 (0.2) | 2.4 (0.1) | |
| ENTR c | 0.77 (0.16) | 0.87 (0.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berry, N.T.; Rhea, C.K.; Wideman, L. Cardio-Hypothalamic-Pituitary Coupling during Rest and in Response to Exercise. Entropy 2022, 24, 1045. https://doi.org/10.3390/e24081045
Berry NT, Rhea CK, Wideman L. Cardio-Hypothalamic-Pituitary Coupling during Rest and in Response to Exercise. Entropy. 2022; 24(8):1045. https://doi.org/10.3390/e24081045
Chicago/Turabian StyleBerry, Nathaniel T., Christopher K. Rhea, and Laurie Wideman. 2022. "Cardio-Hypothalamic-Pituitary Coupling during Rest and in Response to Exercise" Entropy 24, no. 8: 1045. https://doi.org/10.3390/e24081045
APA StyleBerry, N. T., Rhea, C. K., & Wideman, L. (2022). Cardio-Hypothalamic-Pituitary Coupling during Rest and in Response to Exercise. Entropy, 24(8), 1045. https://doi.org/10.3390/e24081045






