Studying the Effect of Cold Rolling and Heat Treatment on the Microstructure and Mechanical Properties of the Fe36Mn20Ni20Cr16Al5Si3 High Entropy Alloy
Abstract
:1. Introduction
2. Experimental Work
3. Results and Discussions
3.1. ThermoCalc® Analysis and Phase Formation
3.2. Microstructure Characterization
3.3. Mechanical Properties
4. Conclusions
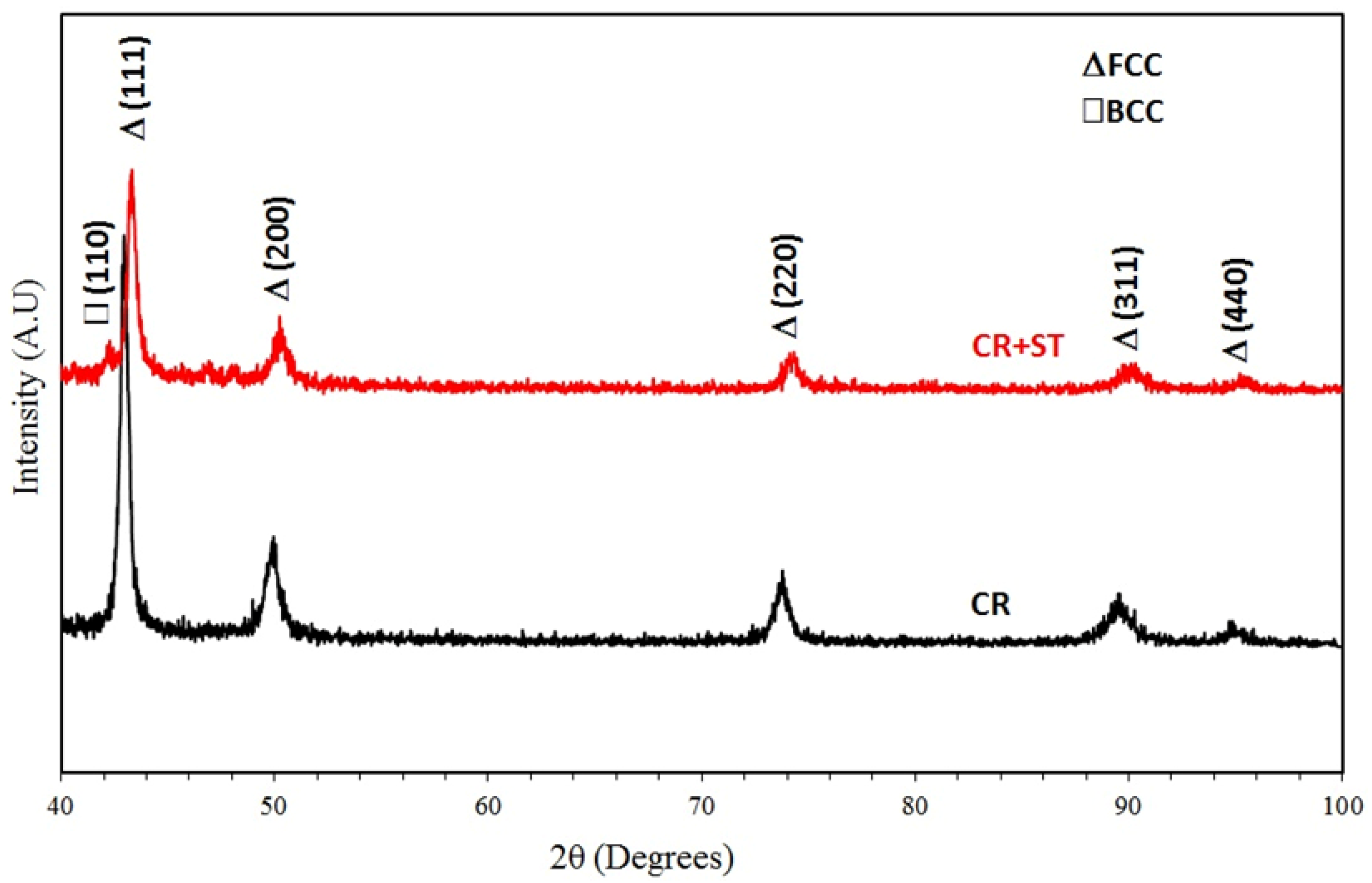
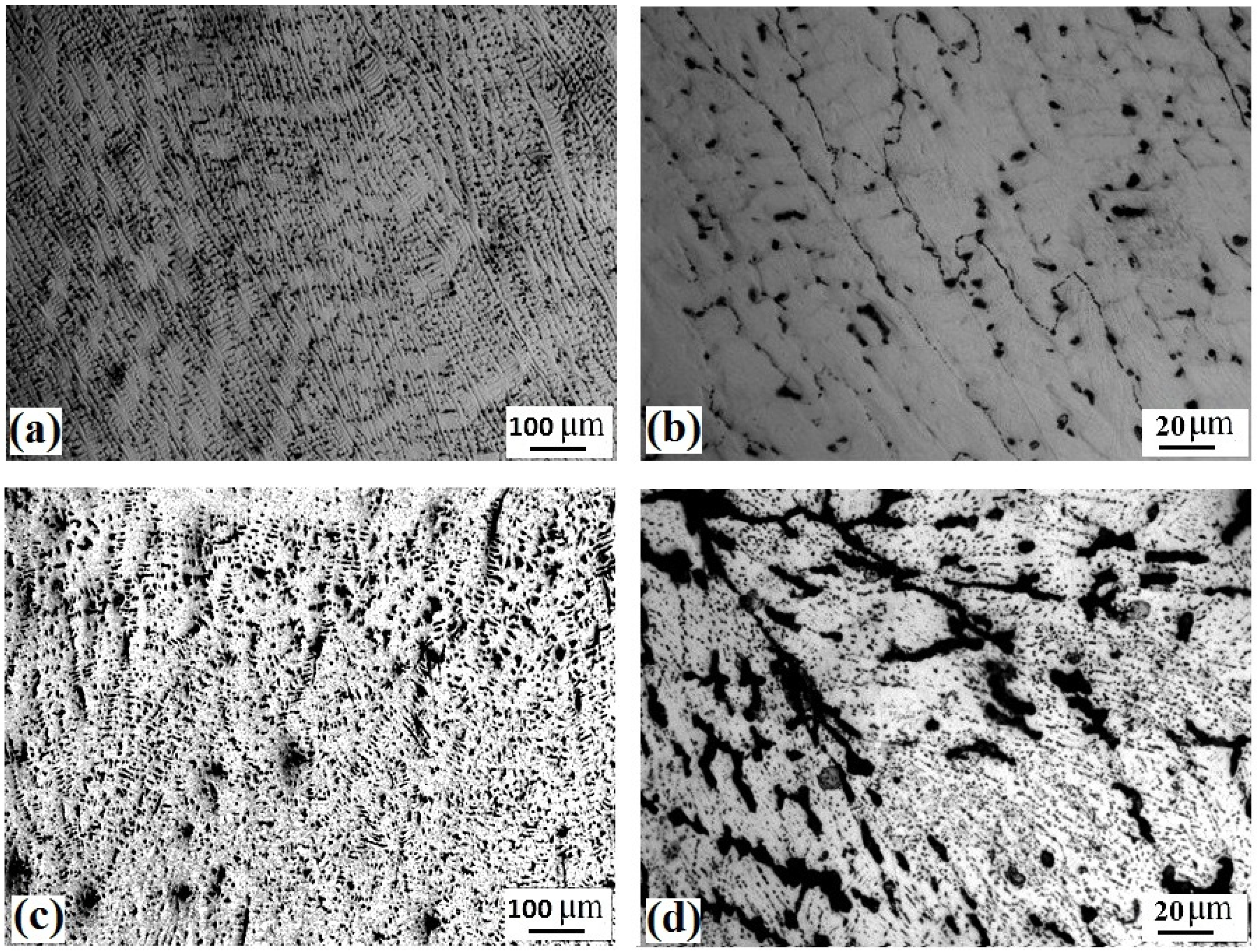
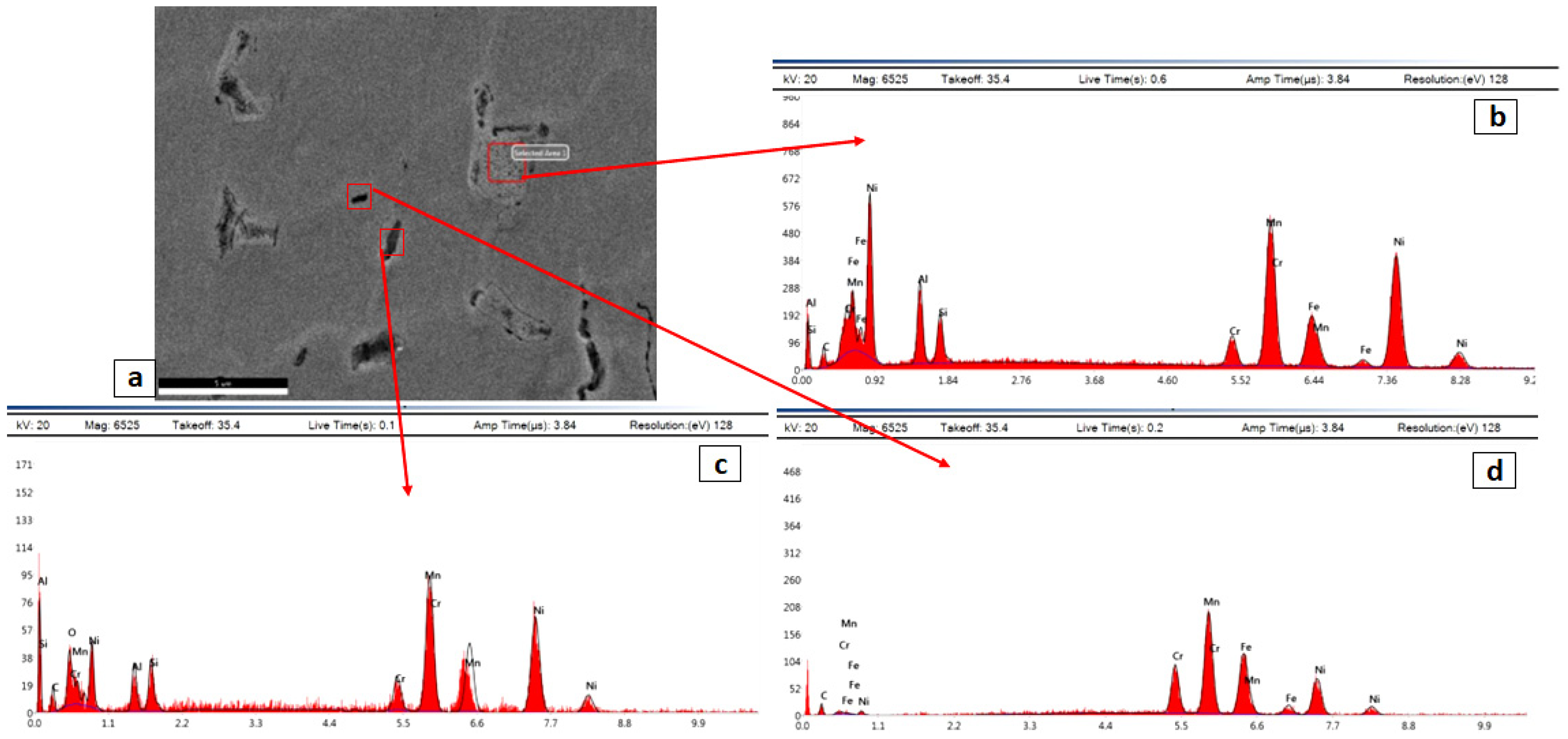
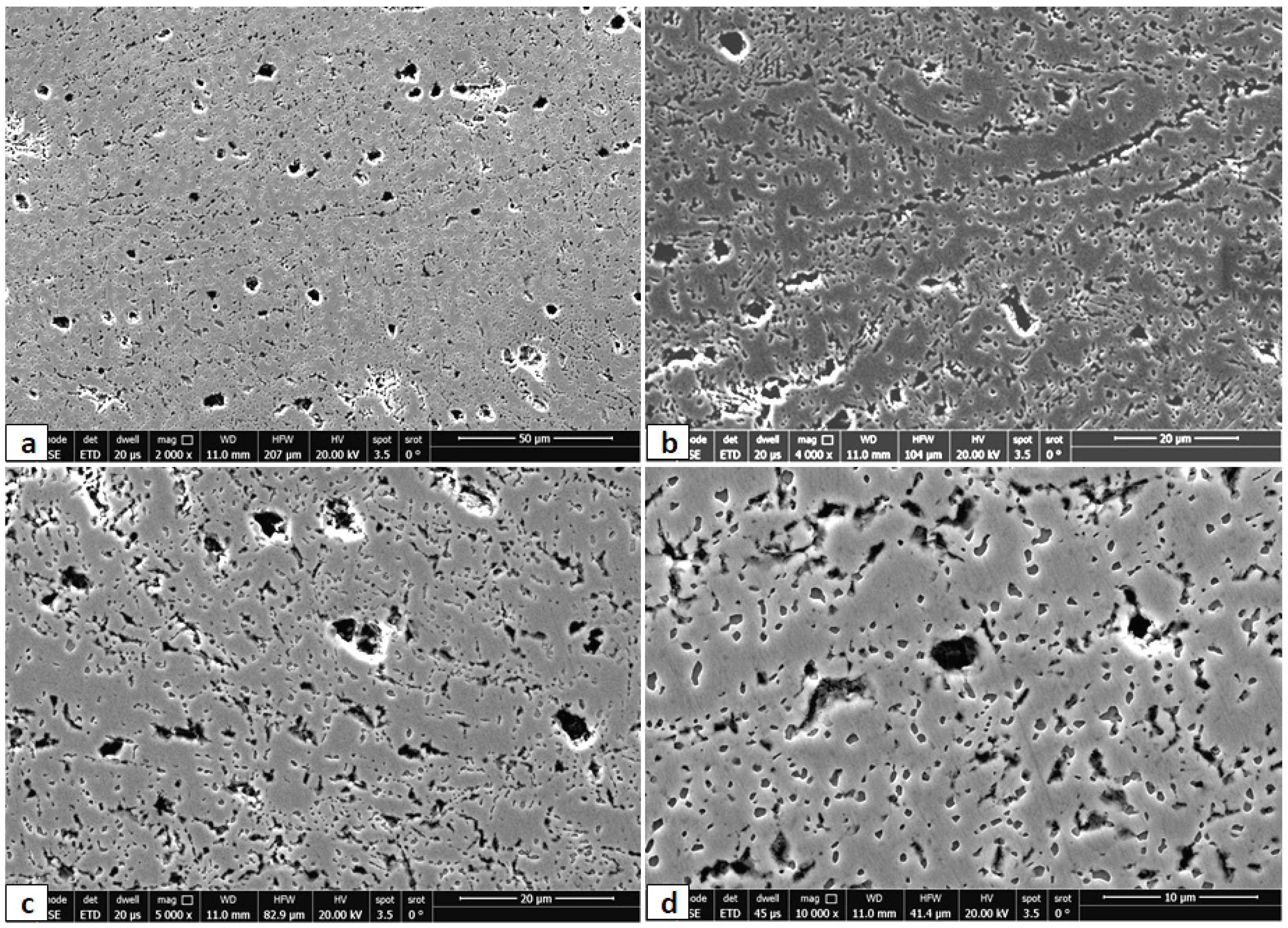
- The as-rolled Fe36Mn20Ni20Cr16Al5Si3 HEA was composed of an elongated FCC phase with evident dendritic structure. Very few amounts of the BCC phase were precipitated in the grain boundaries. These experimentally observed phases are in good agreement with the predictions of ThermoCalc® software.
- By applying annealing treatment to the as-rolled Fe36Mn20Ni20Cr16Al5Si3 HEA, different sizes and features of BCC phases and intermetallics were found in higher ratio throughout the structure.
- Higher compressive yield strength of about 950 MPa was achieved in the as-rolled condition, although the annealed sample lost almost half of its strength.
- The wear resistance of the annealed condition was significantly improved at room and higher temperature. The brittle phases in the annealed condition were found to have a positive impact on the wear resistance.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, H.Y.; Yao, K.F. High Entropy Ti20Zr20Cu20Ni20Be20 Bulk Metallic Glass. J. Non. Cryst. Solids 2013, 364, 9–12. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-Entropy Alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical Properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 Refractory High Entropy Alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A Fracture-Resistant High-Entropy Alloy for Cryogenic Applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Youssef, K.M.; Zaddach, A.J.; Niu, C.; Irving, D.L.; Koch, C.C. A Novel Low-Density, High-Hardness, High-Entropy Alloy with Close-Packed Single-Phase Nanocrystalline Structures. Mater. Res. Lett. 2014, 3, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhou, X.; Wang, W.; Liu, B.; Lv, Y.; Yang, W.; Xu, D.; Liu, Y. A Review on Fundamental of High Entropy Alloys with Promising High–Temperature Properties. J. Alloys Compd. 2018, 760, 15–30. [Google Scholar] [CrossRef]
- Karati, A.; Guruvidyathri, K.; Hariharan, V.S.; Murty, B.S. Thermal Stability of AlCoFeMnNi High-Entropy Alloy. Scr. Mater. 2019, 162, 465–467. [Google Scholar] [CrossRef]
- Koželj, P.; Vrtnik, S.; Jelen, A.; Jazbec, S.; Jagličić, Z.; Maiti, S.; Feuerbacher, M.; Steurer, W.; Dolinšek, J. Discovery of a Superconducting High-Entropy Alloy. Phys. Rev. Lett. 2014, 113, 107001. [Google Scholar] [CrossRef]
- Lee, C.P.; Chen, Y.Y.; Hsu, C.Y.; Yeh, J.W.; Shih, H.C. The Effect of Boron on the Corrosion Resistance of the High Entropy Alloys Al0.5CoCrCuFeNiBx. J. Electrochem. Soc. 2007, 154, C424. [Google Scholar] [CrossRef]
- He, J.Y.; Liu, W.H.; Wang, H.; Wu, Y.; Liu, X.J.; Nieh, T.G.; Lu, Z.P. Effects of Al Addition on Structural Evolution and Tensile Properties of the FeCoNiCrMn High-Entropy Alloy System. Acta Mater. 2014, 62, 105–113. [Google Scholar] [CrossRef]
- Ma, E.; Wu, X. Tailoring Heterogeneities in High-Entropy Alloys to Promote Strength–Ductility Synergy. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-Entropy Alloy: Challenges and Prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miller, J.D.; Miracle, D.B.; Woodward, C. Accelerated Exploration of Multi-Principal Element Alloys with Solid Solution Phases. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. The Generalized Thermodynamic Rule for Phase Selection in Multicomponent Alloys. Intermetallics 2015, 59, 75–80. [Google Scholar] [CrossRef]
- Miracle, D.B.; Miller, J.D.; Senkov, O.N.; Woodward, C.; Uchic, M.D.; Tiley, J. Exploration and Development of High Entropy Alloys for Structural Applications. Entropy 2014, 16, 494–525. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C.; Miracle, D.B. Low-Density, Refractory Multi-Principal Element Alloys of the Cr-Nb-Ti-V-Zr System: Microstructure and Phase Analysis. Acta Mater. 2013, 61, 1545–1557. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Shaysultanov, D.G.; Salishchev, G.A.; Tikhonovsky, M.A. Structure and Mechanical Properties of a Light-Weight AlNbTiV High Entropy Alloy. Mater. Lett. 2015, 142, 153–155. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, Y.; Qiao, Y.; Chen, G.L. Novel Microstructure and Properties of Multicomponent CoCrCuFeNiTix Alloys. Intermetallics 2007, 15, 357–362. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. Design of High Entropy Alloys: A Single-Parameter Thermodynamic Rule. Scr. Mater. 2015, 104, 53–55. [Google Scholar] [CrossRef]
- Nene, S.S.; Frank, M.; Agrawal, P.; Sinha, S.; Liu, K.; Shukla, S.; Mishra, R.S.; McWilliams, B.A.; Cho, K.C. Microstructurally Flexible High Entropy Alloys: Linkages between Alloy Design and Deformation Behavior. Mater. Des. 2020, 194, 108968. [Google Scholar] [CrossRef]
- Nene, S.S.; Frank, M.; Liu, K.; Sinha, S.; Mishra, R.S.; McWilliams, B.A.; Cho, K.C. Corrosion-Resistant High Entropy Alloy with High Strength and Ductility. Scr. Mater. 2019, 166, 168–172. [Google Scholar] [CrossRef]
- Hong, S.I.; Moon, J.; Hong, S.K.; Kim, H.S. Thermally Activated Deformation and the Rate Controlling Mechanism in CoCrFeMnNi High Entropy Alloy. Mater. Sci. Eng. A 2017, 682, 569–576. [Google Scholar] [CrossRef]
- Ma, S.G. Creep Resistance and Strain-Rate Sensitivity of a CoCrFeNiAl0.3 High-Entropy Alloy by Nanoindentation. Mater. Res. Express 2019, 6, 126508. [Google Scholar] [CrossRef]
- Ebied, S.M.; Gepreel, M.A.; Hamada, A. Microstructural Evolution of Two Binary β-Titanium Alloys during Cold Deformation. IOP Conf. Ser. Mater. Sci. Eng. 2017, 201, 012047. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Song, W.; Shan, F.; Song, K.; Zhang, K.; Peng, C.; Sun, H.; Hu, L. Phase Formation, Texture Evolutions, and Mechanical Behaviors of Al0.5CoCr0.8FeNi2.5V0.2 High-Entropy Alloys upon Cold Rolling. Prog. Nat. Sci. Mater. Int. 2022, 32, 196–205. [Google Scholar] [CrossRef]
- Zou, Y.; Li, S.; Liu, S.; Li, J.; Li, Y. Improved Mechanical and Corrosion Properties of CrMnFeCoNi High Entropy Alloy with Cold Rolling and Post Deformation Annealing Process. J. Alloys Compd. 2021, 887, 161416. [Google Scholar] [CrossRef]
- Gouda, M.K.; Salman, S.A.; Ebied, S. Improvement in the Microhardness and Corrosion Behaviour of Ti-14Mn Biomedical Alloy by Cold Working. Mater. Res. Express 2022, 9, 015401. [Google Scholar] [CrossRef]
- Kaushik, L.; Kim, M.S.; Singh, J.; Kang, J.H.; Heo, Y.U.; Suh, J.Y.; Choi, S.H. Deformation Mechanisms and Texture Evolution in High Entropy Alloy during Cold Rolling. Int. J. Plast. 2021, 141, 102989. [Google Scholar] [CrossRef]
- He, Y.; Yang, H.; Zhao, C.; Zhang, Y.; Pan, X.; Li, J.; Wang, J. Enhancing Mechanical Properties of Al0.25CoCrFeNi High-Entropy Alloy via Cold Rolling and Subsequent Annealing. J. Alloys Compd. 2020, 830, 154645. [Google Scholar] [CrossRef]
- Mehranpour, M.S.; Shahmir, H.; Nili-ahmadabadi, M. CoCrFeNiMn High Entropy Alloy Microstructure and Mechanical Properties after Severe Cold Shape Rolling and Annealing. Mater. Sci. Eng. A 2020, 793, 139884. [Google Scholar] [CrossRef]
- Yurchenko, N.Y.; Panina, E.S.; Zherebtsov, S.V.; Tikhonovsky, M.A.; Salishchev, G.A.; Stepanov, N.D. Microstructure Evolution of a Novel Low-Density Ti–Cr–Nb–V Refractory High Entropy Alloy during Cold Rolling and Subsequent Annealing. Mater. Charact. 2019, 158, 109980. [Google Scholar] [CrossRef]
- Mahmoud, E.R.I.; Shaharoun, A.; Gepreel, M.A.; Ebied, S. Phase Prediction, Microstructure and Mechanical Properties of Fe–Mn–Ni–Cr–Al–Si High Entropy Alloys. Metals 2022, 12, 1164. [Google Scholar] [CrossRef]
- Elkatatny, S.; Gepreel, M.A.H.; Hamada, A.; Nakamura, K.; Yamanaka, K.; Chiba, A. Effect of Al Content and Cold Rolling on the Microstructure and Mechanical Properties of Al5Cr12Fe35Mn28Ni20 High-Entropy Alloy. Mater. Sci. Eng. A 2019, 759, 380–390. [Google Scholar] [CrossRef]
- Lin, K.; Chen, S.C.; Lin, H.C.; Yen, H.W. Enhancement in Mechanical Properties through an FCC-to-HCP Phase Transformation in an Fe-17.5Mn-10Co-12.5Cr-5Ni-5Si (in at%) Medium-Entropy Alloy. J. Alloys Compd. 2022, 898, 162765. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, E.R.I.; Shaharoun, A.; Gepreel, M.A.; Ebied, S. Studying the Effect of Cold Rolling and Heat Treatment on the Microstructure and Mechanical Properties of the Fe36Mn20Ni20Cr16Al5Si3 High Entropy Alloy. Entropy 2022, 24, 1040. https://doi.org/10.3390/e24081040
Mahmoud ERI, Shaharoun A, Gepreel MA, Ebied S. Studying the Effect of Cold Rolling and Heat Treatment on the Microstructure and Mechanical Properties of the Fe36Mn20Ni20Cr16Al5Si3 High Entropy Alloy. Entropy. 2022; 24(8):1040. https://doi.org/10.3390/e24081040
Chicago/Turabian StyleMahmoud, Essam R. I., Awaludin Shaharoun, Mohamed A. Gepreel, and Saad Ebied. 2022. "Studying the Effect of Cold Rolling and Heat Treatment on the Microstructure and Mechanical Properties of the Fe36Mn20Ni20Cr16Al5Si3 High Entropy Alloy" Entropy 24, no. 8: 1040. https://doi.org/10.3390/e24081040
APA StyleMahmoud, E. R. I., Shaharoun, A., Gepreel, M. A., & Ebied, S. (2022). Studying the Effect of Cold Rolling and Heat Treatment on the Microstructure and Mechanical Properties of the Fe36Mn20Ni20Cr16Al5Si3 High Entropy Alloy. Entropy, 24(8), 1040. https://doi.org/10.3390/e24081040





