Reaction Mechanism of CO2 with Choline-Amino Acid Ionic Liquids: A Computational Study
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Choline–Anion Ion Pairs
3.2. Reaction of Anions with
3.3. Formation of Carbamic Derivatives for the Glycinate and L-Phenylalanilate Anions
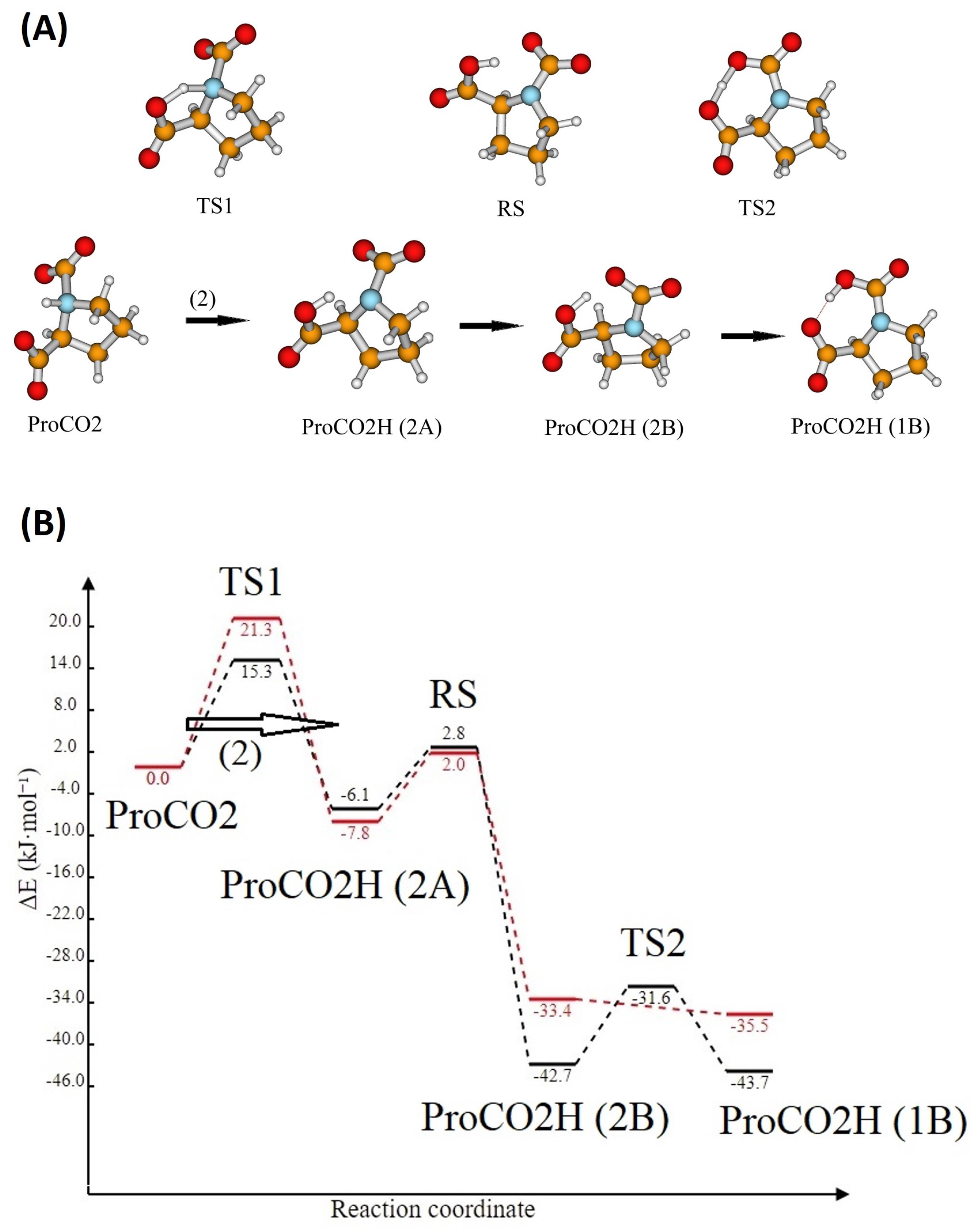
3.4. Formation of Carbamic Derivatives for the L-prolinate Anions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Popp, M.; Schmidt, H.; Marotzke, J. Transition to a Moist Greenhouse with CO2 and solar forcing. Nat. Commun. 2016, 7, 10627–10636. [Google Scholar] [CrossRef] [PubMed]
- Meinshausen, M.; Meinshausen, N.; Hare, W.; Raper, S.C.B.; Frieler, K.; Knutti, R.; Frame, D.J.; Allen, M.R. Greenhouse-gas emission targets for limiting global warming to 2 °C. Nature 2009, 58, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Woodhead Publishing: Sawston, UK, 2020; Chapter 1; pp. 3–28. [Google Scholar]
- Li, B.; Duan, Y.; Luebke, D.; Morreale, B. Advances in CO2 capture technology: A patent review. Appl. Energy 2013, 102, 1439–1447. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shahb, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent Advances in Solid Sorbents for CO2 Capture and New Development Trends. Energy Environ. Sci. 2014, 11, 3478–3518. [Google Scholar] [CrossRef]
- Shen, M.; Tong, L.; Yin, S.; Liu, C.; Wang, L.; Feng, W.; Ding, Y. Cryogenic technology progress for CO2 capture under carbon neutrality goals: A review. Sep. Pur. Techn. 2022, 299, 121734–121740. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-Based CO2 Capture Technology Development from the Beginning of 2013-A Review. ACS Appl. Mater. Interfaces 2015, 7, 2137–2148. [Google Scholar] [CrossRef]
- Yamada, H. Amine-based capture of CO2 for utilization and storage. Polim. J. 2021, 53, 93–101. [Google Scholar] [CrossRef]
- Shao, R.; Stangeland, A. Amines Used in CO2 Capture–Health and Environmental Impacts; The Bellona Foundation: Oslo, Norway, 2009. [Google Scholar]
- Nematollahi, M.H.; Carvalho, P.J. Green solvents for CO2 capture. Curr. Opin. Green Sust. Chem. 2019, 18, 25–30. [Google Scholar] [CrossRef]
- Gabriela, M.E.; Nela, S.; Gabriela, P.D.; Florian, D.C. Novel Technology for CO2 Capture Using Green Solvents. In Proceedings of the 10th International Conference on ENERGY and ENVIRONMENT (CIEM), Bucharest, Romania, 14–15 October 2021; pp. 1–5. [Google Scholar]
- Yang, Z.Z.; Zhao, Y.N.; He, L.N. CO2 chemistry: Task-specific ionic liquids for CO2 capture/activation and subsequent conversion. RSC Adv. 2011, 1, 545–567. [Google Scholar] [CrossRef]
- Sarmad, S.; Mikkola, J.P.; Ji, X. Carbon Dioxide Capture with Ionic Liquids and Deep Eutectic Solvents: A New Generation of Sorbents. ChemSusChem 2017, 10, 324–352. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Wang, J.; Zhang, S. Active chemisorption sites in functionalized ionic liquids for carbon capture. Chem. Soc. Rev. 2016, 45, 4307–4339. [Google Scholar] [CrossRef]
- Shukla, S.K.; Khokarale, S.G.; Bui, T.Q.; Mikkola, J.P.T. Ionic Liquids: Potential Materials for Carbon Dioxide Capture and Utilization. Front. Mater. 2019, 6, 42. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, X.; Bai, L.; Zhang, X.; Wang, H.; Wang, J.; Bao, D.; Li, M.; Liu, X.; Zhang, S. Ionic-Liquid-Based CO2 Capture Systems: Structure, Interaction and Process. Chem. Rev. 2017, 117, 9625–9673. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nature 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H., Jr. CO2 Capture by a Task-Specific Ionic Liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Kasprzak, D.J.; Junk, C.P.; Yokozeki, A. A Phase Behavior of Carbon Dioxide + [bmim][Ac] Mixtures. J. Chem. Thermodyn. 2008, 40, 25–31. [Google Scholar] [CrossRef]
- Jessop, P.G.; Heldebrant, D.J.; Li, X.; Eckert, C.A.; Liotta, C.L. Green Chemistry: Reversible Nonpolar-to-polar Solvent. Nature 2005, 436, 1102. [Google Scholar] [CrossRef]
- Gurkan, B.; Goodrich, B.F.; Mindrup, E.M.; Ficke, L.E.; Massel, M.; Seo, S.; Senftle, T.P.; Wu, H.; Glaser, M.F.; Shah, J.K.; et al. Molecular Design of High Capacity, Low Viscosity, Chemically Tunable Ionic Liquids for CO2 Capture. J. Phys. Chem. Lett. 2010, 1, 3494–3499. [Google Scholar] [CrossRef]
- Wang, C.; Luo, H.; Luo, X.; Li, H.; Dai, S. Equimolar CO2 Capture by Imidazolium-based Ionic Liquids and Superbase Systems. Green Chem. 2010, 12, 2019–2023. [Google Scholar] [CrossRef]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Fukumoto, K. Amino acid ionic liquids. Acc. Che. Rev. 2007, 40, 1122–1129. [Google Scholar] [CrossRef]
- Saravanamurugan, S.; Kunov-Kruse, A.J.; Fehrmann, R.; Riisager, A. Amine-Functionalized Amino Acid-based Ionic Liquids as Efficient and High-Capacity Absorbents for CO2. ChemSusChem 2014, 7, 897–902. [Google Scholar] [CrossRef]
- Gurkan, B.E.; Fuente, J.C.d.; Mindrup, E.M.; Ficke, L.E.; Goodrich, B.F.; Price, E.A.; Schneider, W.F.; Brennecke, J.F. Equimolar CO2 Absorption by Anion-Functionalized Ionic Liquids. J. Am. Chem. Soc. 2010, 132, 2116–2117. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Z.; Bao, Z.; Zhang, Z.; Yang, Y.; Ren, Q.; Xing, H.; Dai, S. New Insights into CO2 Absorption Mechanisms with Amino-Acid Ionic Liquids. ChemSusChem 2016, 9, 806–812. [Google Scholar] [CrossRef]
- Kirchhecker, S.; Esposito, D. Amino acid based ionic liquids: A green and sustainable perspective. Curr. Opin. Green Sustain. Chem. 2016, 2, 28–33. [Google Scholar] [CrossRef]
- Noorani, N.; Mehrdad, A. Experimental and theoretical study of CO2 sorption in biocompatible and biodegradable cholinium-based ionic liquids. J. Sep. Pur. Technol. 2021, 254, 117609–117616. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Zhang, Y.Y.; Yuan, Z.H.; Ji, X.Y.; Liu, C.; Yangt, Z.H.; Lu, X.H. Thermodynamic study for gas absorption in choline-2-pyrrollidine-carboxylic acid + polyethylene glycol. J. Chem. Eng. Data. 2016, 61, 3428–3437. [Google Scholar] [CrossRef]
- Latini, G.; Signorile, M.; Rosso, F.; Fin, A.; d’Amora, M.; Giordani, S.; Pirri, F.; Crocella, V.; Bordiga, S.; Bocchini, S. Efficient and reversible CO2 capture in bio-based ionic liquids solutions. J. CO2 Util. 2022, 55, 101815–101823. [Google Scholar] [CrossRef]
- Luo, X.Y.; Fan, X.; Shi, G.L.; Li, H.R.; Wang, C.M. Decreasing the Viscosity in CO2 Capture by Amino-Functionalized Ionic Liquids through the Formation of Intramolecular Hydrogen Bond. J. Phys. Chem. B 2016, 120, 2807–2813. [Google Scholar] [CrossRef] [PubMed]
- Onofri, S.; Adenusi, H.; Donne, A.L.; Bodo, E. CO2 Capture in Ionic Liquids Based on Amino Acid Anions with Protic Side Chains: A Computational Assessment of Kinetically Efficient Reaction Mechanisms. ChemistryOpen 2020, 9, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Ma, R.; Song, C.; Yang, Z.; Yu, A.; Cai, Y.; He, L.N.; Zhao, Y.N.; Yu, B.; Song, Q.W. Equimolar CO2 Capture by N-Substituted Amino Acid Salts and Subsequent Conversion. Angew. Chem. Int. Ed. 2012, 51, 11306–11310. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, Q.R.; Schneider, W.F.; Maginn, E.J. Role of Molecular Modeling in the Development of CO2–Reactive Ionic Liquids. Chem. Rev. 2018, 118, 5242–5260. [Google Scholar] [CrossRef]
- Firaha, D.S.; Kirchner, B. Tuning the Carbon Dioxide Absorption in Amino Acid Ionic Liquids. ChemSusChem 2016, 9, 1591–1599. [Google Scholar] [CrossRef]
- Onofri, S.; Bodo, E. CO2 Capture in Biocompatible Amino Acid Ionic Liquids: Exploring the Reaction Mechanisms for Bimolecular Absorption Processes. J. Phys. Chem. B 2021, 125, 5611–5619. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Karkhanechi, H.; Kamio, E.; Yoshioka, T.; Matsuyama, H. Quantum Mechanical and Molecular Dynamics Simulations of Dual-Amino-Acid Ionic Liquids for CO2 Capture. J. Phys. Chem. C 2016, 120, 27734–27745. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Ashraf, M.; AlMayef, T.; Chawl, M.; Poater, A.; Cavallo, L. Amino acid ionic liquids as potential candidates for CO2 capture: Combined density functional theory and molecular dynamics simulations. J. Chem. Phys. Lett. 2020, 745, 137239–137247. [Google Scholar] [CrossRef]
- Mercy, M.; de Leeuw, N.H.; Bell, R.G. Mechanisms of CO2 capture in ionic liquids: A computational perspective. Faraday Discuss. 2016, 192, 479–492. [Google Scholar] [CrossRef]
- Prakash, P.; Venkatnathan, A. Site-Specific Interactions in CO2 Capture by Lysinate Anion and Role of Water Using Density Functional Theory. J. Phys. Chem. C 2018, 122, 12647–12656. [Google Scholar] [CrossRef]
- Donne, A.L.; Bodo, E. Cholinium amino acid-based ionic liquids. Biophys. Rev. 2021, 13, 147–160. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Yang, Z.; Ji, X.; Lu, X. Thermodynamic study on carbon dioxide absorption in aqueous solutions of choline-based amino acid ionic liquids. Sep. Purif. Technol. 2019, 214, 128–138. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, Y.; Ji, X.; Yang, Z.; Lu, X. Experimental study of CO2 absorption in aqueous cholinium-based ionic liquids. Fluid Phase Equilibria 2017, 445, 14–24. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density functional thermochemistry. iii. the role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R. Development of the colle-salvetti correlation energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic 525 testing of four M06-class functionals and 12 other functionals. Theor. Chem. Accounts 2008, 120, 215–241. [Google Scholar]
- Walker, M.; Harvey, A.J.A.; Sen, A.; Dessent, C.E.H. Performance of M06, M06-2X, and M06-HF Density Functionals for Conformationally Flexible Anionic Clusters: M06 Functionals Perform Better than B3LYP for a Model System with Dispersion and Ionic Hydrogen-Bonding Interactions. J. Phys. Chem. A 2013, 117, 12590–12600. [Google Scholar] [CrossRef]
- Fukui, K. The path of chemical reactions—The IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Wojnarowska, Z.; Paluch, M. Recent progress on dielectric properties of protic ionic liquids. J. Phys. Condens. Matter. 2015, 27, 073202–073221. [Google Scholar] [CrossRef]
- Bennet, E.L.; Song, C.; Huang, Y.; Xiao, J. Measured relative complex permittivities for multiple series of ionic liquids. J. Mol. Liq. 2019, 294, 111571–111580. [Google Scholar] [CrossRef]
- Donne, A.L.; Adenusi, H.; Porcelli, F.; Bodo, E. Hydrogen Bonding as a Clustering Agent in Protic Ionic Liquids: Like-Charge vs Opposite-Charge Dimer Formation. ACS Omega 2018, 3, 10589–10600. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, L.; Ramondo, F.; Caminiti, R.; Campetella, M.; Luca, A.D.; Gontrani, L. Structural studies on choline-carboxylate bio-ionic liquids by X-ray scattering and molecular dynamics. J. Chem. Phys. 2015, 143, 114506–114515. [Google Scholar] [CrossRef]
- Tanzi, L.; Nardone, M.; Benassi, P.; Ramondo, F.; Caminiti, R.; Gontrani, L. Choline salicylate ionic liquid by X-ray scattering, vibrational spectroscopy and molecular dynamics. J. Mol. Liquid. 2016, 218, 39–49. [Google Scholar] [CrossRef]
- Di Muzio, S.; Ramondo, F.; Gontrani, L.; Ferella, F.; Nardone, M.; Benassi, P. Choline Hydrogen Dicarboxylate Ionic Liquids by X-ray Scattering, Vibrational Spectroscopy and Molecular Dynamics: H-Fumarate and H-Maleate and Their Conformations. Molecules 2021, 25, 4990. [Google Scholar] [CrossRef]
- Kasahara, S.; Kamio, E.; Shaikh, A.R.; Matsuki, T.; Matsuyama, H. Effect of the amino-group densities of functionalized ionic liquids on the facilitated transport properties for CO2 separation. J. Membr. Sci. 2016, 503, 148–157. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Maginn, E.J. Amine-functionalized task-specific ionic liquids: A mechanistic explanation for the dramatic increase in viscosity upon complexation with CO2 from molecular simulation. J. Am. Chem. Soc. 2008, 130, 14690–14704. [Google Scholar] [CrossRef]
- Li, W.; Wen, S.; Shen, L.; Zhang, Y.; Sun, C.; Li, S. Mechanism and Kinetic Study of Carbon Dioxide Absorption into Methyldiethanolamine/1-Hydroxyethyl-3-methylimidazolium Lysine/Water System. Energy Fuels 2018, 32, 10813–10821. [Google Scholar] [CrossRef]










| [Gly] | [Phe] | [Pro] | |
|---|---|---|---|
| −54 | −66 | −67 | |
| (PCM) | −26 (−21) | −39 (−31) | −46 (−38) |
| −48 | −37 | −44 | |
| (PCM) | −41 (−44) | −33 (−44) | −36 (−42) |
| −102 | −103 | −111 | |
| (PCM) | −67 (−65) | −72 (−75) | −82 (−80) |
| [Gly][Ch] | [Phe][Ch] | [Pro][Ch] | |
| (PCM) | −7 (−14) | −28 (−22) | −49 (−41) |
| (PCM) | −42 (−56) | −36 (−42) | −21 (−25)/−31 (−39) |
| (PCM) | −49 (−70) | −64 (−64) | −70 (−66)/−80 (−72) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramondo, F.; Di Muzio, S. Reaction Mechanism of CO2 with Choline-Amino Acid Ionic Liquids: A Computational Study. Entropy 2022, 24, 1572. https://doi.org/10.3390/e24111572
Ramondo F, Di Muzio S. Reaction Mechanism of CO2 with Choline-Amino Acid Ionic Liquids: A Computational Study. Entropy. 2022; 24(11):1572. https://doi.org/10.3390/e24111572
Chicago/Turabian StyleRamondo, Fabio, and Simone Di Muzio. 2022. "Reaction Mechanism of CO2 with Choline-Amino Acid Ionic Liquids: A Computational Study" Entropy 24, no. 11: 1572. https://doi.org/10.3390/e24111572
APA StyleRamondo, F., & Di Muzio, S. (2022). Reaction Mechanism of CO2 with Choline-Amino Acid Ionic Liquids: A Computational Study. Entropy, 24(11), 1572. https://doi.org/10.3390/e24111572





