Hemispheric Asymmetry of Functional Brain Networks under Different Emotions Using EEG Data
Abstract
1. Introduction
2. Materials and Methods
2.1. EEG Data Acquisition
2.2. Data Preprocessing
2.3. Frequency Division
2.4. Network Construction
2.5. Network Analysis
2.6. Asymmetry Score
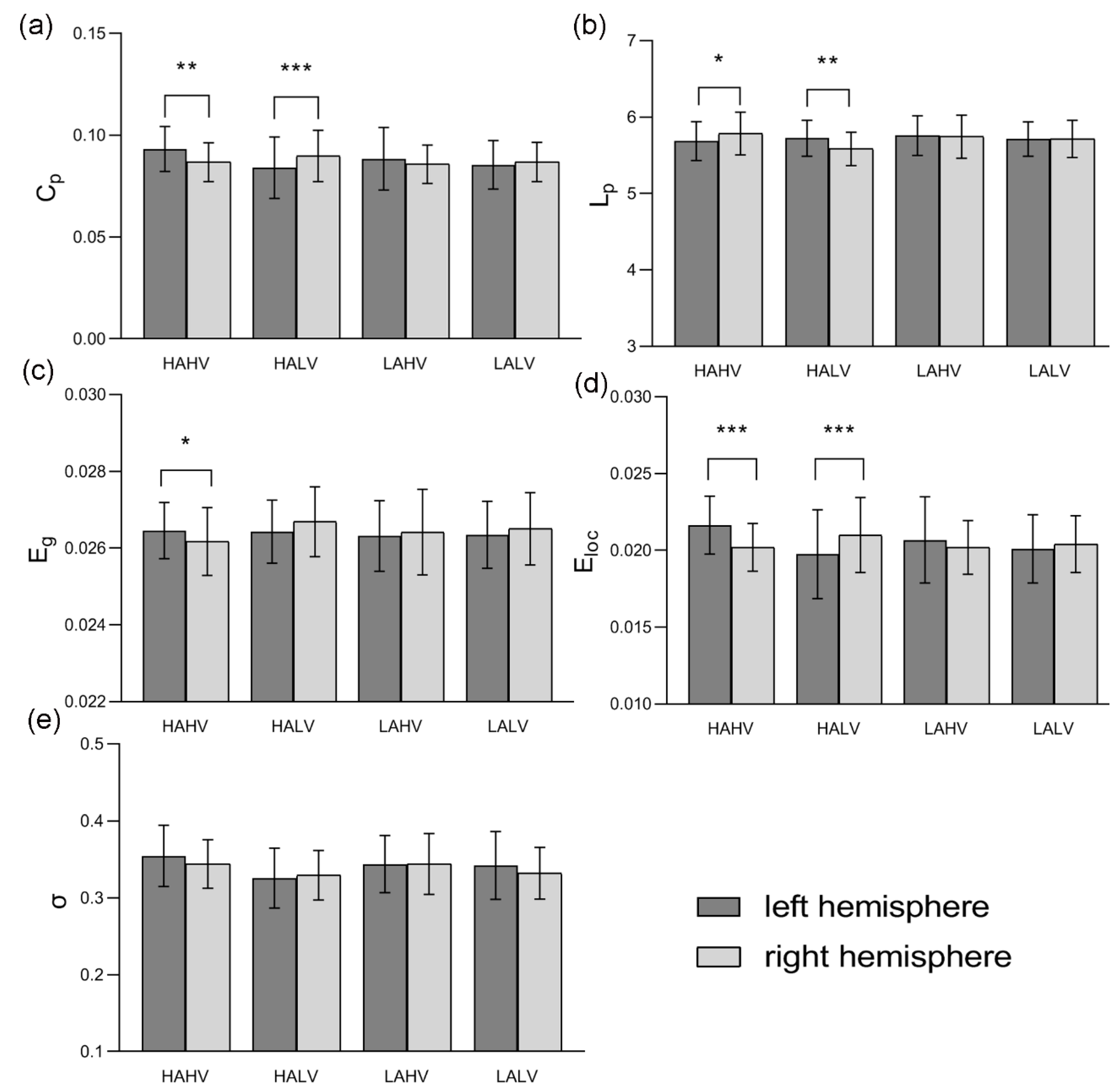
3. Results
3.1. Global Properties of Hemispheric Networks
3.2. Asymmetry Scores of Global Properties
3.3. Regional Properties of Hemispheric Networks
3.4. Asymmetry Score of Nodal Efficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Verma, G.K.; Tiwary, U.S. Multimodal fusion framework: A multiresolution approach for emotion classification and recognition from physiological signals. Neuroimage 2014, 102 Pt 1, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Koelstra, S.; Muhl, C.; Soleymani, M.; Lee, J.-S.; Yazdani, A.; Ebrahimi, T.; Pun, T.; Nijholt, A.; Patras, I. DEAP: A Database for Emotion Analysis Using Physiological Signals. IEEE Trans. Affect. Comput. 2012, 3, 18–31. [Google Scholar] [CrossRef]
- Kop, W.J.; Synowski, S.J.; Newell, M.E.; Schmidt, L.A.; Waldstein, S.R.; Fox, N.A. Autonomic nervous system reactivity to positive and negative mood induction: The role of acute psychological responses and frontal electrocortical activity. Biol. Psychol. 2011, 86, 230–238. [Google Scholar] [CrossRef]
- Alves, N.T.; Aznar-Casanova, J.A.; Fukusima, S.S. Patterns of brain asymmetry in the perception of positive and negative facial expressions. Laterality 2009, 14, 256–272. [Google Scholar] [CrossRef]
- Ahern, G.L.; Schwartz, G.E. Differential lateralization for positive and negative emotion in the human brain: EEG spectral analysis. Neuropsychologia 1985, 23, 745–755. [Google Scholar] [CrossRef]
- Balconi, M.; Mazza, G. Lateralisation effect in comprehension of emotional facial expression: A comparison between EEG alpha band power and behavioural inhibition (BIS) and activation (BAS) systems. Laterality 2010, 15, 361–384. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Ge, Y.; Zheng, Y.; Sun, X.; Zhang, K. Asymmetric hemisphere activation in tenderness: Evidence from EEG signals. Sci. Rep. 2018, 8, 8029. [Google Scholar] [CrossRef] [PubMed]
- Borod, J.C.; Cicero, B.A.; Obler, L.K.; Welkowitz, J.; Erhan, H.M.; Santschi, C.; Grunwald, I.S.; Agosti, R.M.; Whalen, J.R. Right hemisphere emotional perception: Evidence across multiple channels. Neuropsychology 1998, 12, 446–458. [Google Scholar] [CrossRef]
- Gainotti, G. Unconscious processing of emotions and the right hemisphere. Neuropsychologia 2012, 50, 205–218. [Google Scholar] [CrossRef]
- Adolphs, R.; Jansari, A.; Tranel, D. Hemispheric perception of emotional valence from facial expressions. Neuropsychology 2001, 15, 516–524. [Google Scholar] [CrossRef]
- Silberman, E.K.; Weingartner, H.J.B. Hemispheric lateralization of functions related to emotion. Brain Cognit. 1986, 5, 322–353. [Google Scholar] [CrossRef]
- Poole, B.D.; Gable, P.A. Affective motivational direction drives asymmetric frontal hemisphere activation. Exp. Brain Res. 2014, 232, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Peterburs, J.; Mertzen, J.; Schmitz, J.; Güntürkün, O.; Grimshaw, G. Effects of Emotional Valence on Hemispheric Asymmetries in Response Inhibition. Symmetry 2017, 9, 145. [Google Scholar] [CrossRef]
- Zibman, S.; Daniel, E.; Alyagon, U.; Etkin, A.; Zangen, A. Interhemispheric cortico-cortical paired associative stimulation of the prefrontal cortex jointly modulates frontal asymmetry and emotional reactivity. Brain Stimul. 2019, 12, 139–147. [Google Scholar] [CrossRef] [PubMed]
- van Straaten, E.C.W.; Stam, C.J. Structure out of chaos: Functional brain network analysis with EEG, MEG, and functional MRI. Eur. Neuropsychopharmacol. 2013, 23, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Cea-Canas, B.; Gomez-Pilar, J.; Nunez, P.; Rodriguez-Vazquez, E.; de Uribe, N.; Diez, A.; Perez-Escudero, A.; Molina, V. Connectivity strength of the EEG functional network in schizophrenia and bipolar disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 98. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Lee, B.I.; Shin, K.J.; Ha, S.Y.; Park, J.; Kim, T.H.; Mun, C.W.; Kim, S.E. Progressive topological disorganization of brain network in focal epilepsy. Acta Neurol. Scand. 2018, 137, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, V.; Radetz, A.; Ciolac, D.; Muthuraman, M.; Gonzalez-Escamilla, G.; Zipp, F.; Groppa, S. Graph Theoretical Framework of Brain Networks in Multiple Sclerosis: A Review of Concepts. Neuroscience 2019, 403, 35–53. [Google Scholar] [CrossRef]
- Avena-Koenigsberger, A.; Misic, B.; Sporns, O. Communication dynamics in complex brain networks. Nat. Rev. Neurosci. 2017, 19, 17–33. [Google Scholar] [CrossRef]
- Pessoa, L. Understanding emotion with brain networks. Curr. Opin. Behav. Sci. 2018, 19, 19–25. [Google Scholar] [CrossRef]
- Jung, T.P.; Makeig, S.; Humphries, C.; Lee, T.W.; McKeown, M.J.; Iragui, V.; Sejnowski, T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 2000, 37, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Meng, H.; Nandi, A.; Li, M. Emotion Detection from EEG Recordings. In Proceedings of the 2016 12th International Conference on Natural Computation, Fuzzy Systems and Knowledge Discovery, Changsha, China, 13–15 August 2016; pp. 1722–1727. [Google Scholar]
- Qiao, R.; Qing, C.; Zhang, T.; Xing, X.; Xu, X.; IEEE. A Novel Deep-Learning based Framework for Multi-Subject Emotion Recognition. In Proceedings of the 2017 4th International Conference on Information, Cybernetics and Computational Social Systems, Dalian, China, 24–26 July 2017; pp. 181–185. [Google Scholar]
- Groppe, D.M.; Bickel, S.; Keller, C.J.; Jain, S.K.; Hwang, S.T.; Harden, C.; Mehta, A.D. Dominant frequencies of resting human brain activity as measured by the electrocorticogram. Neuroimage 2013, 79, 223–233. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Sehatpour, P.; Molholm, S.; Schwartz, T.H.; Mahoney, J.R.; Mehta, A.D.; Javitt, D.C.; Stanton, P.K.; Foxe, J.J. A human intracranial study of long-range oscillatory coherence across a frontal-occipital-hippocampal brain network during visual object processing. Proc. Natl. Acad. Sci. USA 2008, 105, 4399–4404. [Google Scholar] [CrossRef] [PubMed]
- Stam, C.J.; Dijk, B.W.V. Synchronization likelihood: An unbiased measure of generalized synchronization in multivariate data sets. Phys. D Nonlinear Phenom. 2002, 163, 236–251. [Google Scholar] [CrossRef]
- Peraza, L.R.; Asghar, A.U.; Green, G.; Halliday, D.M. Volume conduction effects in brain network inference from electroencephalographic recordings using phase lag index. J. Neurosci. Methods 2012, 207, 189–199. [Google Scholar] [CrossRef]
- Stam, C.J.; Nolte, G.; Daffertshofer, A. Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 2007, 28, 1178–1193. [Google Scholar] [CrossRef]
- Li, D.; Li, T.; Niu, Y.; Xiang, J.; Cao, R.; Liu, B.; Zhang, H.; Wang, B. Reduced hemispheric asymmetry of brain anatomical networks in attention deficit hyperactivity disorder. Brain Imaging Behav. 2019, 13, 669–684. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xia, M.; Liao, X.; Evans, A.; He, Y. GRETNA: A graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Agosta, F.; Sala, S.; Valsasina, P.; Meani, A.; Canu, E.; Magnani, G.; Cappa, S.F.; Scola, E.; Quatto, P.; Horsfield, M.A.J.N. Brain network connectivity assessed using graph theory in frontotemporal dementia. Neurology 2013, 81, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Marrelec, G.; Bellec, P.; Krainik, A.; Duffau, H.; Pelegrini-Isaac, M.; Lehericy, S.; Benali, H.; Doyon, J. Regions, systems, and the brain: Hierarchical measures of functional integration in fMRI. Med. Image Anal. 2008, 12, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O. Network attributes for segregation and integration in the human brain. Curr. Opin. Neurobiol. 2013, 23, 162–171. [Google Scholar] [CrossRef]
- Shen, R.; Junhua, L.; Taya, F.; deSouza, J.; Thakor, N.V.; Bezerianos, A. Dynamic Functional Segregation and Integration in Human Brain Network During Complex Tasks. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 547–556. [Google Scholar] [CrossRef]
- Brier, M.R.; Thomas, J.B.; Fagan, A.M.; Hassenstab, J.; Holtzman, D.M.; Benzinger, T.L.; Morris, J.C.; Ances, B.M. Functional connectivity and graph theory in preclinical Alzheimer’s disease. Neurobiol. Aging 2014, 35, 757–768. [Google Scholar] [CrossRef]
- Tinaz, S.; Lauro, P.; Hallett, M.; Horovitz, S.G. Deficits in task-set maintenance and execution networks in Parkinson’s disease. Brain Struct. Funct. 2016, 221, 1413–1425. [Google Scholar] [CrossRef]
- Latora, V.; Marchiori, M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef]
- Achard, S.; Bullmore, E.T. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 2007, 3, 174–183. [Google Scholar] [CrossRef]
- Ratnarajah, N.; Rifkin-Graboi, A.; Fortier, M.V.; Chong, Y.S.; Kwek, K.; Saw, S.M.; Godfrey, K.M.; Gluckman, P.D.; Meaney, M.J.; Qiu, A. Structural connectivity asymmetry in the neonatal brain. Neuroimage 2013, 75, 187–194. [Google Scholar] [CrossRef]
- Aydin, S.; Demirtas, S.; Tunga, M.A.; Ates, K. Comparison of hemispheric asymmetry measurements for emotional recordings from controls. Neural Comput. Appl. 2018, 30, 1341–1351. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Papousek, I.; Reiser, E.M.; Weber, B.; Freudenthaler, H.H.; Schulter, G. Frontal brain asymmetry and affective flexibility in an emotional contagion paradigm. Psychophysiology 2012, 49, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Lucchiari, C. Consciousness and arousal effects on emotional face processing as revealed by brain oscillations. A gamma band analysis. Int. J. Psychophysiol. 2008, 67, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Miskovic, V.; Schmidt, L.A. Cross-Regional cortical synchronization during affective image viewing. Brain Res. 2010, 1362, 102–111. [Google Scholar] [CrossRef]
- Danielle, S.; Bassett, A.M.-L.; Sophie, A.; Thomas, D.; Edward, B. Adaptive reconfiguration of fractal small-world human brain functional networks. Proc. Natl. Acad. Sci. USA 2006, 103, 19518–19523. [Google Scholar] [CrossRef]
- Gainotti, G. Emotional behavior and hemispheric side of the lesion. Cortex 1972, 8, 41–55. [Google Scholar] [CrossRef]
- Gawda, B.; Szepietowska, E.; Soluch, P.; Wolak, T. Valence of Affective Verbal Fluency: fMRI Studies on Neural Organization of Emotional Concepts Joy and Fear. J. Psycholinguist. Res. 2017, 46, 731–746. [Google Scholar] [CrossRef]





| Category | HAHV | HALV | LAHV | LALV |
|---|---|---|---|---|
| Number of EEG data | 429 | 298 | 253 | 260 |
| Network Properties | Definitions | Measurement and Interpretation |
|---|---|---|
| Clustering coefficient (Cp) | is the number of neighbors of node i, and is the number of edges between neighbors of node i. N is the number of nodes in the network G (N = 18 in this work). | |
| Characteristic path length (Lp) | is the shortest path length between nodes i and j. | |
| Global efficiency (Eg) | reflects the information transmission efficiency of the whole network. | |
| Local efficiency (Eloc) | is local efficiency of node i, and is a measure of the average local efficiency of network. | |
| Small-world property (σ) | represents the normalized clustering coefficient. represents the normalized characteristic path length. | |
| Nodal efficiency (Enodal) | measures the information transmission ability of node i in network G. |
| Properties | HAHV Mean (p-Value) | HALV Mean (p-Value) | LAHV Mean (p-Value) | LALV Mean (p-Value) |
|---|---|---|---|---|
| AS (Cp) | −3.565 (0.001) | 3.668 (<0.001) | −2.239 (0.204) | 0.983 (0.270) |
| AS (Lp) | 0.850 (0.037) | −1.234 (0.007) | −0.131 (0.735) | −0.002 (0.996) |
| AS (Eg) | −0.549 (0.022) | 0.485 (0.138) | 0.174 (0.542) | 0.291 (0.338) |
| AS (Eloc) | −3.386 (<0.001) | 3.266 (0.001) | −1.789 (0.771) | 0.861 (0.306) |
| AS (σ) | −1.369 (0.244) | 1.076 (0.470) | −0.447 (0.725) | −1.316 (0.378) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, R.; Shi, H.; Wang, X.; Huo, S.; Hao, Y.; Wang, B.; Guo, H.; Xiang, J. Hemispheric Asymmetry of Functional Brain Networks under Different Emotions Using EEG Data. Entropy 2020, 22, 939. https://doi.org/10.3390/e22090939
Cao R, Shi H, Wang X, Huo S, Hao Y, Wang B, Guo H, Xiang J. Hemispheric Asymmetry of Functional Brain Networks under Different Emotions Using EEG Data. Entropy. 2020; 22(9):939. https://doi.org/10.3390/e22090939
Chicago/Turabian StyleCao, Rui, Huiyu Shi, Xin Wang, Shoujun Huo, Yan Hao, Bin Wang, Hao Guo, and Jie Xiang. 2020. "Hemispheric Asymmetry of Functional Brain Networks under Different Emotions Using EEG Data" Entropy 22, no. 9: 939. https://doi.org/10.3390/e22090939
APA StyleCao, R., Shi, H., Wang, X., Huo, S., Hao, Y., Wang, B., Guo, H., & Xiang, J. (2020). Hemispheric Asymmetry of Functional Brain Networks under Different Emotions Using EEG Data. Entropy, 22(9), 939. https://doi.org/10.3390/e22090939






