Effect of Enhanced Gravity on the Microstructure and Mechanical Properties of Al0.9CoCrFeNi High-Entropy Alloy
Abstract
1. Introduction
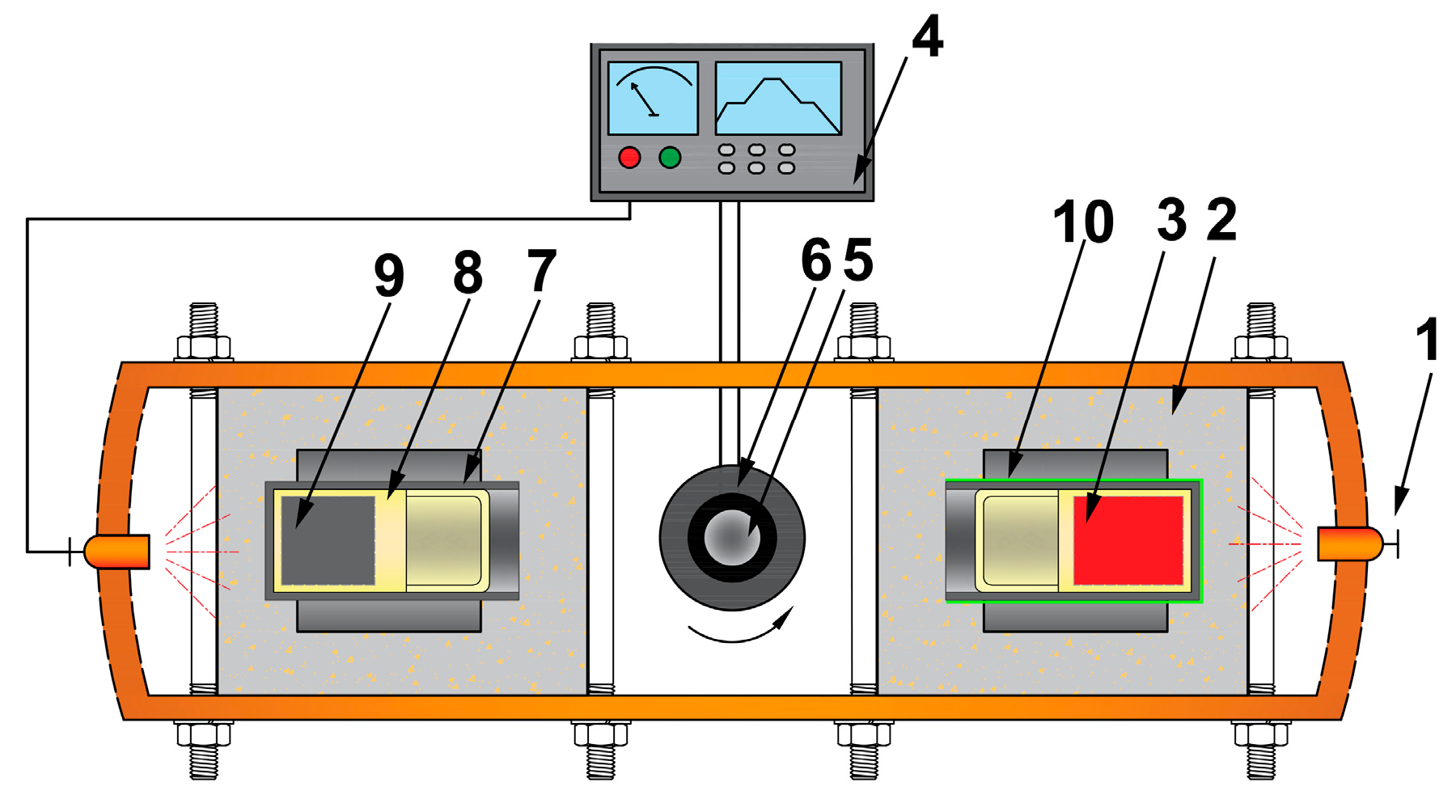
2. Materials and Methods
3. Results
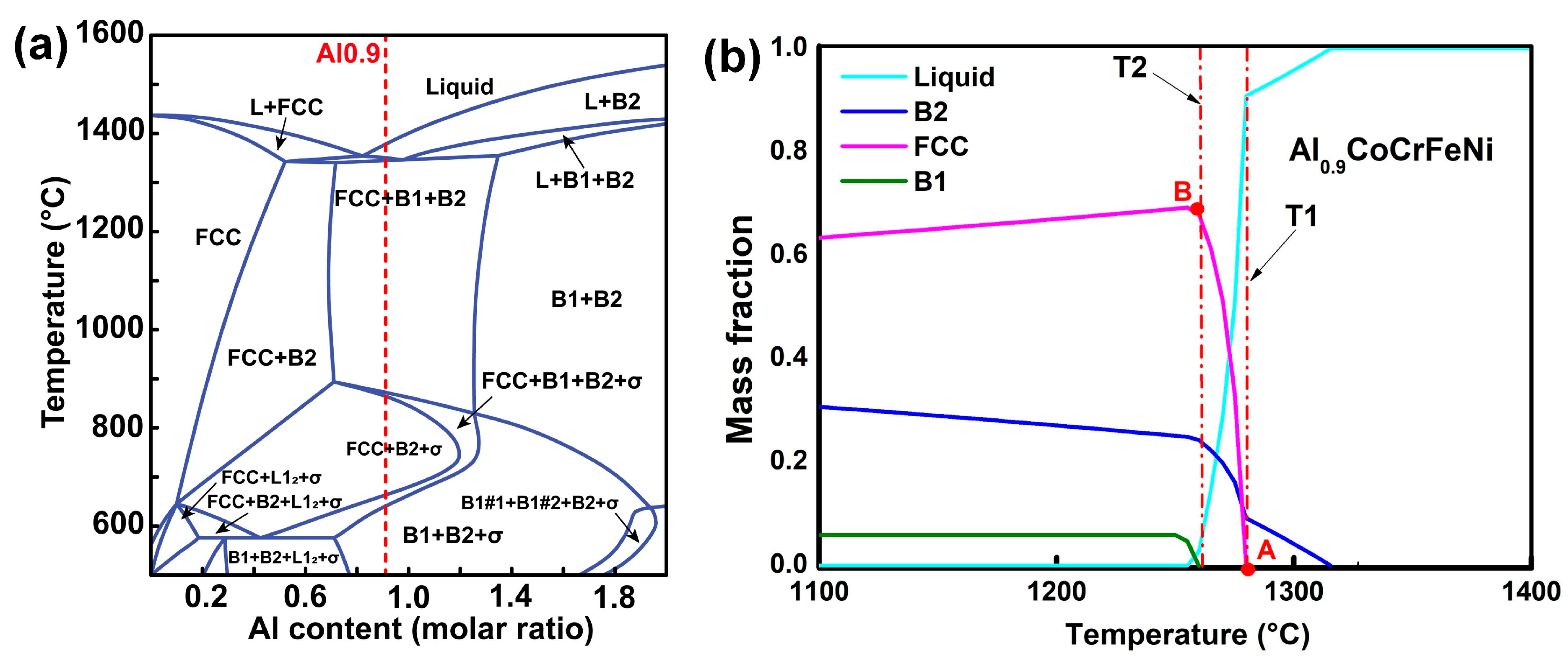
3.1. Phase Composition and Microstructure
3.2. Mechanical Properties
4. Discussion
4.1. Phase Transition in the Al0.9CoCrFeNi HEA Samples
4.2. Microstructure and Mechanical Properties
5. Conclusions
- (1)
- Al0.9CoCrFeNi alloys processed under normal and enhanced gravity fields exhibited similar columnar nondendritic grain structures.
- (2)
- High centrifugal pressure shifted the corresponding eutectic point to the higher Al contents. As a result, the FCC phase content decreased, and that of the B2 phase increased.
- (3)
- The B1 phase content increases in the system eventually due to the solid phase transition under enhanced gravity, and the main contribution of the increase amount of BCC structure phases comes from the B2 phase.
- (4)
- The mass transfer enhanced by the enhanced gravity promoted element diffusion and phase enrichment. The Al and Ni contents in the B2 phase increased obviously with higher acceleration value and Cr contents in the B1 phase increased slightly with higher acceleration value, while Co, Cr, and Fe contents increased in the FCC phase.
- (5)
- At acceleration 360 g, the compressive strength and plasticity of the corresponding sample were significantly enhanced and reached 2845 MPa and 36.4%, respectively.
Author Contributions
Funding
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Kozak, R.; Sologubenko, A.; Steurer, W. Single-phase high-entropy alloys—An overview. Z. Krist Cryst. Mater. 2015, 230, 55–68. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Miracle, D.; Majumdar, B.; Wertz, K.; Gorsse, S. New strategies and tests to accelerate discovery and development of multi-principal element structural alloys. Scr. Mater. 2017, 127, 195–200. [Google Scholar] [CrossRef]
- Li, G.; Xiao, D.; Yu, P.; Zhang, L.; Liaw, P.K.; Li, Y.; Liu, R. Equation of state of an AlCoCrCuFeNi high-entropy alloy. JOM 2015, 67, 2310–2313. [Google Scholar] [CrossRef]
- Gao, M.C. Progress in high-entropy alloys. JOM 2014, 66, 1964–1965. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y. The ultrahigh charpy impact toughness of forged AlxCoCrFeNi high entropy alloys at room and cryogenic temperatures. Intermetallics 2016, 70, 24–28. [Google Scholar] [CrossRef]
- Santodonato, L.J.; Zhang, Y.; Feygenson, M.; Parish, C.M.; Gao, M.C.; Weber, R.J.; Neuefeind, J.C.; Tang, Z.; Liaw, P.K. Deviation from high-entropy configurations in the atomic distributions of a multi-principal-element alloy. Nat. Commun. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Chen, J.; Niu, P.; Wei, T.; Hao, L.; Liu, Y.; Wang, X.; Peng, Y. Fabrication and mechanical properties of AlCoNiCrFe high-entropy alloy particle reinforced Cu matrix composites. J. Alloys Compd. 2015, 649, 630–634. [Google Scholar] [CrossRef]
- Kao, Y.-F.; Chen, T.-J.; Chen, S.-K.; Yeh, J.-W. Microstructure and mechanical property of as-cast, -homogenized, and -deformed AlxCoCrFeNi (0≤ x ≤ 2) high-entropy alloys. J. Alloys Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Lin, C.-M.; Tsai, H.-L. Evolution of microstructure, hardness, and corrosion properties of high-entropy Al0.5CoCrFeNi alloy. Intermetallics 2011, 19, 288–294. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Wang, Y.; Chen, G. Solid solution alloys of Al Co Cr Fe Ni Ti x with excellent room-temperature mechanical properties. Appl. Phys. Lett. 2007, 90, 181904. [Google Scholar] [CrossRef]
- He, J.; Liu, W.; Wang, H.; Wu, Y.; Liu, X.; Nieh, T.; Lu, Z. Effects of Al addition on structural evolution and tensile properties of the FeCoNiCrMn high-entropy alloy system. Acta Mater. 2014, 62, 105–113. [Google Scholar] [CrossRef]
- Cieslak, J.; Tobola, J.; Berent, K.; Marciszko, M. Phase composition of AlxFeNiCrCo high entropy alloys prepared by sintering and arc-melting methods. J. Alloys Compd. 2018, 740, 264–272. [Google Scholar] [CrossRef]
- Joseph, J.; Stanford, N.; Hodgson, P.; Fabijanic, D.M. Understanding the mechanical behaviour and the large strength/ductility differences between FCC and BCC AlxCoCrFeNi high entropy alloys. J. Alloys Compd. 2017, 726, 885–895. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, S.; Qiao, J.; Wang, Z.; Gao, M.; Jiao, Z.; Yang, H.; Zhang, Y. Superior high tensile elongation of a single-crystal Al0.3CoCrFeNi high-entropy alloy by Bridgman solidification. Intermetallics 2014, 54, 104–109. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, S.; Wang, Q.; Liu, Z.; Wang, J.; Yang, Y.; Liu, C. Nanoindentation characterized initial creep behavior of a high-entropy-based alloy CoFeNi. Intermetallics 2014, 53, 183–186. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, P.; Cheng, H.; Zhang, H.; Diao, H.; Shi, Y.; Chen, B.; Chen, P.; Feng, R.; Bai, J. Nanoindentation creep behavior of an Al0.3CoCrFeNi high-entropy alloy. Metall. Mater. Trans. A 2016, 47, 5871–5875. [Google Scholar] [CrossRef]
- Manzoni, A.; Daoud, H.; Völkl, R.; Glatzel, U.; Wanderka, N. Phase separation in equiatomic AlCoCrFeNi high-entropy alloy. Ultramicroscopy 2013, 132, 212–215. [Google Scholar] [CrossRef]
- Xia, S.; Gao, M.; Zhang, Y. Abnormal temperature dependence of impact toughness in AlxCoCrFeNi system high entropy alloys. Mater. Chem. Phys. 2018, 210, 213–221. [Google Scholar] [CrossRef]
- Ren, B.; Liu, Z.; Cai, B.; Wang, M.; Shi, L. Aging behavior of a CuCr2Fe2NiMn high-entropy alloy. Mater. Des. 2012, 33, 121–126. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, Y.; Chen, X.; Wang, T.; Si, J.; Wang, L.; Wang, Y.; Hui, X. Phase composition and solid solution strengthening effect in TiZrNbMoV high-entropy alloys. Mater. Des. 2015, 83, 651–660. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, Z.; Wang, Z.; Wang, M. Influences of super-gravity field on aluminum grain refining. Metall. Mater. Trans. A 2010, 41, 670–675. [Google Scholar] [CrossRef]
- Yang, Y.; Song, B.; Cheng, J.; Song, G.; Yang, Z.; Cai, Z. Effect of Super-gravity Field on Grain Refinement and Tensile Properties of Cu–Sn Alloys. ISIJ Int. 2018, 58, 98–106. [Google Scholar] [CrossRef]
- Li, R.; Wang, Z.; Guo, Z.; Liaw, P.K.; Zhang, T.; Li, L.; Zhang, Y. Graded microstructures of Al-Li-Mg-Zn-Cu entropic alloys under enhanced gravity. Sci. China Mater. 2019, 62, 736–744. [Google Scholar] [CrossRef]
- Shiratori, H.; Fujieda, T.; Yamanaka, K.; Koizumi, Y.; Kuwabara, K.; Kato, T.; Chiba, A. Relationship between the microstructure and mechanical properties of an equiatomic AlCoCrFeNi high-entropy alloy fabricated by selective electron beam melting. Mater. Sci. Eng. A 2016, 656, 39–46. [Google Scholar] [CrossRef]
- Chen, K.; Hu, Z.; Ding, B. Nucleation in metallic melt on the ground and under elevated gravity. J. Mater. Sci. Technal. 1994, 10, 307–309. [Google Scholar]
- Trivedi, R.; Jin, F.; Anderson, I. Dynamical evolution of microstructure in finely atomized droplets of Al-Si alloys. Acta Mater. 2003, 51, 289–300. [Google Scholar] [CrossRef]
- Sr, C.; Jm, K. Numerical simulation of microstructure evolution of Al alloys in centrifugal casting. ISIJ Int. 2001, 41, 738–747. [Google Scholar]
- Yang, Y.; Song, B.; Yang, Z.; Song, G.; Cai, Z.; Guo, Z. The refining mechanism of super gravity on the solidification structure of Al-Cu alloys. Materials 2016, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, J.; Shi, A.; Meng, L.; Guo, Z. Recovery of zinc from galvanizing dross by a method of super-gravity separation. J. Alloys Compd. 2018, 735, 1997–2006. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, K.; Lu, Y.; Gao, X.; Wang, T.; Li, T. Effect of vanadium addition on the microstructure and properties of AlCoCrFeNi high entropy alloy. Mater. Des. 2014, 57, 67–72. [Google Scholar] [CrossRef]
- Chen, J.; Niu, P.; Liu, Y.; Lu, Y.; Wang, X.; Peng, Y.; Liu, J. Effect of Zr content on microstructure and mechanical properties of AlCoCrFeNi high entropy alloy. Mater. Des. 2016, 94, 39–44. [Google Scholar] [CrossRef]
- Kuwabara, K.; Shiratori, H.; Fujieda, T.; Yamanaka, K.; Koizumi, Y.; Chiba, A. Mechanical and corrosion properties of AlCoCrFeNi high-entropy alloy fabricated with selective electron beam melting. Addit. Manuf. 2018, 23, 264–271. [Google Scholar] [CrossRef]
- Li, R.; Liaw, P.; Zhang, Y. Synthesis of AlxCoCrFeNi high-entropy alloys by high-gravity combustion from oxides. Mater. Sci. Eng. A 2017, 707, 668–673. [Google Scholar] [CrossRef]
- Xian, X.; Zhong, Z.-H.; Lin, L.-J.; Zhu, Z.-X.; Chen, C.; Wu, Y.-C. Tailoring strength and ductility of high-entropy CrMnFeCoNi alloy by adding Al. Rare Met. 2018, 1–7. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Guo, Z. Deposit structure and kinetic behavior of metal electrodeposition under enhanced gravity-induced convection. J. Electroanal. Chem. 2015, 744, 25–31. [Google Scholar] [CrossRef]





| Al | Co | Cr | Fe | Ni |
|---|---|---|---|---|
| 9.5 | 23.2 | 20.7 | 21.8 | 24.8 |
| Al0.9CoCrFeNi | Regions | Chemical Composition | ||||
|---|---|---|---|---|---|---|
| Al | Co | Cr | Fe | Ni | ||
| G = 1 | A (BCC structure phases) | 10.58 | 21.57 | 20.61 | 20.09 | 23.17 |
| B (FCC structure phases) | 7.13 | 23.88 | 22.89 | 22.50 | 23.60 | |
| B2 phase | 11.35 | 20.60 | 19.93 | 18.73 | 24.00 | |
| B1 phase | 8.41 | 24.31 | 22.54 | 23.92 | 20.82 | |
| G = 360 | C (BCC structure phases) | 16.36 | 22.40 | 10.20 | 14.91 | 33.07 |
| D (FCC structure phases) | 3.54 | 24.54 | 25.06 | 26.78 | 17.40 | |
| B2 phase | 19.48 | 18.14 | 5.80 | 16.54 | 35.91 | |
| B1 phase | 7.41 | 34.61 | 22.81 | 10.24 | 24.93 | |
| Different Acceleration Value | σ (MPa) | ε (%) |
|---|---|---|
| acceleration 1 g | 2106 | 38.2 |
| acceleration 140 g | 2324 | 27.9 |
| acceleration 210 g | 2595 | 34.9 |
| acceleration 360 g | 2845 | 36.4 |
| Samples | σmax | εp | Phase | Ref. | Preparation |
|---|---|---|---|---|---|
| (MPa) | (%) | ||||
| Al0.9CoCrFeNi | 2845 | 36.4 | FCC+BCC | Our work | Enhanced gravity solidification |
| AlCoCrFeNi | 2864 | 22.7 | BCC | [33] | Normal gravity solidification |
| AlCoCrFeNi | 2670 | 22.5 | BCC | [34] | Normal gravity solidification |
| AlCoCrFeNi | 1668 | 26.4 | FCC+BCC | [30] | Additive manufacturing |
| AlCoCrFeNi | 1447 | 14.5 | FCC+BCC | [35] | Additive manufacturing |
| Al0.75CoCrFeNi | 1950 | 33.2 | FCC+BCC | [27] | Suction-casting |
| Al0.75CoCrFeNi | 1870 | 22 | FCC+BCC | [36] | Combustion synthesis under enhanced gravity |
| Al0.75CoCrFeNi | 1193 | 27.2 | FCC+BCC | [37] | Normal gravity solidification |
| Al0.5CoCrFeNi | 1750 | 32 | FCC+BCC | [38] | Combustion synthesis under enhanced gravity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, A.; Li, R.; Zhang, Y.; Wang, Z.; Guo, Z. Effect of Enhanced Gravity on the Microstructure and Mechanical Properties of Al0.9CoCrFeNi High-Entropy Alloy. Entropy 2020, 22, 1318. https://doi.org/10.3390/e22111318
Shi A, Li R, Zhang Y, Wang Z, Guo Z. Effect of Enhanced Gravity on the Microstructure and Mechanical Properties of Al0.9CoCrFeNi High-Entropy Alloy. Entropy. 2020; 22(11):1318. https://doi.org/10.3390/e22111318
Chicago/Turabian StyleShi, Anjun, Ruixuan Li, Yong Zhang, Zhe Wang, and Zhancheng Guo. 2020. "Effect of Enhanced Gravity on the Microstructure and Mechanical Properties of Al0.9CoCrFeNi High-Entropy Alloy" Entropy 22, no. 11: 1318. https://doi.org/10.3390/e22111318
APA StyleShi, A., Li, R., Zhang, Y., Wang, Z., & Guo, Z. (2020). Effect of Enhanced Gravity on the Microstructure and Mechanical Properties of Al0.9CoCrFeNi High-Entropy Alloy. Entropy, 22(11), 1318. https://doi.org/10.3390/e22111318






