3.2. Retrieval from Single Reservoir
Instead of directly retrieving work from the reservoir material, we are now interested in engines operating in contact with reservoirs. Here, the reservoirs remain intact, but interact with a thermodynamic engine, in which a working substance undergoes a cyclic change of properties. For simplicity we study systems where the working substance is the same as the reservoir material, so that both share their thermodynamic properties.
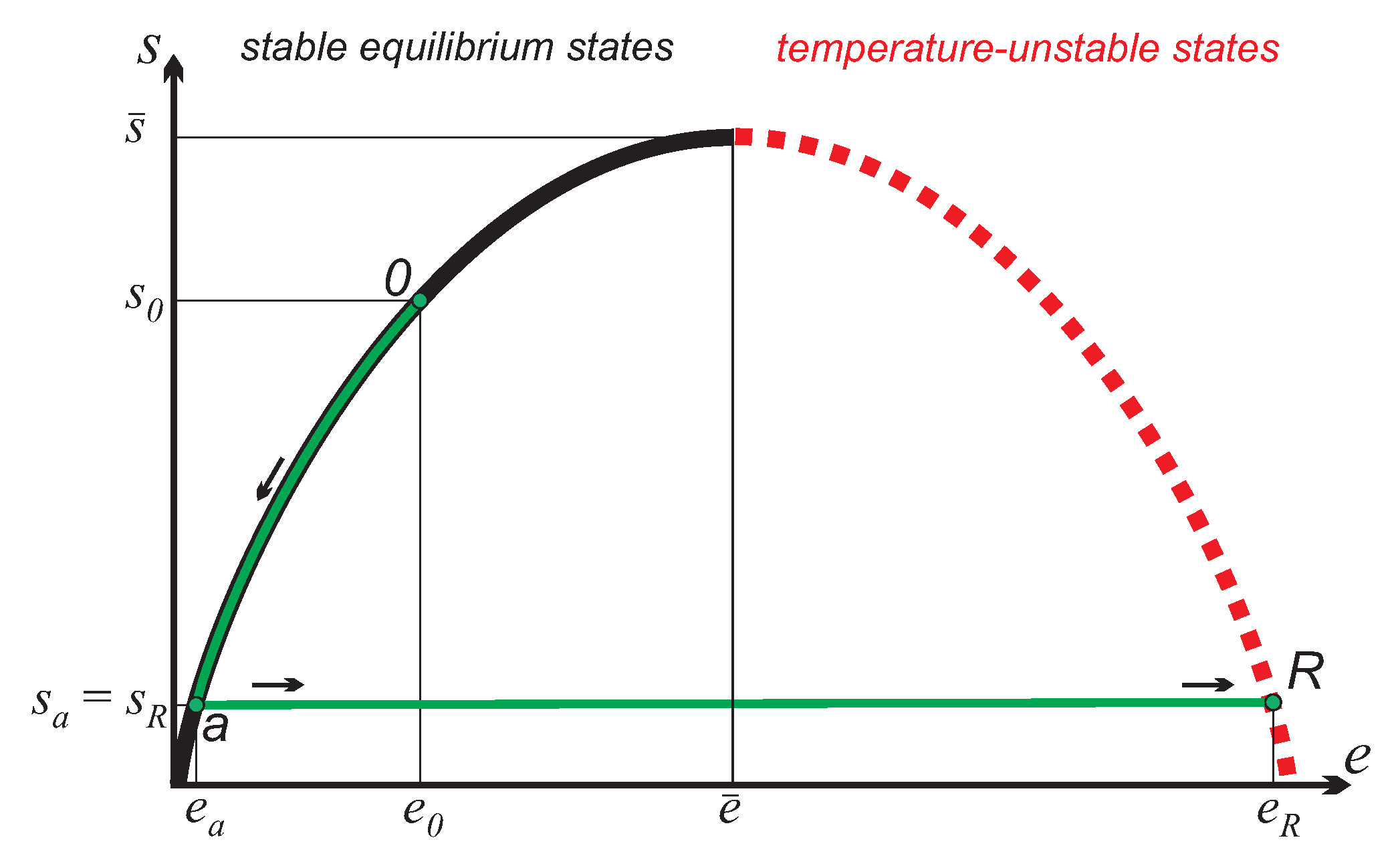
Since work can be directly retrieved from inverted states, the simplest cycle possible does not require a second reservoir, but just uses a single inverted reservoir of
N moles and the working substance of
moles. As a starting point for the process, we consider a reservoir at state
and the working substance at state
, where
. The cycle consists of the following two processes, as depicted in
Figure 2:
a-
, charge: Working substance (state
a) and reservoir (state
R) equilibrate irreversibly to the common equilibrium state
while adiabatically isolated from their surroundings. The first law (3)
reduces to:
so that the the energy transferred from reservoir to the working substance is:
With
, the second law (3)
reduces to:
-
, work production: The working substance returns to non-inverted state
, in an adiabatic reversible process, where
as indicated in
Figure 2. The first law (3)
gives the work produced as:
and the second law (3)
simply states isentropicity,
.
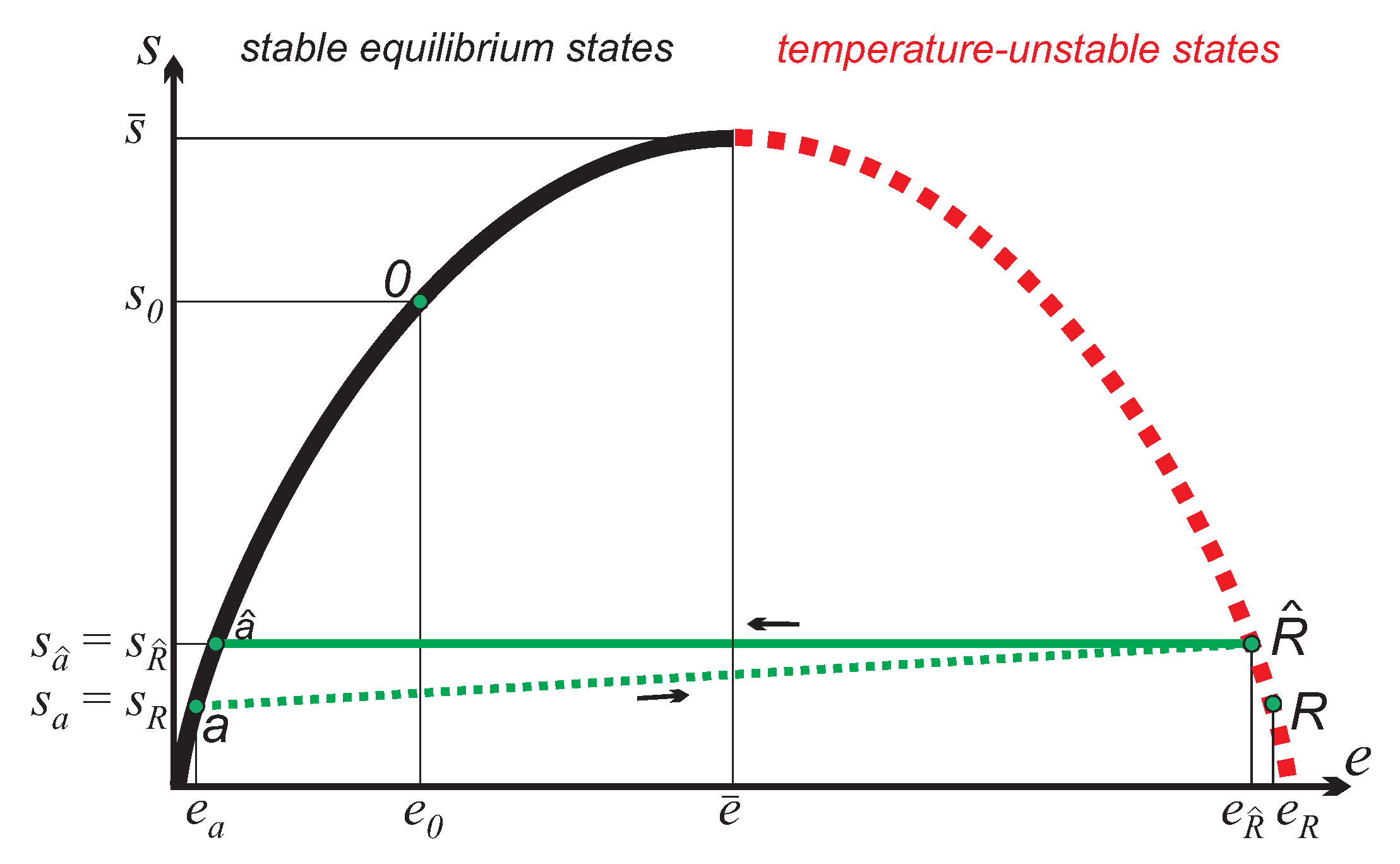
Accordingly, the endpoint of the equilibration process has slightly larger entropy and slightly smaller energy than the initial reservoir state R. As the cycle is repeated, overtime the reservoir state moves up on the curve, towards larger entropies and lower energies. If the reservoir is much larger than the working system, , the final state of the cycle almost coincides with the initial state. However, the small difference between the two states R and will be important for a full understanding of the process.
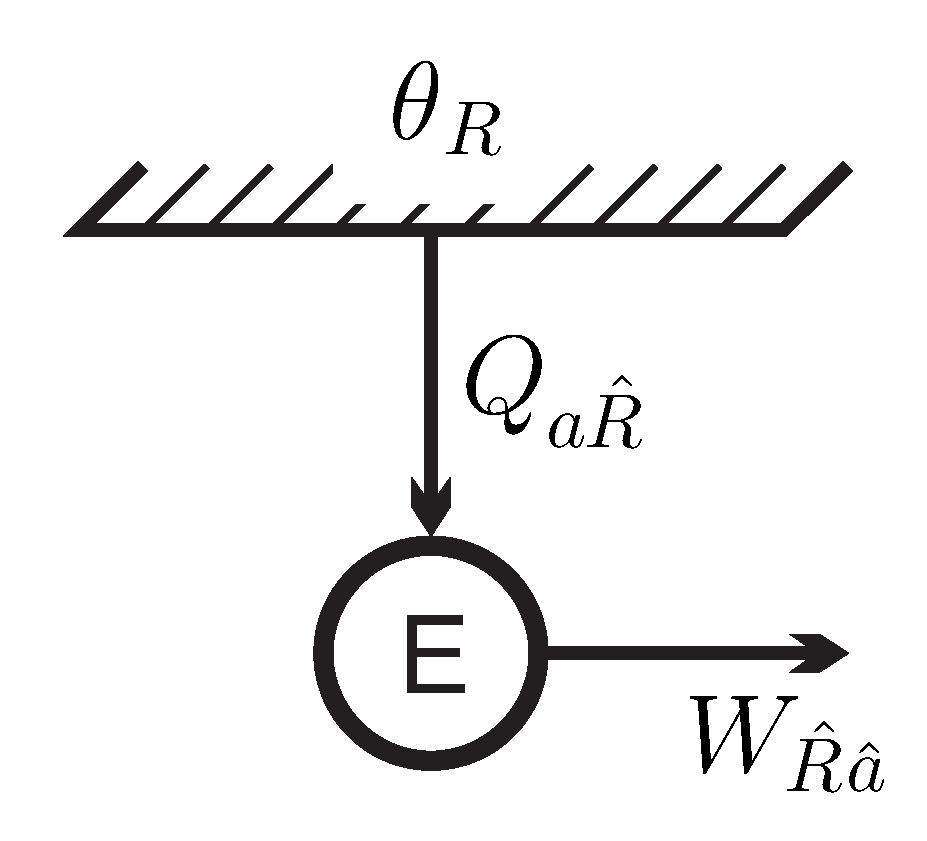
Figure 3 shows the energy flows for the engine
E in contact with the inverted reservoir at apparent negative temperature
. As discussed before [
12], in the present interpretation this process does not contradict the Kelvin–Planck statement, since the reservoir is not in a stable equilibrium state.
Following usual definitions from the thermodynamics of engines [
13], the energy efficiency of this simple back and forth (b-f) process is:
In this definition, the energy drawn from the reservoir is considered as the expense incurred, while the work produced is considered as the gain. Due to the (very small) difference between start and end state, the resulting efficiency is just a bit below unity, reaching unity for
.
A proper efficiency measure should be useful for evaluating the quality of the processes in the engine. The value found here, , is simply reflecting conservation of energy for the cycle, and does not give useful information on the quality of the process.
An efficiency of (almost) unity seemingly contradicts classical thermodynamic statements on heat engine efficiency [
13], but, of course, heat engine efficiency is based on the discussion of thermal reservoirs, while the reservoir of inverted states is better seen as a work reservoir that stores the work required for its creation [
12], and this work should be seen as the associated cost.
Accordingly, one should not just ask for the energy transferred from the reservoir, but also for the depletion of the reservoir’s work potential. Since we study a process that is always detached from the free environment, the work potential of the reservoir in the original state
R and the new state
is given by the work that can be obtained from adiabatic reversible re-inversion of the reservoir to states
a or
, respectively. That is,
Thus, the change of the reservoir’s work potential for one cycle is the difference:
Since
, we can use linear approximations, and find with Equation (1),
and, with
,
, and Equation (2),
Due to the assumed symmetry of the curve, we have
, so that, combining the above with Equation (11), we find the change in work potential as:
Hence, the change of the reservoir’s work potential is equal to twice the energy transferred from the reservoir to the working substance.
From an engineering perspective, the expense related to the cycle is the work for the creation of the inverted reservoir. Thus, a better measure for the efficiency is the relative amount of work potential used,
Only half of the change in work potential is realized in the cyclic back-and-forth process, while the other half is lost in the irreversible equilibration process
a-
. Indeed, the work loss to irreversibilities is related to the entropy generation (12) as (for
):
The efficiency
compares actual work produced to work potential consumed and hence belongs to the class of “thermodynamic efficiencies” [
18] or “second law efficiencies” [
13]. Unambiguously, a thermodynamic efficiency is always below unity, unless the process is fully reversible, and is considered to be a better measure for the quality of a process than an energy efficiency such as
[
18].
However, a better interpretation of the efficiency is as the round trip efficiency of a storage system, where the work retrieved is compared to the work for charging (the change in work potential).
3.3. “Otto Engine”
Next, inspired by [
19], we consider a process similar to the classical Otto cycle, where heat is exchanged with two thermal reservoirs at different temperatures. The Otto cycle consists of the following four processes [
13]: (i) Work production in adiabatic expansion, (ii) isochoric heat exchange with free environment (no work), (iii) work input for adiabatic compression, and (iv) isochoric heat exchange within a high temperature environment (no work).
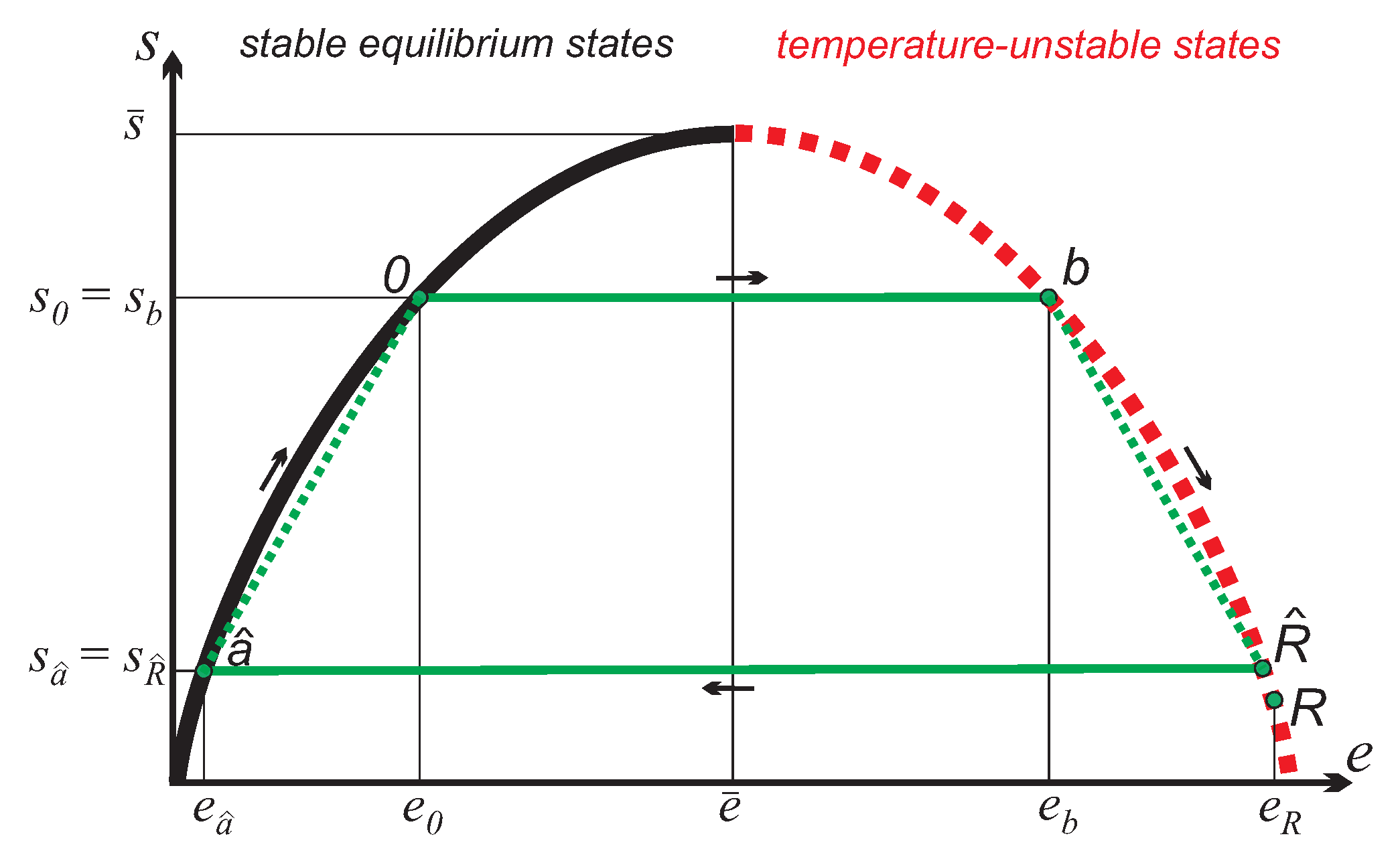
The cycle to be studied, shown in
Figure 4, similarly involves two adiabatic work processes, and two irreversible exchange processes with the reservoirs without work. For the same working substance and reservoir as before, the four processes are as follows (symmetry of the entropy curve used for some results):
0-
b: Adiabatic reversible state inversion; work input:
b-
: Irreversible equilibration with inverted reservoir to common equilibrium
; no work, energy transfer:
-
: Adiabatic reversible return from inverted states; work produced:
-0: Irreversible equilibration with free environment at
; no work, energy transfer:
Due to the assumed symmetry of the entropy curve, the net work produced by the cycle is:
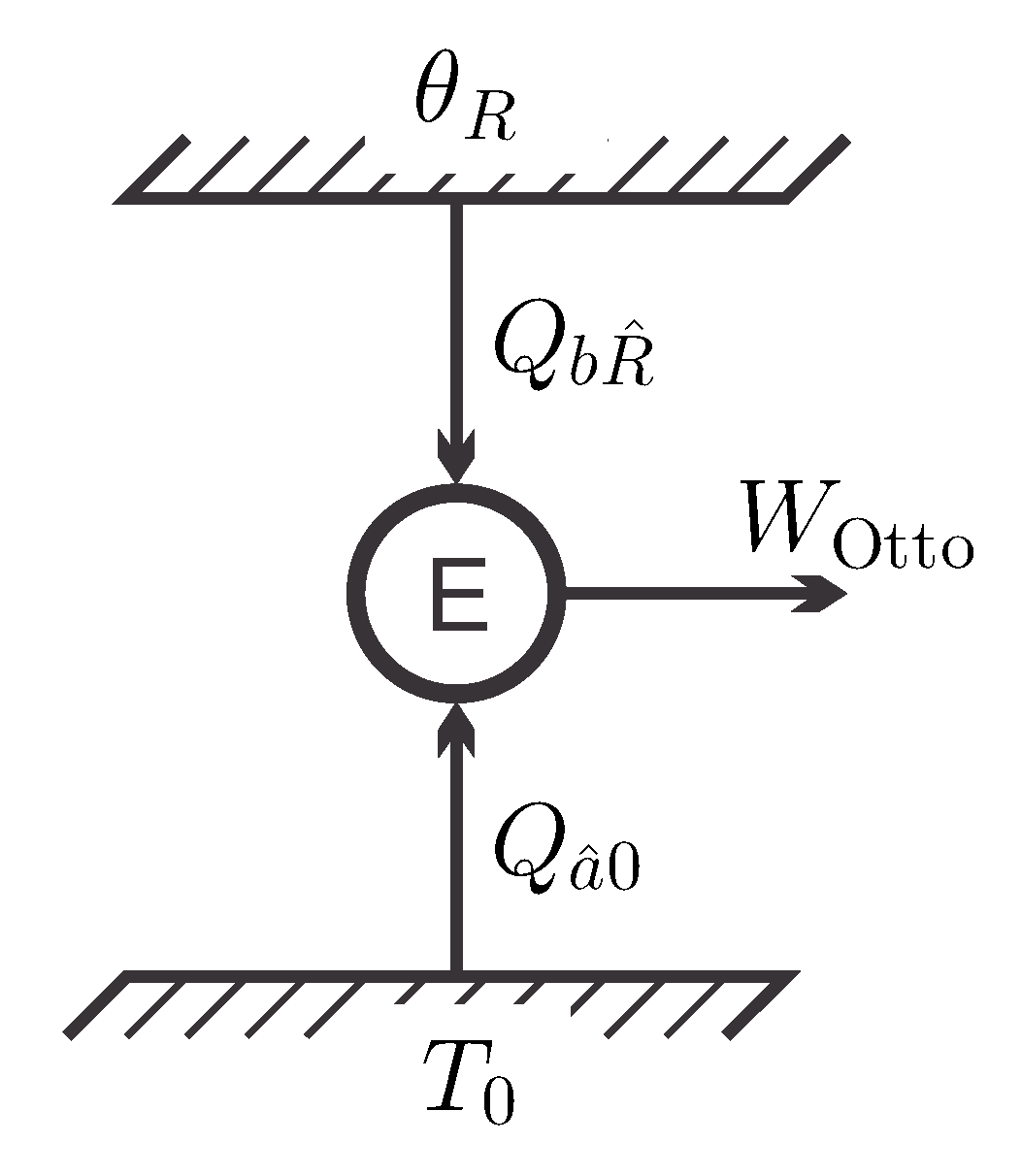
Figure 5 shows the energy flows for this process and again we consider the question of meaningful definitions for the efficiency of the cycle.
A natural choice for the definition of energy efficiency is to compare the work produced to the energy drawn into the cycle [
7], so that:
This gives an efficiency at unity, just as for the simple back-and-forth cycle, see Equation (14). Again, this is simply a reflection of energy conservation for the cycle, with energy drawn from the reservoirs fully converted to work.
Both cycles draw energy from the same inverted reservoir and have energy efficiency of unity. That is, from the viewpoint on energy efficiency, one cannot decide which of the processes is the better choice for the exploitation of the reservoir.
As before, the storage efficiency, which compares work produced to change of work potential, provides a clearer picture and deeper insight. The work potential of the inverted reservoir relative to the free environment is given by Equation (8), hence the change of work potential between states
R and
, after one cycle of the “Otto” engine, is:
Insertion of Equations (18) and (23) yields the change of work potential due to one run of the cycle as:
where we used
, due to the assumed symmetry of the curve
.
Comparing work produced to work potential used gives:
Note that in the limit
the storage efficiency
approaches unity, while the net work of the cycle vanishes.
As expected, the cycle does not fully realize the work potential drawn from the reservoir. The difference is due to generation of entropy in the energy transfer processes
b-
and
-0 (equilibration between working substance and reservoirs, without work). A short calculation yields the overall entropy generation as:
which is, as ususal in thermodynamics [
13], related to the work lost through the environmental temperature
as:
In summary, we state that energy based efficiencies of unity for cycles in contact with an inverted reservoir only reflect the conservation of energy and do not give insight into cycle performance. Indeed, based on this information, one would have difficulty choosing.
However, if one considers the inverted reservoir as a storage system, one can compare the work produced to the change of work potential of the reservoir, as in Equations (20) and (30). Indeed, comparing these values shows that the Otto engine performs better than the simple back-forth process, as long as .