Temperature Effects and Entropy Generation of Pressure Retarded Osmosis Process
Abstract
:1. Introduction
2. Mathematical Model
3. Results and Discussion
4. Model Validation
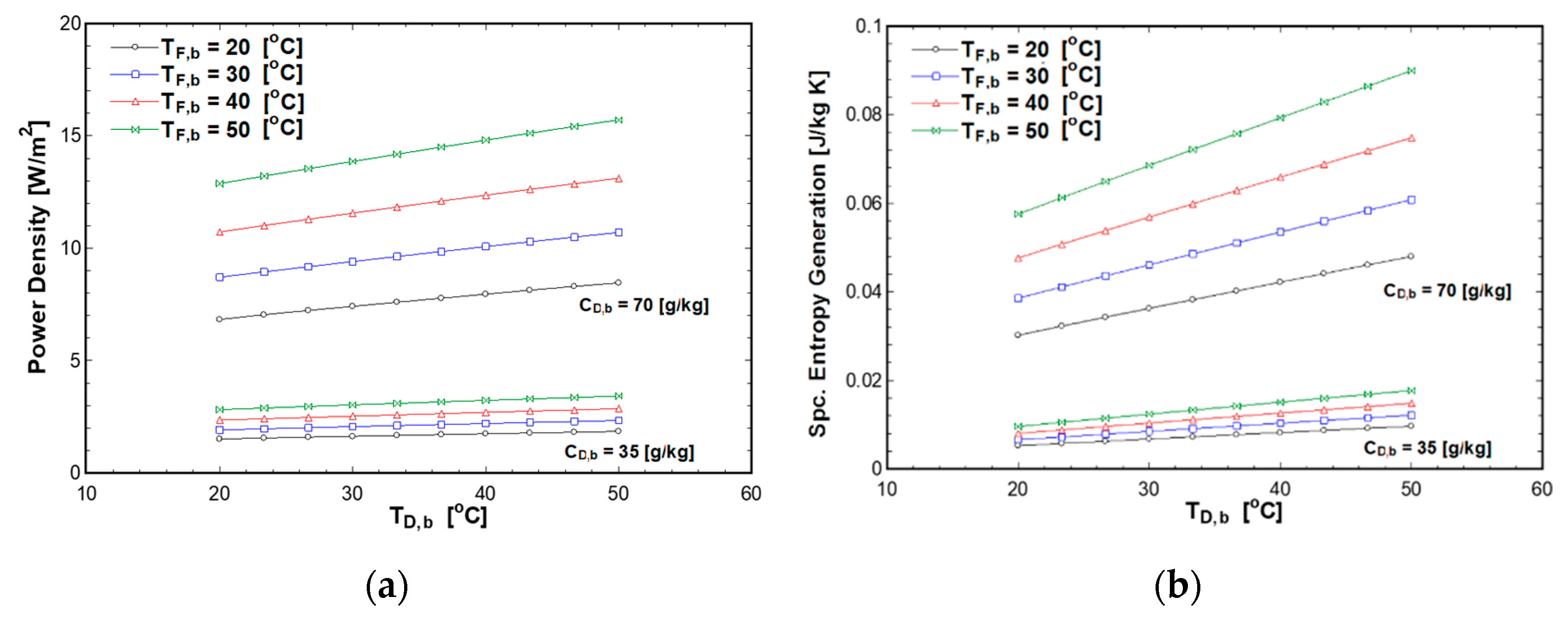
4.1. Effect of Feed and Draw Solutions Temperatures on PRO Performance
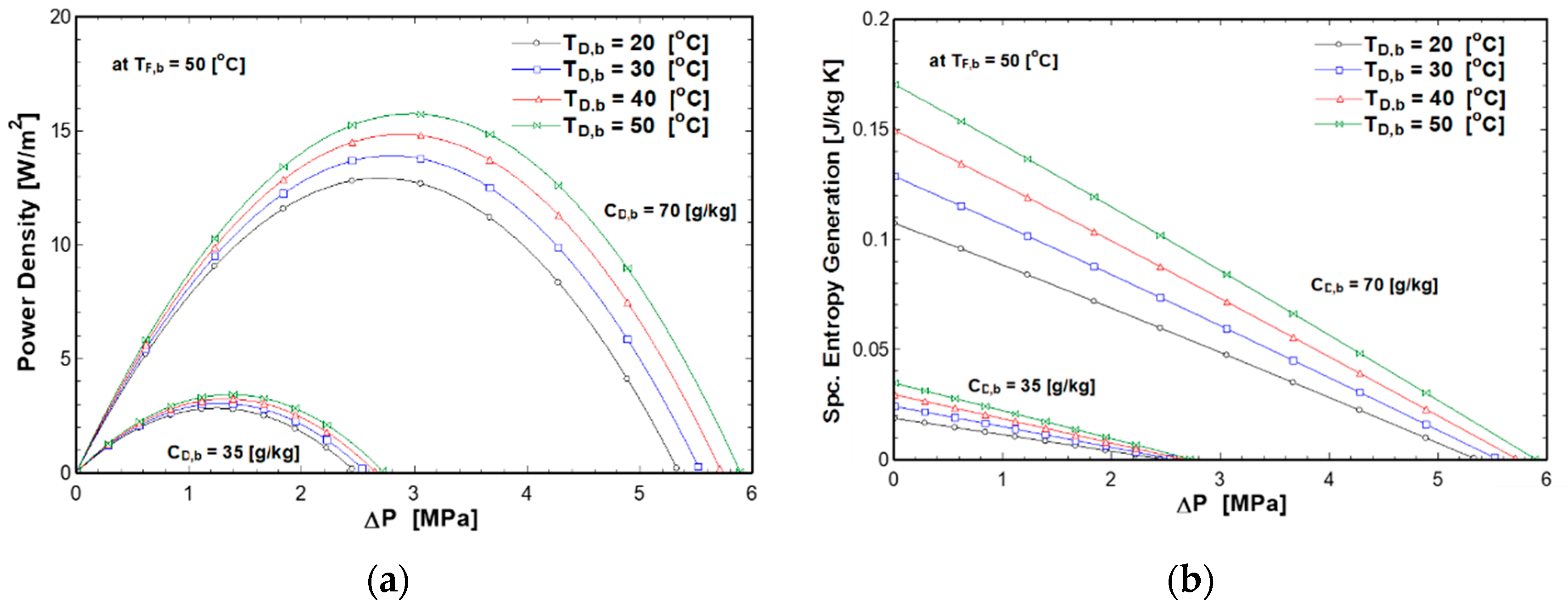
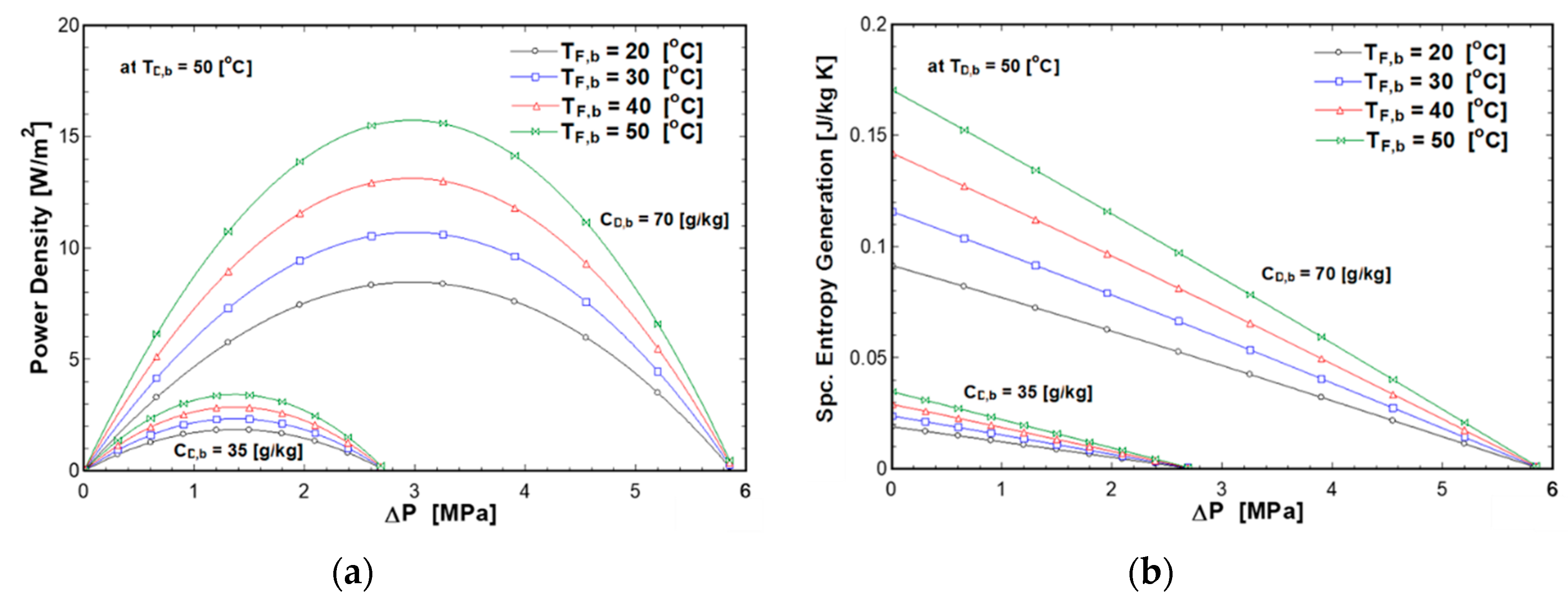
4.2. Effect of Applied Pressure and Solutions Temperatures on PRO Performance
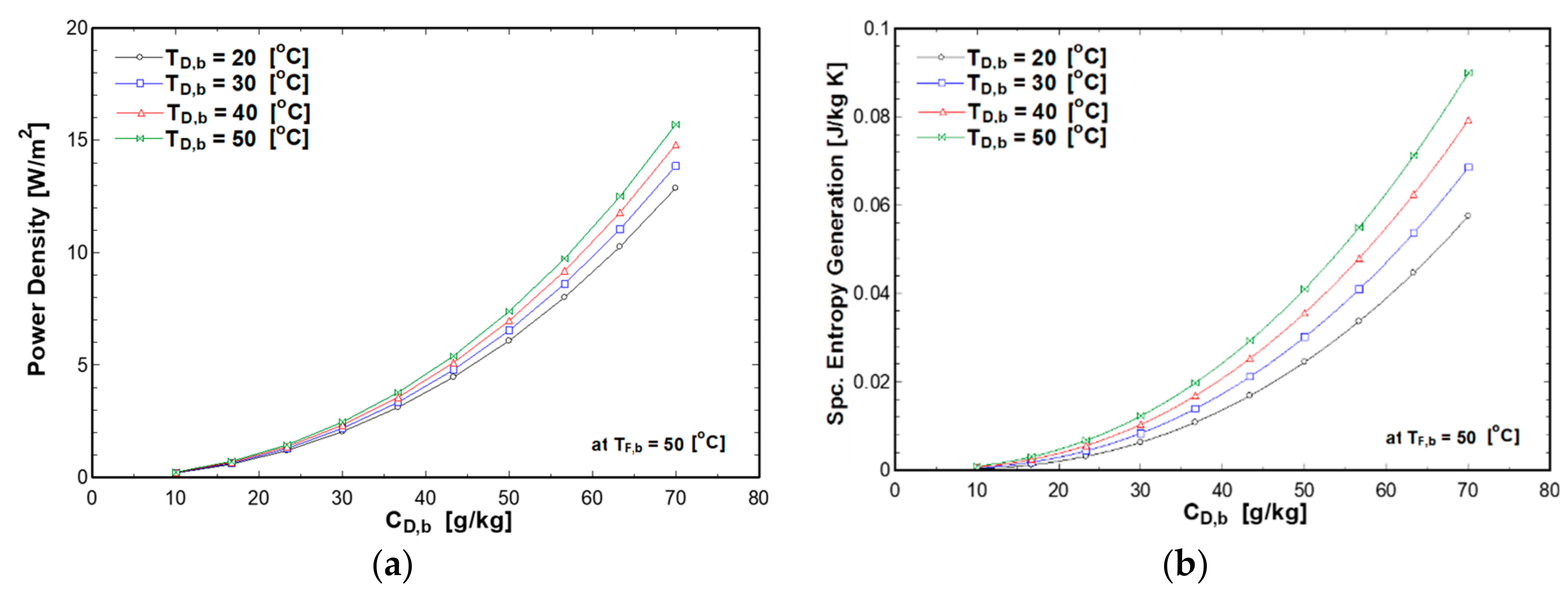
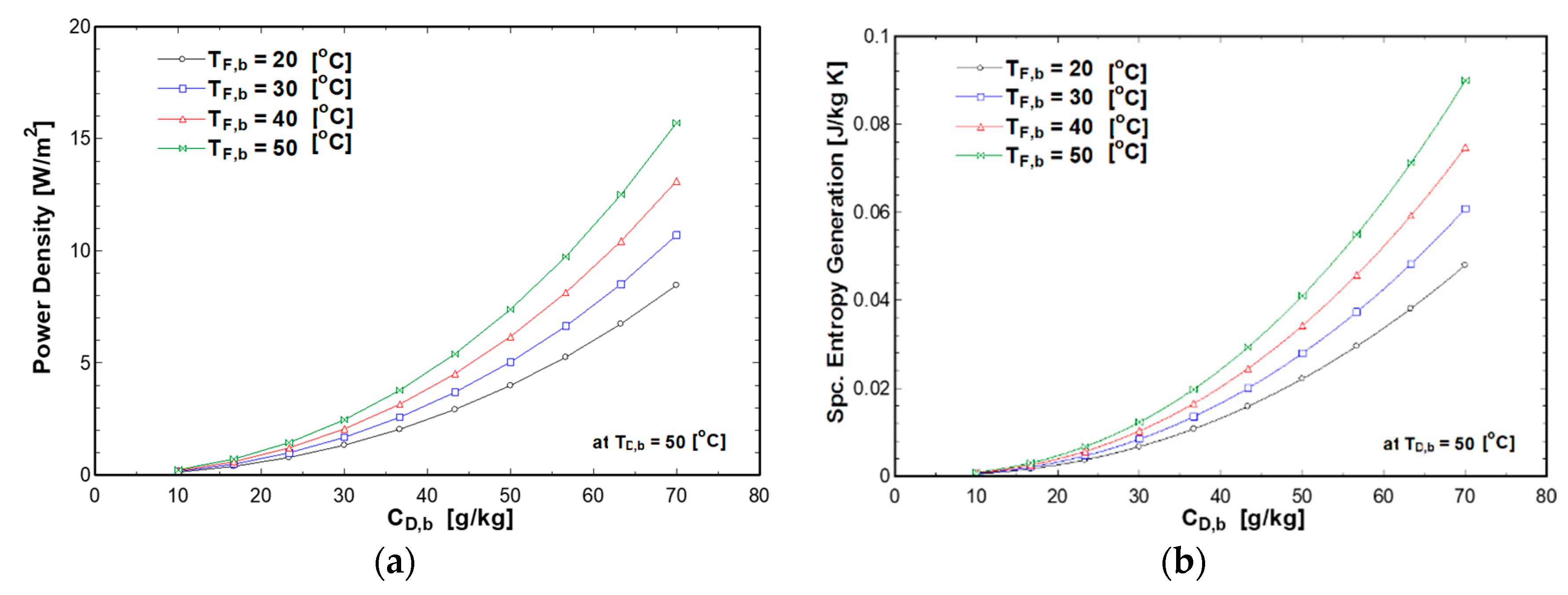
4.3. Effect of Draw Concentration and Solutions Temperatures on PRO Performance
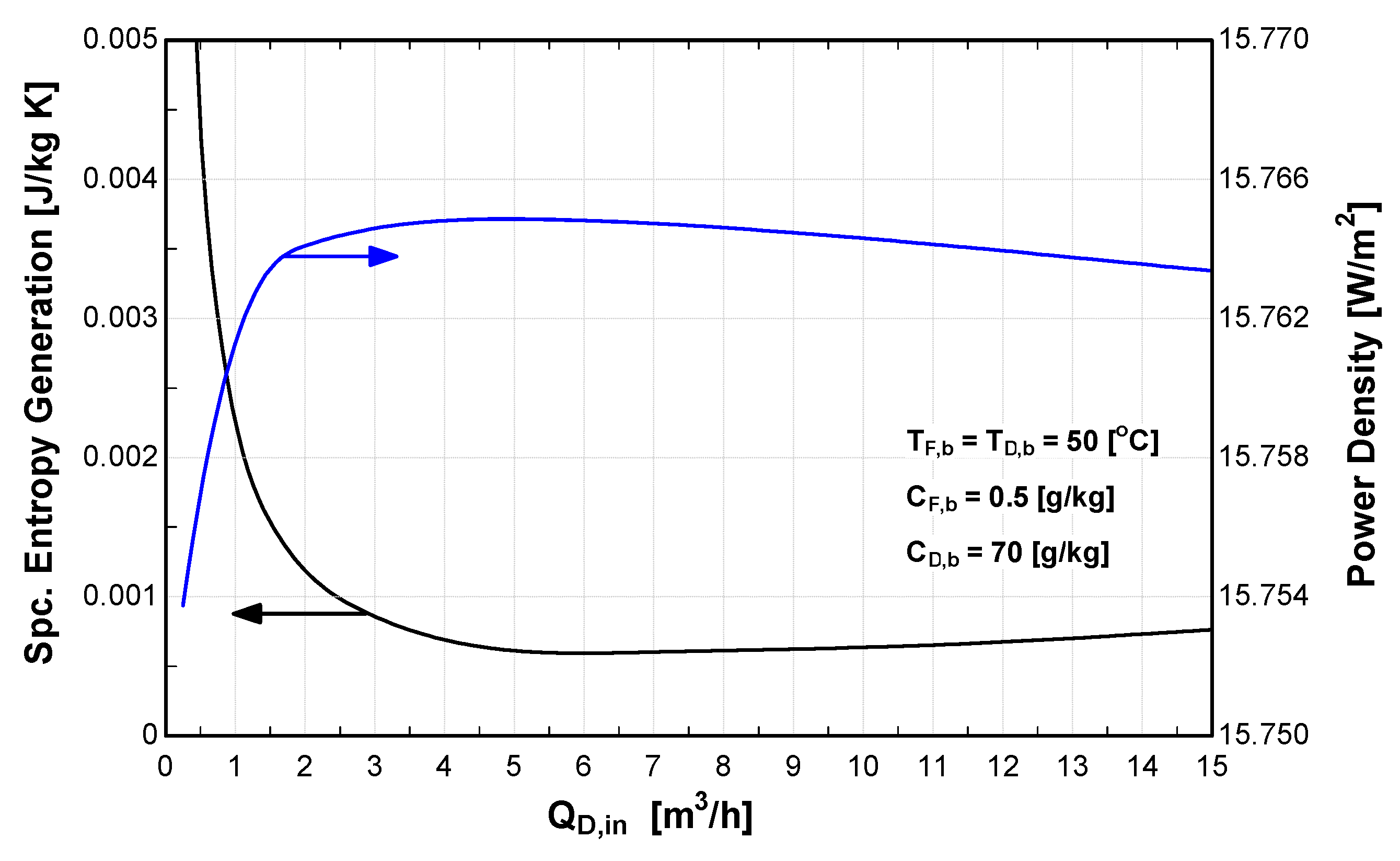
4.4. Effect of Flow Rate on PRO Performance
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| A | membrane water permeability | m/s-kPa |
| Am | membrane surface area | m2 |
| B | membrane salt permeability | m/s |
| C | solute concentration or salinity | g/kg |
| D | diffusion coefficient | m2/s |
| dh | hydraulic diameter | m |
| J | flow flux | m/s |
| solute resistivity | s/m | |
| mass transfer coefficient | m/s | |
| L | membrane length | m |
| mass flow rate | kg/s | |
| transmembrane pressure difference between feed and draw | kPa | |
| pressure drop along flow channel | kPa | |
| Q | volumetric flow rate | m3/s |
| R | universal gas constant | J/mole K |
| Re | Reynolds number | - |
| rp | pore size | m |
| S | structure parameter | m |
| specific entropy generation | J/kg K | |
| rate of entropy generation | W/K | |
| s | specific entropy | J/kg K |
| Sc | Schmidt number | - |
| Sh | Sherwood number | - |
| T | temperature | °C |
| Tw | average water temperature | °C |
| tm | membrane thickness | m |
| V | velocity | m/s |
| W | power density | W/ |
| Greek symbols | ||
| β | van’t Hoff coefficient | - |
| ε | porosity of the membrane | - |
| μ | viscosity | kg/ms |
| π | osmotic pressure | kPa |
| ρ | density | kg/m3 |
| Subscripts | ||
| b | bulk | |
| D | draw solution | |
| F | feed solution | |
| i | cell number | |
| icp | surface between active layer and support layer | |
| m | membrane surface | |
| s | salt or solute | |
| w | water |
References
- Nagy, E. Basic Equations of Mass Transport Through a Membrane Layer, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Loeb, S. Large-scale power production by pressure-retarded osmosis, using river water and sea water passing through spiral modules. Desalination 2002, 143, 115–122. [Google Scholar] [CrossRef]
- Touati, K.; Tadeo, F.; Chae, S.H.; Kim, J.H.; Alvarez-Silva, O. Pressure Retarded Osmosis: Renewable Energy Generation and Recovery; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Senthil, S.; Senthilmurugan, S. Reverse Osmosis–Pressure Retarded Osmosis hybrid system: Modelling, simulation and optimization. Desalination 2016, 389, 78–97. [Google Scholar]
- Helfer, F.; Lemckert, C.; Anissimov, Y.G. Osmotic power with Pressure Retarded Osmosis: Theory, performance and trends—A review. J. Membr. Sci. 2014, 453, 337–358. [Google Scholar] [CrossRef]
- Cath, T.; Childress, A.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Sarp, S.; Li, Z.; Saththasivam, J. Pressure Retarded Osmosis (PRO): Past experiences, current developments, and future prospects. Desalination 2016, 389, 2–14. [Google Scholar] [CrossRef]
- Chung, T.S.; Luo, L.; Wan, C.F.; Cui, Y.; Amy, G. What is next for forward osmosis (FO) and pressure retarded osmosis (PRO). Sep. Purif. Technol. 2015, 156, 856–860. [Google Scholar] [CrossRef]
- Bogler, A.; Lin, S.; Bar-Zeev, E. Biofouling of membrane distillation, forward osmosis and pressure retarded osmosis: Principles, impacts and future directions. J. Membr. Sci. 2017, 542, 378–398. [Google Scholar] [CrossRef]
- Altaee, A.; Sharif, A. Pressure retarded osmosis: Advancement in the process applications for power generation and desalination. Desalination 2015, 356, 31–46. [Google Scholar] [CrossRef]
- Yip, N.Y.; Elimelech, M. Thermodynamic and Energy Efficiency Analysis of Power Generation from Natural Salinity Gradients by Pressure Retarded Osmosis. Environ. Sci. Technol. 2012, 46, 5230–5239. [Google Scholar] [CrossRef]
- Maisonneuve, C.B.; Pillay, J.; Laflamme, P. Osmotic power potential in remote regions of Quebec. Renew. Energy 2015, 81, 62–70. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, K.; Park, M.J.; Shon, H.K.; Kim, J.H. Recent Advances in Osmotic Energy Generation via Pressure-Retarded Osmosis (PRO): A Review. Energies 2015, 8, 11821–11845. [Google Scholar] [CrossRef]
- Bajraktari, N.; Hélix-Nielsen, C.; Madsen, H.T. Pressure retarded osmosis from hypersaline sources—A review. Desalination 2017, 413, 65–85. [Google Scholar] [CrossRef]
- Liu, J.; Xie, L.; Wang, Z.; Yuan, J. Dual-stage nanofiltration seawater desalination: Water quality, scaling and energy consumption. Desalin. Water Treat. 2014, 52, 134–144. [Google Scholar] [CrossRef]
- Mänttäri, M.; Pihlajamäki, A.; Kaipainen, E.; Nyström, M. Effect of temperature and membrane pre-treatment by pressure on the filtration properties of nanofiltration membranes. Desalination 2002, 145, 81–86. [Google Scholar] [CrossRef]
- Nilsson, M.; Trägårdh, G.; Östergren, K. The influence of pH, salt and temperature on nanofiltration performance. J. Membr. Sci. 2008, 312, 97–106. [Google Scholar] [CrossRef]
- Abdelkader, B.A.; Antar, M.A.; Khan, Z. Nanofiltration as a Pretreatment Step in Seawater Desalination: A Review. Arab. J. Sci. Eng. 2018, 43, 4413–4432. [Google Scholar] [CrossRef]
- Farooque, A.M.; Al-amoudi, A.S.; Hassan, A.M. Chemical Cleaning Experiments for Performance Restoration of Nf Membranes Operated on Seawater Feed. In Proceedings of the IDA Conference, Manama, Bahrain, 8–13 March 2002. [Google Scholar]
- Flora, J.R. Stochastic approach to modeling surface fouling of ultrafiltration membranes. J. Membr. Sci. 1993, 76, 85–88. [Google Scholar] [CrossRef]
- Al-Amoudi, A.S.; Farooque, A.M. Performance restoration and autopsy of NF membranes used in seawater pretreatment. Desalination 2005, 178, 261–271. [Google Scholar] [CrossRef]
- Bar-Zeev, E.; Perreault, F.; Straub, A.P.; Elimelech, M. Impaired Performance of Pressure-Retarded Osmosis due to Irreversible Biofouling. Environ. Sci. Technol. 2015, 49, 13050–13058. [Google Scholar] [CrossRef]
- Sheikholeslami, R. Mixed salts—Scaling limits and propensity. Desalination 2003, 154, 117–127. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Lee, C.H. Analysis of CaSO4 scale formation mechanism in various nanofiltration modules. J. Membr. Sci. 1999, 163, 63–74. [Google Scholar] [CrossRef]
- Lee, S.; Lee, C.H. Effect of operating conditions on CaSO4 scale formation mechanism in nanofiltration for water softening. Water Res. 2000, 34, 3854–3866. [Google Scholar] [CrossRef]
- Her, N.; Amy, G.; Jarusutthirak, C. Seasonal variations of nanofiltration (NF) foulants: Identification and control. Desalination 2000, 132, 143–160. [Google Scholar] [CrossRef]
- Andritsos, N.; Kontopoulou, M.; Karabelas, A.J.; Koutsoukos, P.G. Calcium carbonate deposit formation under isothermal conditions. Can. J. Chem. Eng. 1996, 74, 911–919. [Google Scholar] [CrossRef]
- Touati, K.; Tadeo, F.; Schiestel, T. Impact of Temperature on Power Recovery in Osmotic Power Production by Pressure Retarded Osmosis. Energy Procedia 2014, 50, 960–969. [Google Scholar] [CrossRef]
- Sivertsen, E.; Holt, T.; Thelin, W.R. Concentration and Temperature Effects on Water and Salt Permeabilities in Osmosis and Implications in Pressure-Retarded Osmosis. Membranes 2018, 8, 39. [Google Scholar] [CrossRef]
- Touati, K.; Hänel, C.; Tadeo, F.; Schiestel, T. Effect of the feed and draw solution temperatures on PRO performance: Theoretical and experimental study. Desalination 2015, 365, 182–195. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Z.; Li, J.; Tang, Q.; Hu, Y. Investigation of the reduced specific energy consumption of the RO-PRO hybrid system based on temperature-enhanced pressure retarded osmosis. J. Membr. Sci. 2019, 581, 439–452. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Childress, A.E. Power generation with pressure retarded osmosis: An experimental and theoretical investigation. J. Membr. Sci. 2009, 343, 42–52. [Google Scholar] [CrossRef]
- Seppälä, A.; Lampinen, M.J. Thermodynamic optimizing of pressure-retarded osmosis power generation systems. J. Membr. Sci. 1999, 161, 115–138. [Google Scholar] [CrossRef]
- Sharqawy, M.H.; Zubair, S.M.; Lienhard V, J.H. Energy utilization from disposed brine of desalination plants. In Proceedings of the International Mechanical Engineering Congress & Exposition, Houston, TX, USA, 9–15 November 2012; pp. 1–9. [Google Scholar]
- Nayar, K.G.; Sharqawy, M.H.; Banchik, L.D.; Lienhard, J.H. Thermophysical properties of sweater: A review and new correlations that include pressure dependence. Desalination 2016, 390, 1–24. [Google Scholar] [CrossRef]
- Bowen, W.R.; Welfoot, J.S. Modelling the performance of membrane nanofiltration critical assessment and model development. Chem. Eng. Sci. 2002, 57, 1121–1137. [Google Scholar] [CrossRef]
- Maisonneuve, J.; Pillay, P.; Laflamme, C.B. Pressure-retarded osmotic power system model considering non-ideal effects. Renew. Energy 2015, 75, 416–424. [Google Scholar] [CrossRef]
- Sharqawy, M.H.; Zubair, S.M.; Lienhard, J.H. Second law analysis of reverse osmosis desalination plants: An alternative design using pressure retarded osmosis. Energy 2011, 36, 6617–6626. [Google Scholar] [CrossRef]
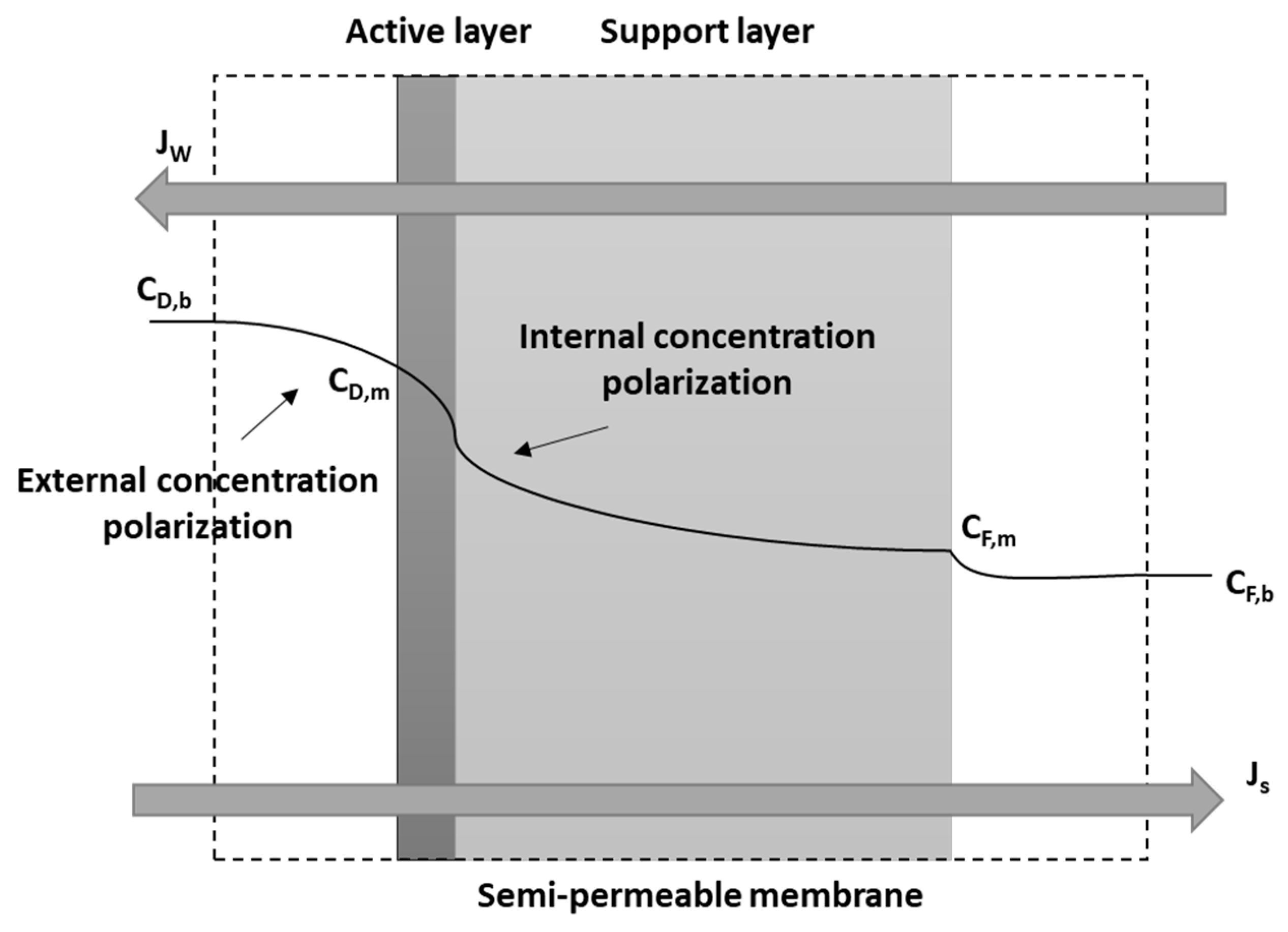
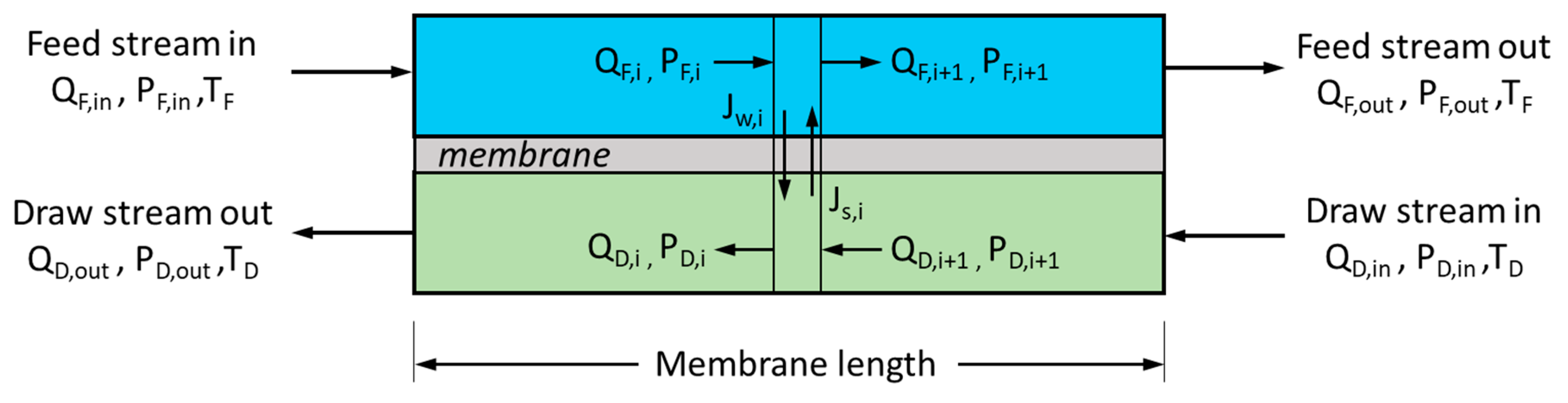
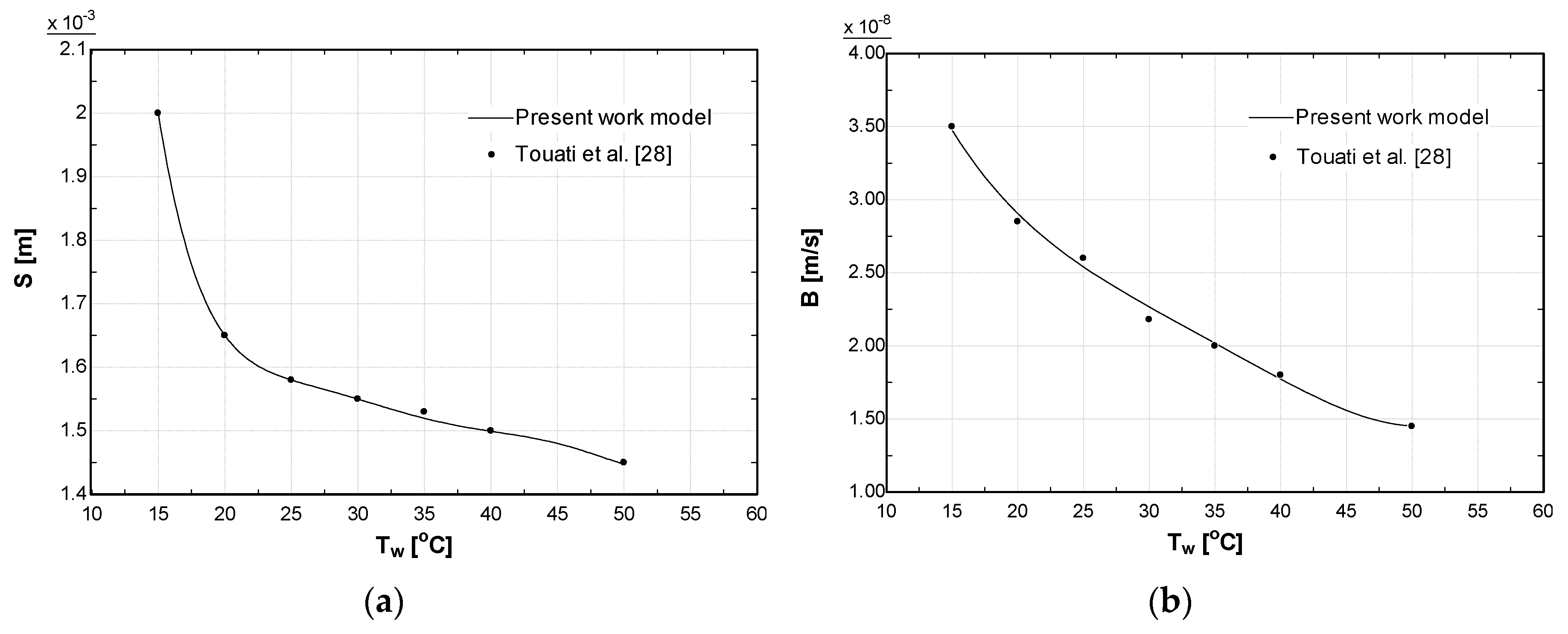
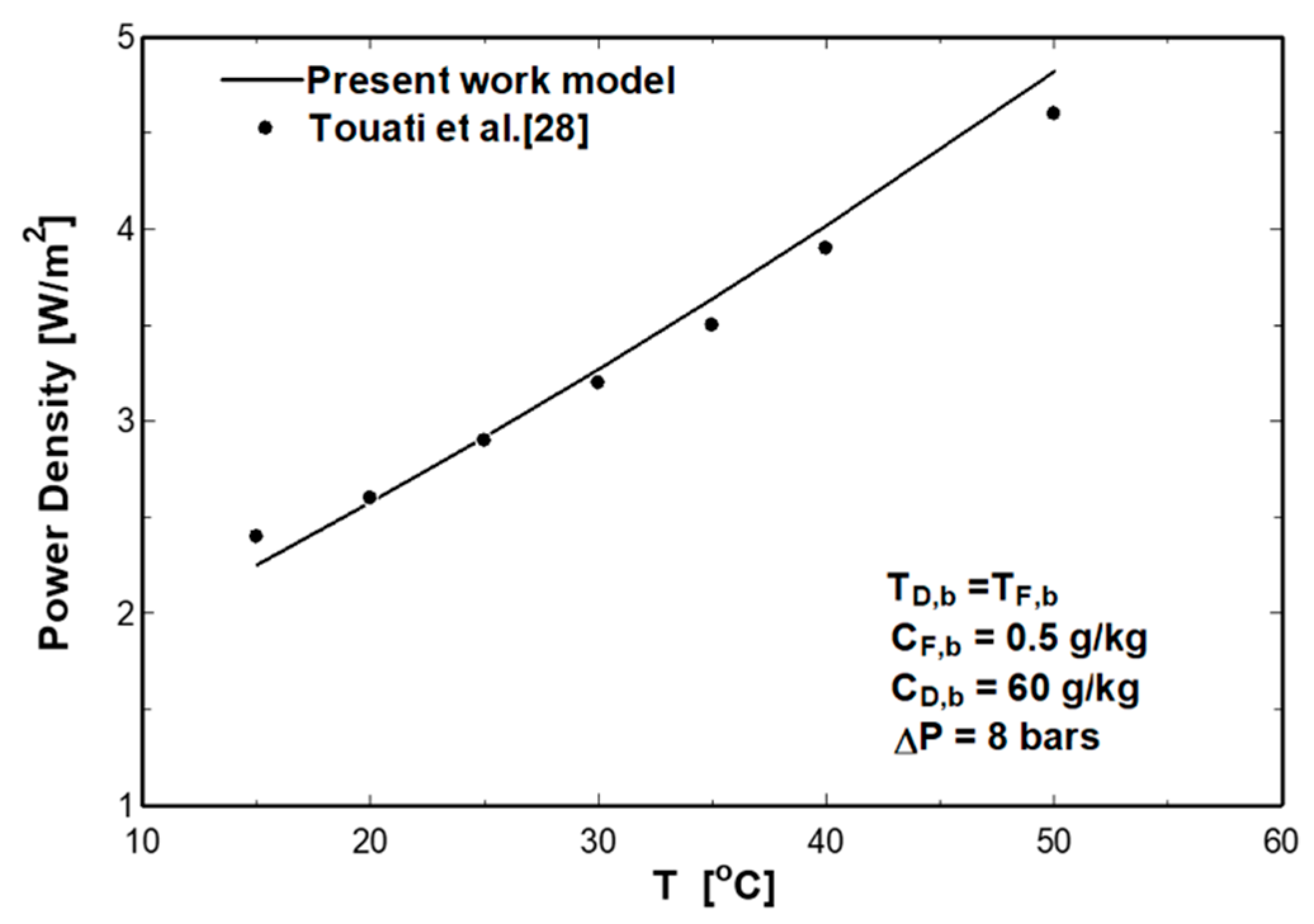
| Parameter | Value |
|---|---|
| Pore size | 0.35 nm |
| Porosity of the support layer | 80% |
| Porosity of the active layer | 0.6% |
| Support layer thickness | 12 μm |
| Active layer thickness | 100 nm |
| Effective membrane area | 18 cm2 |
| Channel dimensions (for feed and draw solutions) | 40 mm–25 mm–2.5 mm |
| Water permeability coefficient A | 1.06 × 10−9 m/s kPa |
| Salt permeability coefficient B | 2.62 × 10−8 m/s |
| Variable | Value |
|---|---|
| Feed temperature | 20–50 °C |
| Draw temperature | 20–50 °C |
| Feed concentration | 0.5 g/kg |
| Draw concentration | 35 g/kg–70 g/kg |
| Volume flow rate | 0.1–15 m3/hr |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelkader, B.; Sharqawy, M.H. Temperature Effects and Entropy Generation of Pressure Retarded Osmosis Process. Entropy 2019, 21, 1158. https://doi.org/10.3390/e21121158
Abdelkader B, Sharqawy MH. Temperature Effects and Entropy Generation of Pressure Retarded Osmosis Process. Entropy. 2019; 21(12):1158. https://doi.org/10.3390/e21121158
Chicago/Turabian StyleAbdelkader, Bassel, and Mostafa H. Sharqawy. 2019. "Temperature Effects and Entropy Generation of Pressure Retarded Osmosis Process" Entropy 21, no. 12: 1158. https://doi.org/10.3390/e21121158
APA StyleAbdelkader, B., & Sharqawy, M. H. (2019). Temperature Effects and Entropy Generation of Pressure Retarded Osmosis Process. Entropy, 21(12), 1158. https://doi.org/10.3390/e21121158





