Potential Exergy Storage Capacity of Salt Caverns in the Cheshire Basin Using Adiabatic Compressed Air Energy Storage
Abstract
1. Introduction
2. Salt Cavern and CAES System Description
2.1. Salt Caverns
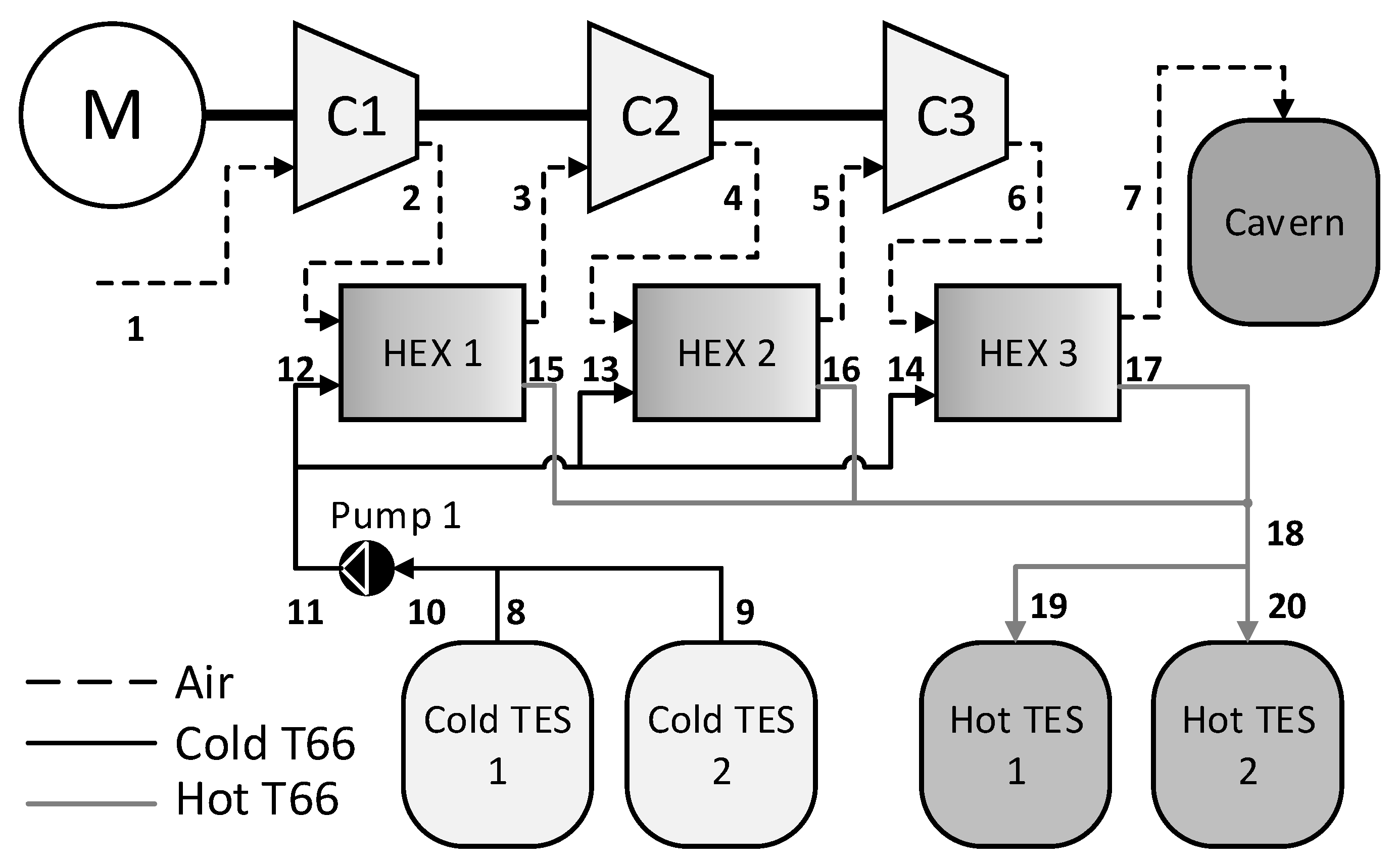
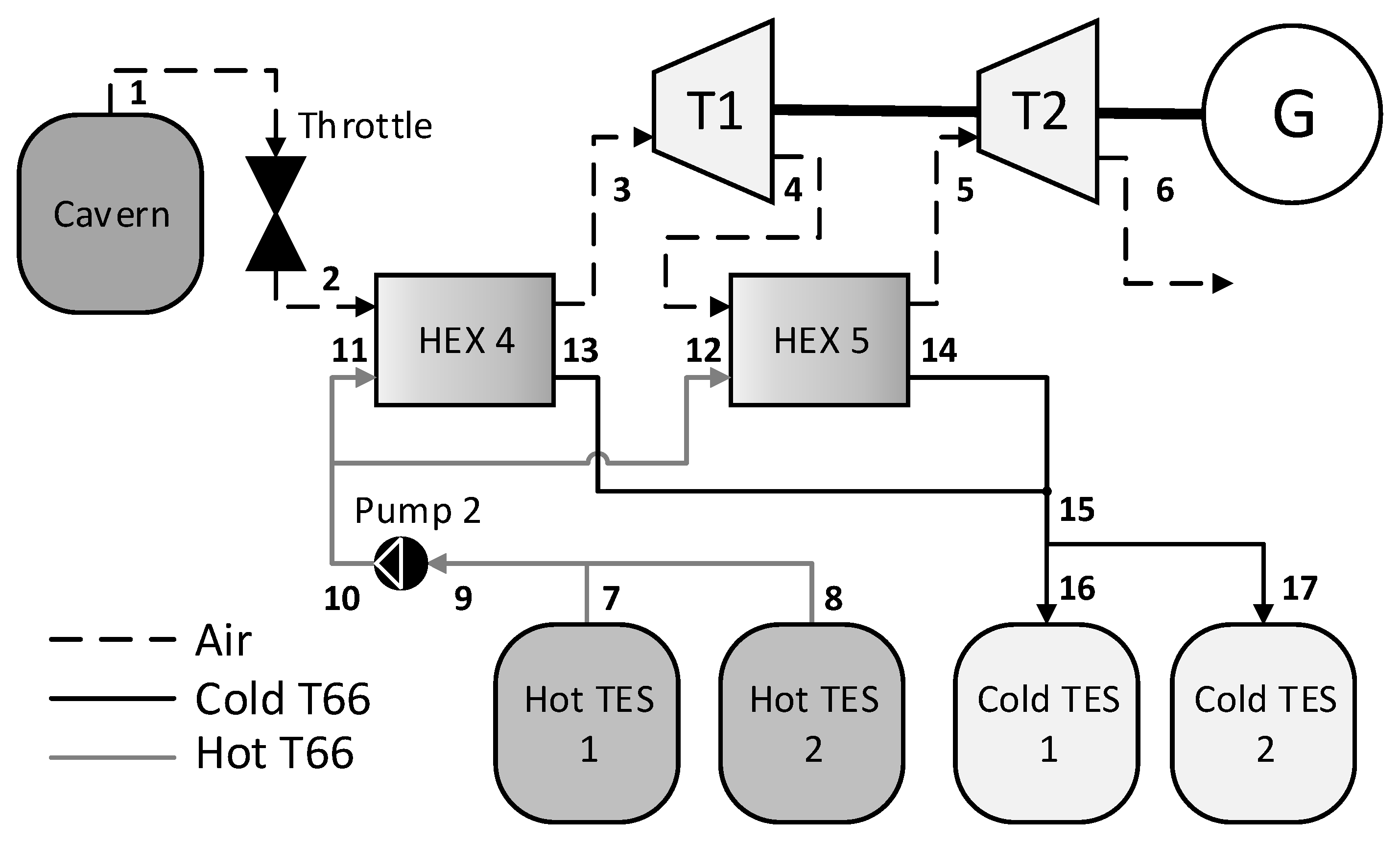
2.2. A-CAES System Structure
2.3. Simulation Study
3. Mathematical Model
- The air acts as an ideal gas.
- The system in any operation reaches steady state.
- The kinetic and potential energy changes are negligible.
- The isentropic efficiencies of compressors, pumps, and turbines are fixed. Their values are given in Table 2.
- The throttling process is isenthalpic.
- The heat and pressure loss in the pipes connecting the components is negligible.
- The ambient pressure and temperature are constant and equal to kPa and K, respectively.
3.1. Energy Analysis
3.1.1. Compressor Model
3.1.2. Turbine Model
3.1.3. Pump Model
3.1.4. Heat Exchanger Model
3.1.5. Throttle Model
3.1.6. Cavern Model
3.1.7. Thermal Energy Storage Model
3.2. Exergy Analysis
3.3. Performance Analysis
4. Results
4.1. System Results
4.2. Case Study
5. Discussion
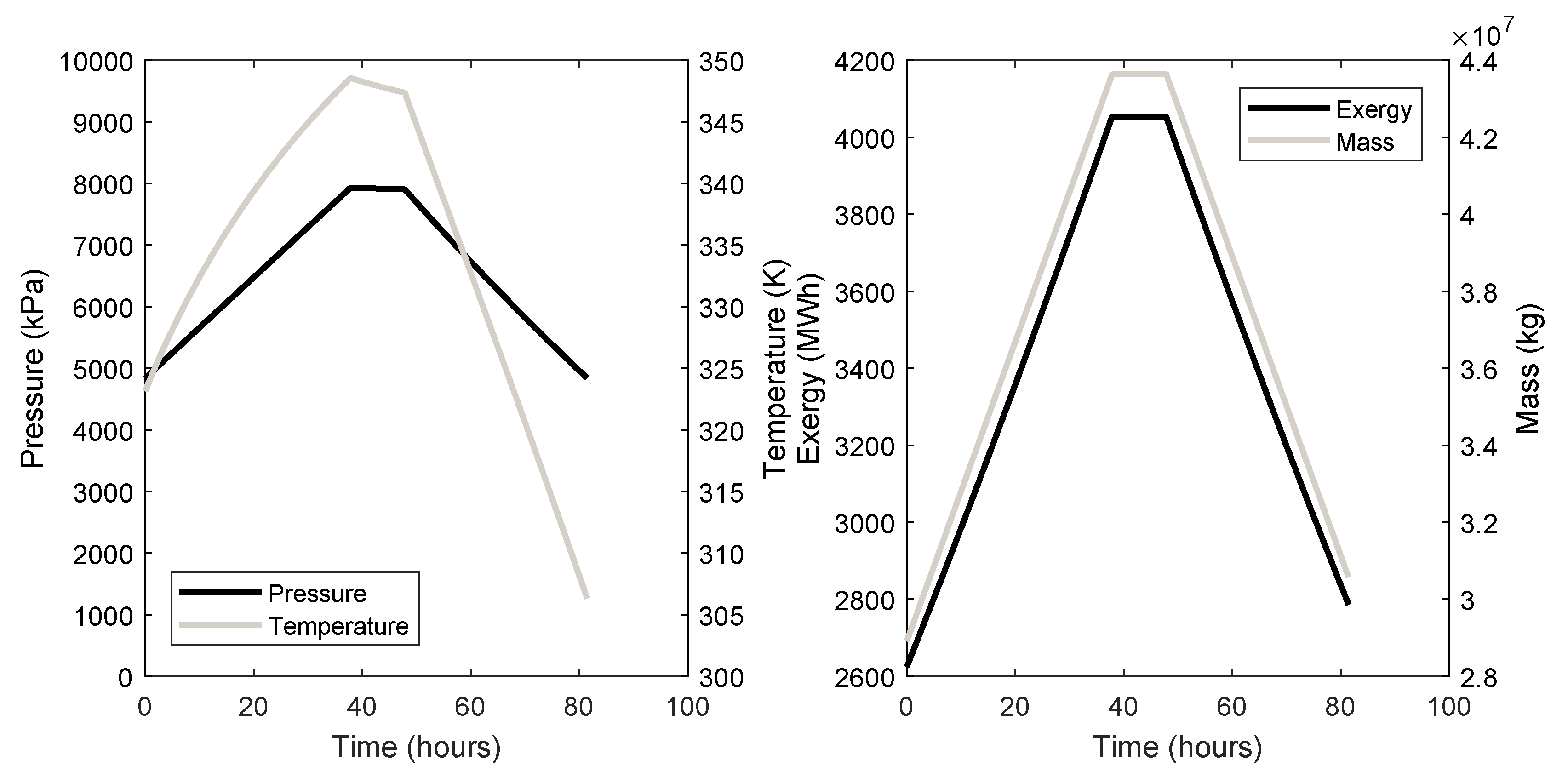
5.1. Total Exergy Storage Capacity
5.2. Work and Power
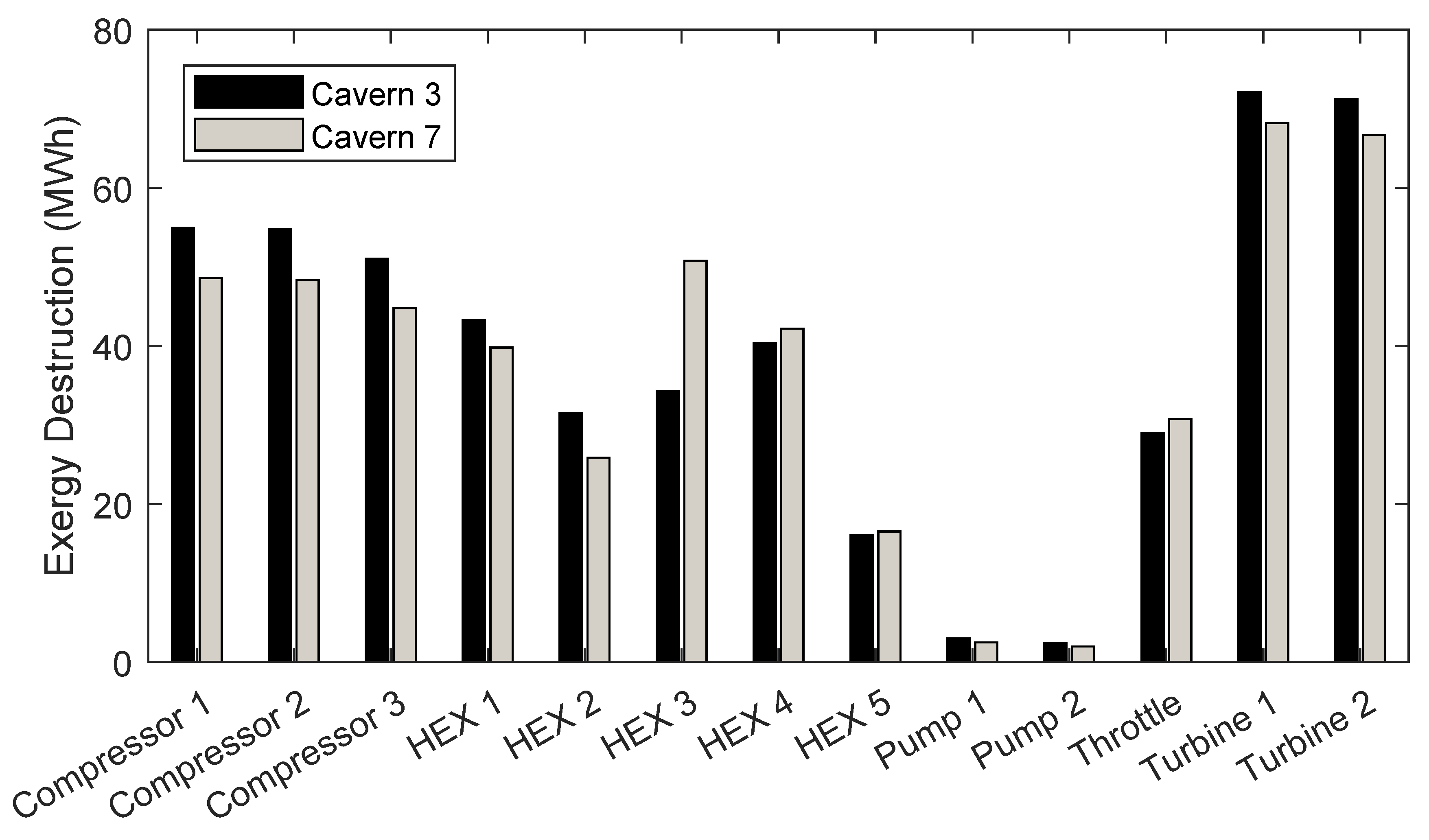
5.3. Component Exergy Destruction
5.4. Case Study
5.5. Future Work
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A-CAES | Adiabatic-CAES |
| BGS | British Geological Survey |
| D-CAES | Diabatic-CAES |
| CAES | Compressed Air Energy Storage |
| CHT | Convective Heat Transfer |
| ES | Energy Storage |
| GIS | Graphical Information System |
| HEX | Heat Exchanger |
| I-CAES | Isothermal CAES |
| MOL | Method of Lines |
| PBTES | Packed Bed Thermal Energy Storage |
| RTE | Round Trip Efficiency |
| SC-CAES | Super Critical-CAES |
| TES | Thermal Energy Storage |
References
- Geissbühler, L.; Becattini, V.; Zanganeh, G.; Zavattoni, S.; Barbato, M.; Haselbacher, A.; Steinfeld, A. Pilot-scale demonstration of advanced adiabatic compressed air energy storage, Part 1: Plant description and tests with sensible thermal-energy storage. J. Energy Storage 2018, 17, 129–139. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Yang, L.; Zhou, Y.; Wang, J. Experimental study of compressed air energy storage system with thermal energy storage. Energy 2016, 103, 182–191. [Google Scholar] [CrossRef]
- Kim, Y.M.; Shin, D.G.; Favrat, D. Operating characteristics of constant-pressure compressed air energy storage (CAES) system combined with pumped hydro storage based on energy and exergy analysis. Energy 2011, 36, 6220–6233. [Google Scholar] [CrossRef]
- Guanwei, J.; Weiqing, X.; Maolin, C.; Yan, S. Micron-sized water spray-cooled quasi-isothermal compression for compressed air energy storage. Exp. Therm. Fluid Sci. 2018, 96, 470–481. [Google Scholar] [CrossRef]
- Heidari, M.; Mortazavi, M.; Rufer, A. Design, modeling and experimental validation of a novel finned reciprocating compressor for Isothermal Compressed Air Energy Storage applications. Energy 2017, 140, 1252–1266. [Google Scholar] [CrossRef]
- Guo, H.; Xu, Y.; Chen, H.; Zhou, X. Thermodynamic characteristics of a novel supercritical compressed air energy storage system. Energy Conv. Manag. 2016, 115, 167–177. [Google Scholar] [CrossRef]
- Guo, H.; Xu, Y.; Chen, H.; Guo, C.; Qin, W. Thermodynamic analytical solution and exergy analysis for supercritical compressed air energy storage system. Appl. Energy 2017, 199, 96–106. [Google Scholar] [CrossRef]
- Cheung, B.C.; Carriveau, R.; Ting, D.S. Parameters affecting scalable underwater compressed air energy storage. Appl. Energy 2014, 134, 239–247. [Google Scholar] [CrossRef]
- Pimm, A.; Garvey, S.; de Jong, M. Commercial grid scaling of Energy Bags for underwater compressed air energy storage. Int. J. Environ. Stud. 2014, 71, 804–811. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J.; Krupke, C. Overview of current development in compressed air energy storage technology. Energy Procedia 2014, 62, 603–611. [Google Scholar] [CrossRef]
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in electrical energy storage system: A critical review. Pro. Nat. Sci. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- Sabihuddin, S.; Kiprakis, A.E.; Mueller, M. A numerical and graphical review of energy storage technologies. Energies 2015, 8, 172–216. [Google Scholar] [CrossRef]
- Venkataramani, G.; Parankusam, P.; Ramalingam, V.; Wang, J. A review on compressed air energy storage—A pathway for smart grid and polygeneration. Renew. Sustain. Energy Rev. 2016, 62, 895–907. [Google Scholar] [CrossRef]
- Guney, M.S.; Tepe, Y. Classification and assessment of energy storage systems. Renew. Sustain. Energy Rev. 2017, 75, 1187–1197. [Google Scholar] [CrossRef]
- Aneke, M.; Wang, M. Energy storage technologies and real life applications—A state of the art review. Appl. Energy 2016, 179, 350–377. [Google Scholar] [CrossRef]
- Amirante, R.; Cassone, E.; Distaso, E.; Tamburrano, P. Overview on recent developments in energy storage: Mechanical, electrochemical and hydrogen technologies. Energy Conv. Manag. 2017, 132, 372–387. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef]
- Mahlia, T.M.; Saktisahdan, T.J.; Jannifar, A.; Hasan, M.H.; Matseelar, H.S. A review of available methods and development on energy storage; Technology update. Renew. Sustain. Energy Rev. 2014, 33, 532–545. [Google Scholar] [CrossRef]
- Akinyele, D.O.; Rayudu, R.K. Review of energy storage technologies for sustainable power networks. Sustain. Energy Techn. 2014, 8, 74–91. [Google Scholar] [CrossRef]
- Zakeri, B.; Syri, S. Electrical energy storage systems: A comparative life cycle cost analysis. Renew. Sustain. Energy Rev. 2015, 42, 569–596. [Google Scholar] [CrossRef]
- Raju, M.; Kumar Khaitan, S. Modeling and simulation of compressed air storage in caverns: A case study of the Huntorf plant. Appl. Energy 2012, 89, 474–481. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, J.H.; Kim, S.J.; Favrat, D. Potential and evolution of compressed air energy storage: Energy and exergy analyses. Entropy 2012, 14, 1501–1521. [Google Scholar] [CrossRef]
- Szablowski, L.; Krawczyk, P.; Badyda, K.; Karellas, S.; Kakaras, E.; Bujalski, W. Energy and exergy analysis of adiabatic compressed air energy storage system. Energy 2017, 138, 12–18. [Google Scholar] [CrossRef]
- Liu, J.L.; Wang, J.H. A comparative research of two adiabatic compressed air energy storage systems. Energy Conv. Manag. 2016, 108, 566–578. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Krupke, C.; Wang, Y.; Sheng, Y.; Li, J.; Xu, Y.; Wang, D.; Miao, S.; Chen, H. Modelling study, efficiency analysis and optimisation of large-scale Adiabatic Compressed Air Energy Storage systems with low-temperature thermal storage. Appl. Energy 2016, 162, 589–600. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, Y.; Li, X.; Xu, J. Theoretical evaluation on the impact of heat exchanger in Advanced Adiabatic Compressed Air Energy Storage system. Energy Conv. Manag. 2014, 86, 1031–1044. [Google Scholar] [CrossRef]
- Peng, H.; Yang, Y.; Li, R.; Ling, X. Thermodynamic analysis of an improved adiabatic compressed air energy storage system. Appl. Energy 2016, 183, 1361–1373. [Google Scholar] [CrossRef]
- Barbour, E.; Mignard, D.; Ding, Y.; Li, Y. Adiabatic Compressed Air Energy Storage with packed bed thermal energy storage. Appl. Energy 2015, 155, 804–815. [Google Scholar] [CrossRef]
- He, W.; Wang, J.; Wang, Y.; Ding, Y.; Chen, H.; Wu, Y.; Garvey, S. Study of cycle-to-cycle dynamic characteristics of adiabatic Compressed Air Energy Storage using packed bed Thermal Energy Storage. Energy 2017, 141, 2120–2134. [Google Scholar] [CrossRef]
- Mazloum, Y.; Sayah, H.; Nemer, M. Exergy analysis and exergoeconomic optimization of a constant-pressure adiabatic compressed air energy storage system. J. Energy Storage 2017, 14, 192–202. [Google Scholar] [CrossRef]
- Chen, L.X.; Xie, M.N.; Zhao, P.P.; Wang, F.X.; Hu, P.; Wang, D.X. A novel isobaric adiabatic compressed air energy storage (IA-CAES) system on the base of volatile fluid. Appl. Energy 2018, 210, 198–210. [Google Scholar] [CrossRef]
- Zhao, P.; Gao, L.; Wang, J.; Dai, Y. Energy efficiency analysis and off-design analysis of two different discharge modes for compressed air energy storage system using axial turbines. Renew. Energy 2016, 85, 1164–1177. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, W.; Ting, D.S.; Carriveau, R.; Wang, Z. Conventional and advanced exergy analyses of an underwater compressed air energy storage system. Appl. Energy 2016, 180, 810–822. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ahmadi, M.H.; Bidi, M.; Joda, F.; Valero, A.; Uson, S. Exergy analysis of a Combined Cooling, Heating and Power system integrated with wind turbine and compressed air energy storage system. Energy Conv. Manag. 2017, 131, 69–78. [Google Scholar] [CrossRef]
- Parkes, D.; Evans, D.J.; Williamson, P.; Williams, J.D.O. Estimating available salt volume for potential CAES development: A case study using the Northwich Halite of the Cheshire Basin. J. Energy Storage 2018, 18, 50–61. [Google Scholar] [CrossRef]
- He, W.; Luo, X.; Evans, D.; Busby, J.; Garvey, S.; Parkes, D.; Wang, J. Exergy storage of compressed air in cavern and cavern volume estimation of the large-scale compressed air energy storage system. Appl. Energy 2017, 208, 745–757. [Google Scholar] [CrossRef]
- Dincer, I.; Rosen, M. Exergy: Energy, Environment and Sustainable Development; Elsevier: Amsterdam, Netherlands, 2007. [Google Scholar]

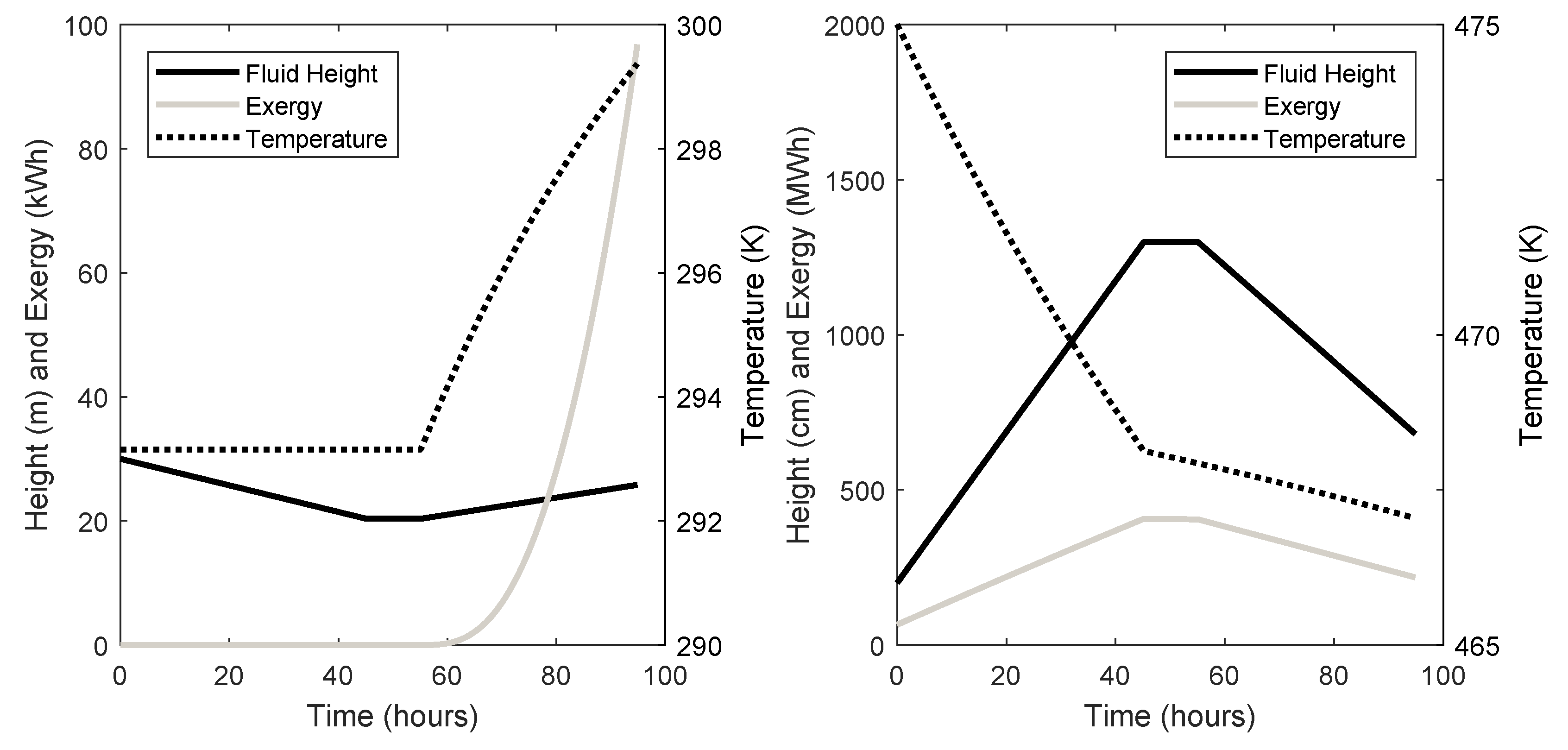
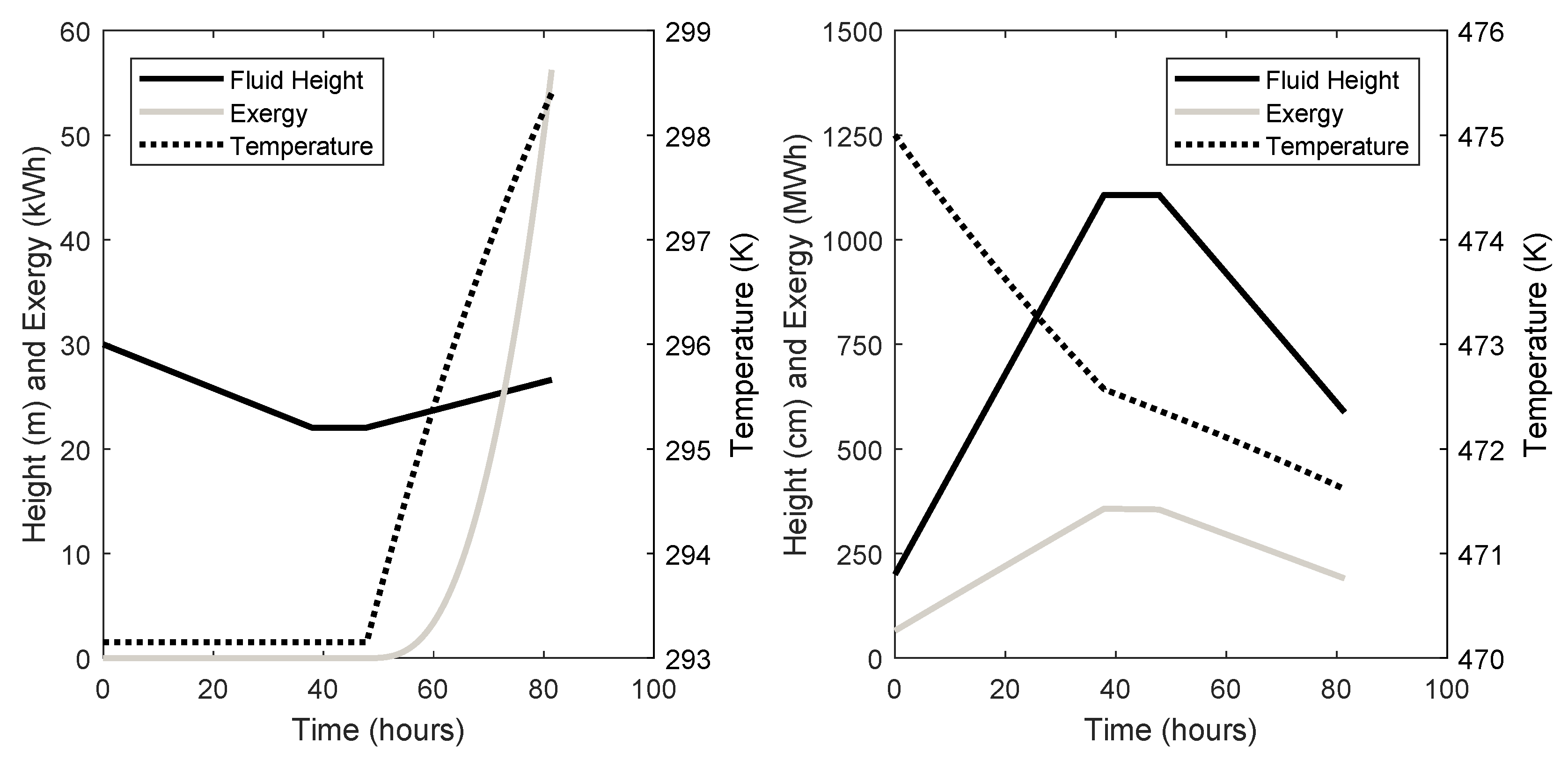
| Cavern ID | Initial Air Pressure (kPa) | Initial Air Mass (kg) | Cavern Volume (m3) | Cavern Surface Area (m2) | Maximum Pressure (kPa) | Ambient Rock Temp (K) | Height (m) |
|---|---|---|---|---|---|---|---|
| 1 | 8667.65 | 7.09E + 07 | 7.58E + 05 | 47,251.08 | 14,209.27 | 307.47 | 138.01 |
| 2 | 3379.81 | 2.62E + 07 | 7.18E + 05 | 45,302.41 | 5540.68 | 292.12 | 130.59 |
| 3 | 3732.66 | 3.40E + 07 | 8.44E + 05 | 51,347.23 | 6119.12 | 294.21 | 153.60 |
| 4 | 2937.49 | 2.25E + 07 | 7.10E + 05 | 44,954.27 | 4815.55 | 290.91 | 129.26 |
| 5 | 8101.01 | 6.67E + 07 | 7.64E + 05 | 47,514.61 | 13,280.34 | 293.97 | 139.01 |
| 6 | 6789.32 | 6.09E + 07 | 8.32E + 05 | 50,778.41 | 11,130.03 | 291.36 | 151.43 |
| 7 | 4840.24 | 2.89E + 07 | 5.54E + 05 | 37,483.65 | 7934.82 | 295.37 | 100.83 |
| 8 | 8946.56 | 6.08E + 07 | 6.31E + 05 | 41,143.08 | 14,666.49 | 311.82 | 114.76 |
| 9 | 6943.81 | 4.21E + 07 | 5.62E + 05 | 37,855.57 | 11,383.30 | 301.81 | 102.24 |
| 10 | 9766.95 | 6.42E + 07 | 6.10E + 05 | 40,145.57 | 16,011.39 | 316.56 | 110.96 |
| Parameter | Value | Unit |
|---|---|---|
| Compressor Isentropic Efficiency, | 0.9 | N/A |
| Compressor Pressure Ratio, | 3.8–6.4 | N/A |
| Turbine Isentropic Efficiency, | 0.9 | N/A |
| Turbine Pressure Ratio, | 4.4–9.4 | N/A |
| HEX Effectiveness, | 0.95 | N/A |
| HEX Pressure Drop | 4% | N/A |
| Pump Isentropic Efficiency, | 0.7 | N/A |
| Pump Pressure Ratio, | 1.8 | N/A |
| Cold Store Initial Temp | 293.15 | K |
| Cold Store Initial Liquid Height | 30 | m |
| Cold Store Max Height, | 30 | m |
| Cold Store Area, | 1500 | m2 |
| Cold Store Thermal Conductivity, | 10 | Wm−1K−1 |
| Hot Store Initial Temp | 475 | K |
| Hot Store Initial Liquid Height | 2 | m |
| Hot Store Max Height, | 30 | m |
| Hot Store Area, | 1500 | m2 |
| Hot Store Thermal Conductivity, | 0.1 | Wm−1K−1 |
| Throttle Output Pressure | 2937.49–9766.95 | kPa |
| Cavern ID | Initial Exergy (MWh) | Final Exergy (MWh) | Net Exergy (MWh) |
|---|---|---|---|
| 1 | 7400.80 | 11,332.41 | 3931.61 |
| 2 | 2154.44 | 3351.41 | 1196.97 |
| 3 | 2877.50 | 4471.98 | 1594.48 |
| 4 | 1779.68 | 2775.31 | 995.63 |
| 5 | 6861.64 | 10,543.50 | 3681.86 |
| 6 | 6012.71 | 9269.01 | 3256.30 |
| 7 | 2625.12 | 4054.37 | 1429.25 |
| 8 | 6397.11 | 9780.59 | 3383.49 |
| 9 | 4174.10 | 6409.30 | 2235.20 |
| 10 | 6884.55 | 10,506.05 | 3621.51 |
| Totals | 47,167.65 | 72,493.95 | 25,326.30 |
| Cavern ID | Comp 1 (MWh) | Comp 2 (MWh) | Comp 3 (MWh) | Pump 1 (MWh) | Pump 2 (MWh) | Turb 2 (MWh) | Turb 2 (MWh) | Total In (MWh) | Total Out (MWh) | RTE |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2150.16 | 2272.66 | 2411.97 | 6.94 | 8.81 | 1893.03 | 1775.67 | 6850.53 | 3668.70 | 53.55% |
| 2 | 627.01 | 653.06 | 675.81 | 2.82 | 3.21 | 535.74 | 520.88 | 1961.90 | 1056.62 | 53.86% |
| 3 | 832.25 | 867.74 | 899.53 | 3.64 | 4.17 | 712.56 | 690.52 | 2607.32 | 1403.07 | 53.81% |
| 4 | 516.8 | 537.12 | 553.96 | 2.45 | 2.77 | 433.03 | 433.03 | 1613.10 | 866.07 | 53.69% |
| 5 | 1986.15 | 2095.46 | 2216.99 | 6.65 | 8.22 | 1736.74 | 1633.69 | 6313.47 | 3370.43 | 53.38% |
| 6 | 1743.29 | 1834.04 | 1930.94 | 6.19 | 7.48 | 1509.38 | 1430.75 | 5521.95 | 2940.13 | 53.24% |
| 7 | 757.49 | 793.03 | 827.81 | 3.00 | 3.55 | 652.81 | 627.17 | 2384.87 | 1279.97 | 54.67% |
| 8 | 1837.09 | 1941.76 | 2060.78 | 5.93 | 7.47 | 1630.69 | 1526.25 | 5853.04 | 3156.94 | 54.94% |
| 9 | 1208.14 | 1272.24 | 1341.69 | 4.20 | 5.15 | 1048.65 | 992.82 | 3831.43 | 2041.47 | 54.28% |
| 10 | 1984.30 | 2101.16 | 2236.48 | 6.18 | 7.93 | 1643.9 | 1763.43 | 6336.06 | 3407.40 | 54.75% |
| Totals | 13,642.66 | 14,368.29 | 15,155.96 | 48.00 | 58.76 | 11,796.59 | 11,394.21 | 43,273.68 | 23,190.80 | N/A |
| Cavern ID | Compressors (MWh) | Charge HEXs (MWh) | Pumps (MWh) | Throttle (MWh) | Turbines (MWh) | Discharge HEXs (MWh) | Total (MWh) |
|---|---|---|---|---|---|---|---|
| 1 | 380.42 | 450.09 | 10.50 | 116.85 | 408.28 | 233.86 | 1600.00 |
| 2 | 121.28 | 79.57 | 4.13 | 20.32 | 106.73 | 40.03 | 372.06 |
| 3 | 160.70 | 108.91 | 5.35 | 28.99 | 143.26 | 56.38 | 503.60 |
| 4 | 101.59 | 60.44 | 3.58 | 15.37 | 87.14 | 30.90 | 299.02 |
| 5 | 355.26 | 395.34 | 9.98 | 105.83 | 372.38 | 210.16 | 1448.94 |
| 6 | 316.61 | 318.23 | 9.24 | 85.66 | 320.41 | 166.08 | 1216.23 |
| 7 | 141.70 | 116.41 | 4.45 | 30.74 | 134.86 | 58.71 | 486.86 |
| 8 | 325.04 | 384.56 | 8.98 | 101.95 | 353.57 | 205.50 | 1379.59 |
| 9 | 218.77 | 226.83 | 6.31 | 59.65 | 223.74 | 115.36 | 850.66 |
| 10 | 348.55 | 432.63 | 9.40 | 112.73 | 382.03 | 232.44 | 1517.78 |
| State | Pressure (kPa) | Temperature (K) | Enthalpy (kJ/kg) | Mass Flow Rate (kg/s) | Specific Exergy (kJ/kg) |
|---|---|---|---|---|---|
| 1 | 101.32 | 293.15 | 294.95 | 108.00 | 0.00 |
| 2 | 415.41 | 454.88 | 465.68 | 108.00 | 152.13 |
| 3 | 398.80 | 301.24 | 304.57 | 108.00 | 115.32 |
| 4 | 1635.06 | 467.42 | 482.59 | 108.00 | 272.13 |
| 5 | 1569.66 | 301.86 | 310.74 | 108.00 | 230.29 |
| 6 | 6435.60 | 468.40 | 495.28 | 108.00 | 388.13 |
| 7 | 6178.18 | 301.91 | 332.74 | 108.00 | 344.55 |
| 8 | 101.32 | 293.15 | 0.01 | 89.89 | 0.00 |
| 9 | 101.32 | 293.15 | 0.01 | 89.89 | 0.00 |
| 10 | 101.32 | 293.15 | 0.01 | 179.79 | 0.00 |
| 11 | 182.38 | 293.40 | 0.46 | 179.79 | 0.08 |
| 12 | 182.38 | 293.40 | 0.46 | 62.04 | 0.08 |
| 13 | 182.38 | 293.40 | 0.46 | 60.70 | 0.08 |
| 14 | 182.38 | 293.40 | 0.46 | 57.05 | 0.08 |
| 15 | 175.08 | 446.80 | 280.93 | 62.04 | 57.43 |
| 16 | 175.08 | 458.72 | 306.20 | 60.70 | 66.34 |
| 17 | 175.08 | 459.65 | 308.17 | 57.05 | 67.05 |
| 18 | 175.08 | 454.90 | 298.11 | 179.79 | 63.41 |
| 19 | 175.08 | 454.90 | 298.11 | 89.89 | 63.41 |
| 20 | 175.08 | 454.90 | 298.11 | 89.89 | 63.41 |
| State | Pressure (kPa) | Temperature (K) | Enthalpy (kJ/kg) | Mass Flow Rate (kg/s) | Specific Exergy (kJ/kg) |
|---|---|---|---|---|---|
| 1 | 101.32 | 293.15 | 294.95 | 108.00 | 0.00 |
| 2 | 455.94 | 468.01 | 480.21 | 108.00 | 164.88 |
| 3 | 437.70 | 301.89 | 305.42 | 108.00 | 123.16 |
| 4 | 1969.66 | 481.97 | 499.37 | 108.00 | 293.57 |
| 5 | 1890.87 | 302.59 | 312.98 | 108.00 | 245.90 |
| 6 | 8508.93 | 483.09 | 515.44 | 108.00 | 417.91 |
| 7 | 8168.58 | 302.65 | 342.55 | 108.00 | 367.77 |
| 8 | 101.32 | 293.15 | 0.01 | 88.35 | 0.00 |
| 9 | 101.32 | 293.15 | 0.01 | 88.35 | 0.00 |
| 10 | 101.32 | 293.15 | 0.01 | 176.71 | 0.00 |
| 11 | 182.38 | 293.40 | 0.46 | 176.71 | 0.08 |
| 12 | 182.38 | 293.40 | 0.46 | 61.50 | 0.08 |
| 13 | 182.38 | 293.40 | 0.46 | 59.97 | 0.08 |
| 14 | 182.38 | 293.40 | 0.46 | 55.24 | 0.08 |
| 15 | 175.08 | 459.28 | 307.40 | 61.50 | 66.77 |
| 16 | 175.08 | 472.54 | 336.15 | 59.97 | 77.43 |
| 17 | 175.08 | 473.60 | 338.47 | 55.24 | 78.31 |
| 18 | 175.08 | 468.26 | 326.87 | 176.71 | 73.90 |
| 19 | 175.08 | 468.26 | 326.87 | 88.35 | 73.90 |
| 20 | 175.08 | 468.26 | 326.87 | 88.35 | 73.90 |
| State T = 0 | Pressure (kPa) | Temp (K) | Enthalpy (kJ/kg) | Mass Flow Rate (kg/s) | Specific Exergy (kJ/kg) | State T = end | Pressure (kPa) | Temp (K) | Enthalpy (kJ/kg) | Mass Flow Rate (kg/s) | Specific Exergy (kJ/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6094.52 | 346.10 | 371.99 | 108.00 | 347.91 | 1 | 3732.66 | 305.00 | 323.97 | 108.00 | 302.76 |
| 2 | 3732.66 | 354.52 | 371.99 | 108.00 | 308.45 | 2 | 3732.66 | 305.00 | 323.97 | 108.00 | 302.76 |
| 3 | 3583.36 | 462.26 | 481.88 | 108.00 | 336.25 | 3 | 3583.36 | 459.78 | 479.23 | 108.00 | 335.31 |
| 4 | 731.30 | 310.42 | 315.38 | 108.00 | 166.63 | 4 | 731.30 | 308.76 | 313.71 | 108.00 | 166.54 |
| 5 | 702.05 | 460.05 | 472.11 | 108.00 | 198.19 | 5 | 702.05 | 459.97 | 472.02 | 108.00 | 198.16 |
| 6 | 143.27 | 308.94 | 311.21 | 108.00 | 29.55 | 6 | 143.27 | 308.89 | 311.15 | 108.00 | 29.55 |
| 7 | 101.32 | 467.93 | 326.02 | 57.22 | 73.56 | 7 | 101.32 | 467.93 | 326.02 | 58.00 | 73.56 |
| 8 | 101.32 | 467.93 | 326.02 | 57.22 | 73.56 | 8 | 101.32 | 467.93 | 326.02 | 58.00 | 73.56 |
| 9 | 101.32 | 467.93 | 326.02 | 114.44 | 73.56 | 9 | 101.32 | 467.93 | 326.02 | 116.01 | 73.56 |
| 10 | 182.38 | 468.32 | 326.93 | 114.44 | 73.96 | 10 | 182.38 | 468.32 | 326.93 | 116.01 | 73.96 |
| 11 | 182.38 | 468.32 | 326.93 | 55.35 | 73.96 | 11 | 182.38 | 468.32 | 326.93 | 56.86 | 73.96 |
| 12 | 182.38 | 468.32 | 326.93 | 59.09 | 73.96 | 12 | 182.38 | 468.32 | 326.93 | 59.15 | 73.96 |
| 13 | 175.08 | 360.21 | 112.52 | 55.35 | 11.50 | 13 | 175.08 | 313.17 | 32.03 | 56.86 | 1.12 |
| 14 | 175.08 | 318.32 | 40.47 | 59.09 | 1.73 | 14 | 175.08 | 316.74 | 37.87 | 59.15 | 1.53 |
| 15 | 175.08 | 338.58 | 75.32 | 114.44 | 5.39 | 15 | 175.08 | 314.27 | 35.00 | 116.01 | 1.24 |
| 16 | 175.08 | 338.58 | 75.32 | 57.22 | 5.39 | 16 | 175.08 | 314.27 | 35.00 | 58.00 | 1.24 |
| 17 | 175.08 | 338.58 | 75.32 | 57.22 | 5.39 | 17 | 175.08 | 314.27 | 35.00 | 58.00 | 1.24 |
| State T = 0 | Pressure (kPa) | Temp (K) | Enthalpy (kJ/kg) | Mass Flow Rate (kg/s) | Specific Exergy (kJ/kg) | State T = end | Pressure (kPa) | Temp (K) | Enthalpy (kJ/kg) | Mass Flow Rate (kg/s) | Specific Exergy (kJ/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7905.10 | 347.37 | 379.70 | 108.00 | 369.82 | 1 | 4840.24 | 306.34 | 330.36 | 108.00 | 324.44 |
| 2 | 4840.24 | 357.88 | 379.70 | 108.00 | 330.78 | 2 | 4840.24 | 306.34 | 330.36 | 108.00 | 324.44 |
| 3 | 4646.63 | 466.64 | 489.19 | 108.00 | 359.87 | 3 | 4646.63 | 464.06 | 486.43 | 108.00 | 358.87 |
| 4 | 829.76 | 303.38 | 308.76 | 108.00 | 176.91 | 4 | 829.76 | 301.71 | 307.08 | 108.00 | 176.85 |
| 5 | 796.56 | 463.92 | 308.76 | 108.00 | 210.27 | 5 | 796.56 | 463.83 | 476.50 | 108.00 | 210.23 |
| 6 | 142.24 | 301.61 | 308.76 | 108.00 | 28.66 | 6 | 142.24 | 301.56 | 303.69 | 108.00 | 28.65 |
| 7 | 101.32 | 472.37 | 335.71 | 56.67 | 77.21 | 7 | 101.32 | 472.37 | 335.71 | 57.46 | 77.21 |
| 8 | 101.32 | 472.37 | 335.71 | 56.67 | 77.21 | 8 | 101.32 | 472.37 | 335.71 | 57.46 | 77.21 |
| 9 | 101.32 | 472.37 | 335.71 | 113.34 | 77.21 | 9 | 101.32 | 472.37 | 335.71 | 114.93 | 77.21 |
| 10 | 182.38 | 472.76 | 336.63 | 113.34 | 77.62 | 10 | 182.38 | 472.76 | 336.63 | 114.93 | 77.62 |
| 11 | 182.38 | 472.76 | 336.63 | 54.25 | 77.62 | 11 | 182.38 | 472.76 | 336.63 | 55.78 | 77.62 |
| 12 | 182.38 | 472.76 | 336.63 | 59.09 | 77.62 | 12 | 182.38 | 472.76 | 336.63 | 59.15 | 77.62 |
| 13 | 175.08 | 363.62 | 118.67 | 54.25 | 12.66 | 13 | 175.08 | 314.66 | 34.45 | 55.78 | 1.29 |
| 14 | 175.08 | 311.85 | 29.88 | 59.09 | 0.99 | 14 | 175.08 | 310.26 | 27.29 | 59.15 | 0.84 |
| 15 | 175.08 | 336.63 | 72.38 | 113.34 | 4.95 | 15 | 175.08 | 311.35 | 30.76 | 114.93 | 0.94 |
| 16 | 175.08 | 336.63 | 72.38 | 56.67 | 4.95 | 16 | 175.08 | 311.35 | 30.76 | 57.46 | 0.94 |
| 17 | 175.08 | 336.63 | 72.38 | 56.67 | 4.95 | 17 | 175.08 | 311.35 | 30.76 | 57.46 | 0.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dooner, M.; Wang, J. Potential Exergy Storage Capacity of Salt Caverns in the Cheshire Basin Using Adiabatic Compressed Air Energy Storage. Entropy 2019, 21, 1065. https://doi.org/10.3390/e21111065
Dooner M, Wang J. Potential Exergy Storage Capacity of Salt Caverns in the Cheshire Basin Using Adiabatic Compressed Air Energy Storage. Entropy. 2019; 21(11):1065. https://doi.org/10.3390/e21111065
Chicago/Turabian StyleDooner, Mark, and Jihong Wang. 2019. "Potential Exergy Storage Capacity of Salt Caverns in the Cheshire Basin Using Adiabatic Compressed Air Energy Storage" Entropy 21, no. 11: 1065. https://doi.org/10.3390/e21111065
APA StyleDooner, M., & Wang, J. (2019). Potential Exergy Storage Capacity of Salt Caverns in the Cheshire Basin Using Adiabatic Compressed Air Energy Storage. Entropy, 21(11), 1065. https://doi.org/10.3390/e21111065






