Enthalpy Relaxation of Polyamide 11 of Different Morphology Far Below the Glass Transition Temperature
Abstract
:1. Introduction
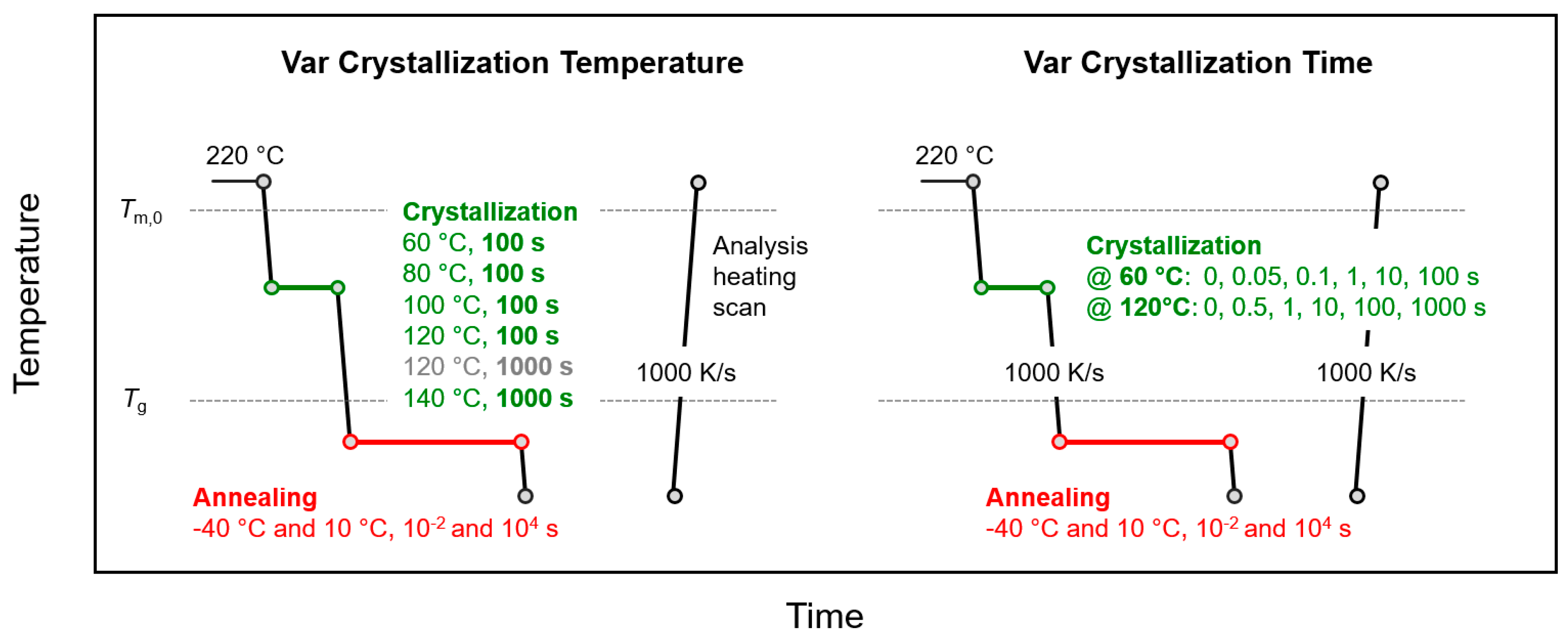
2. Materials and Methods
3. Results and Discussion
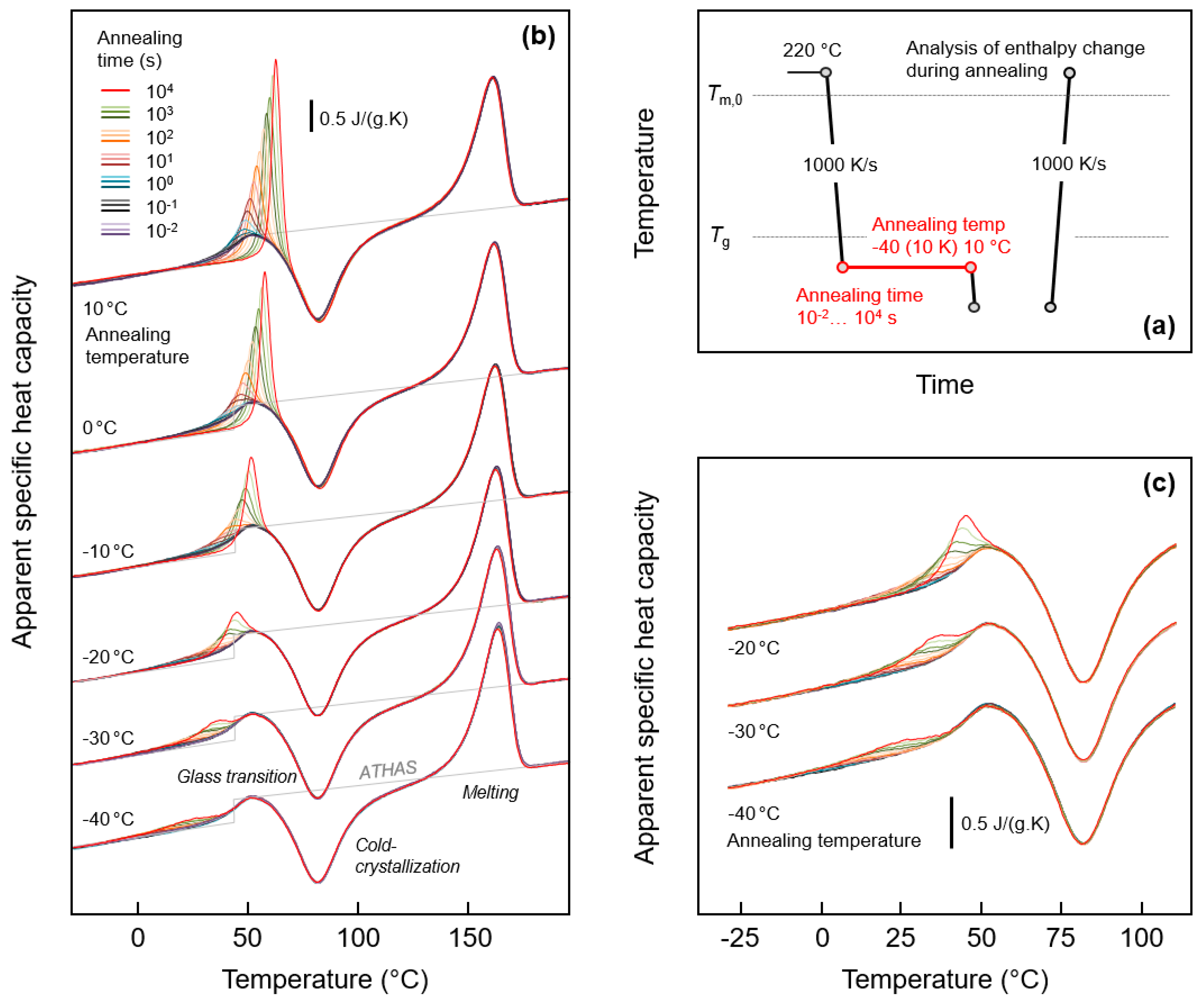
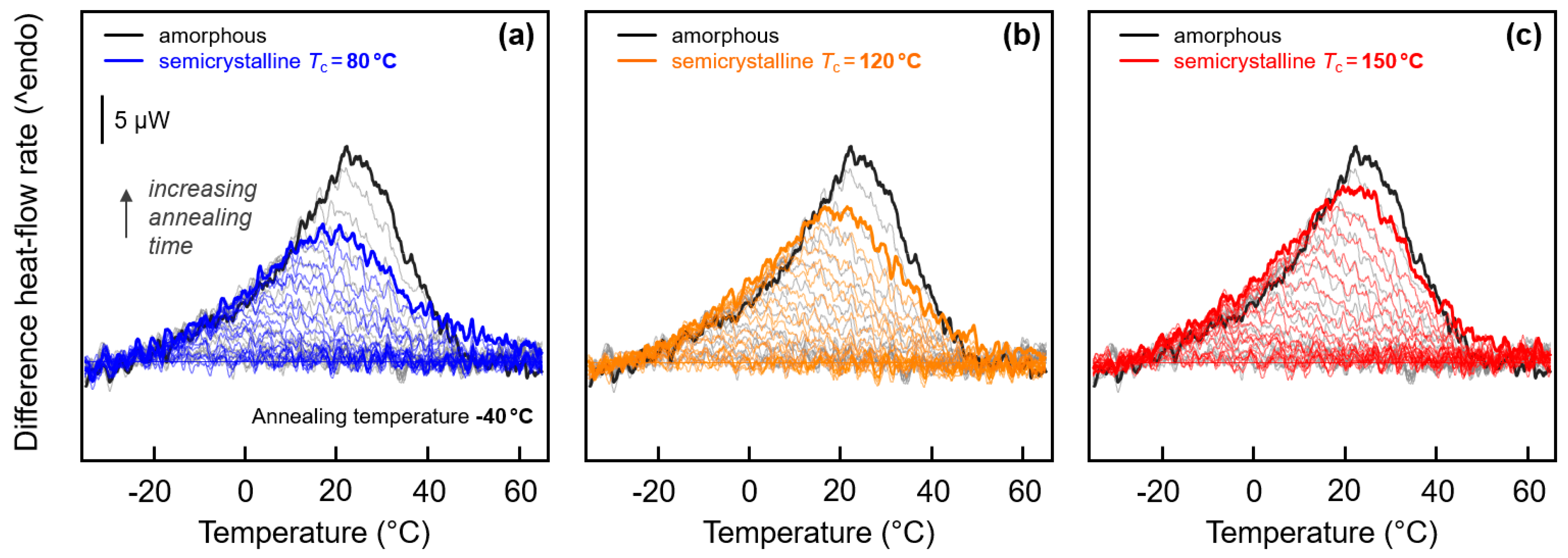
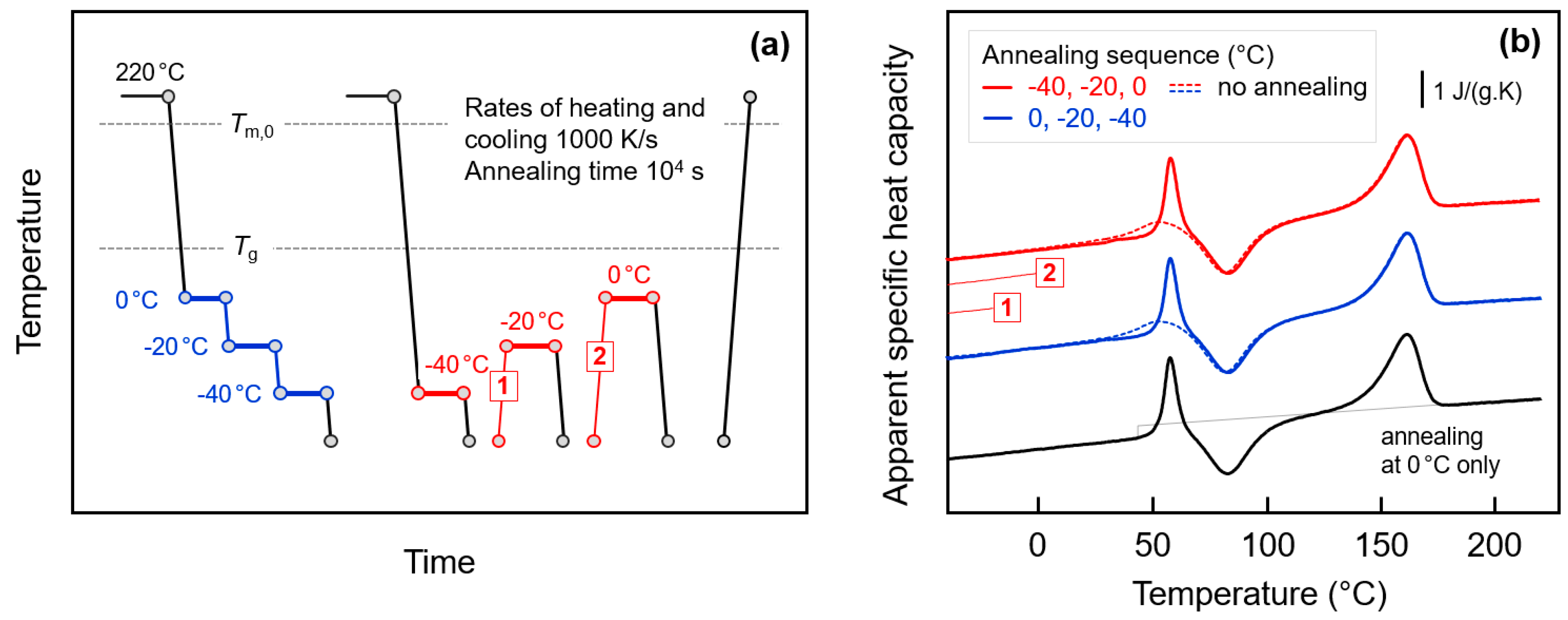
3.1. Glass Relaxation and/or Ordering of Fully Amorphous PA 11
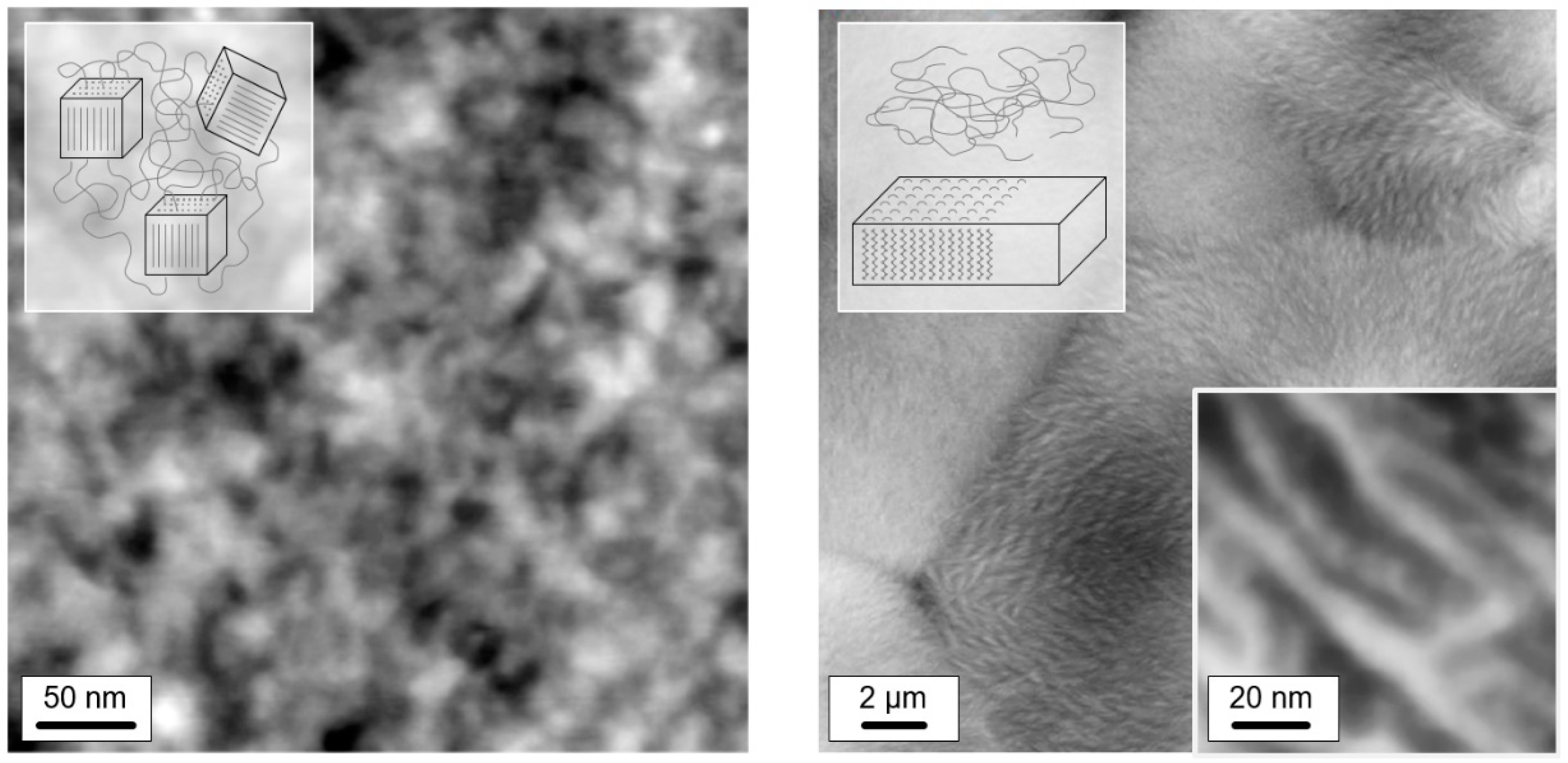
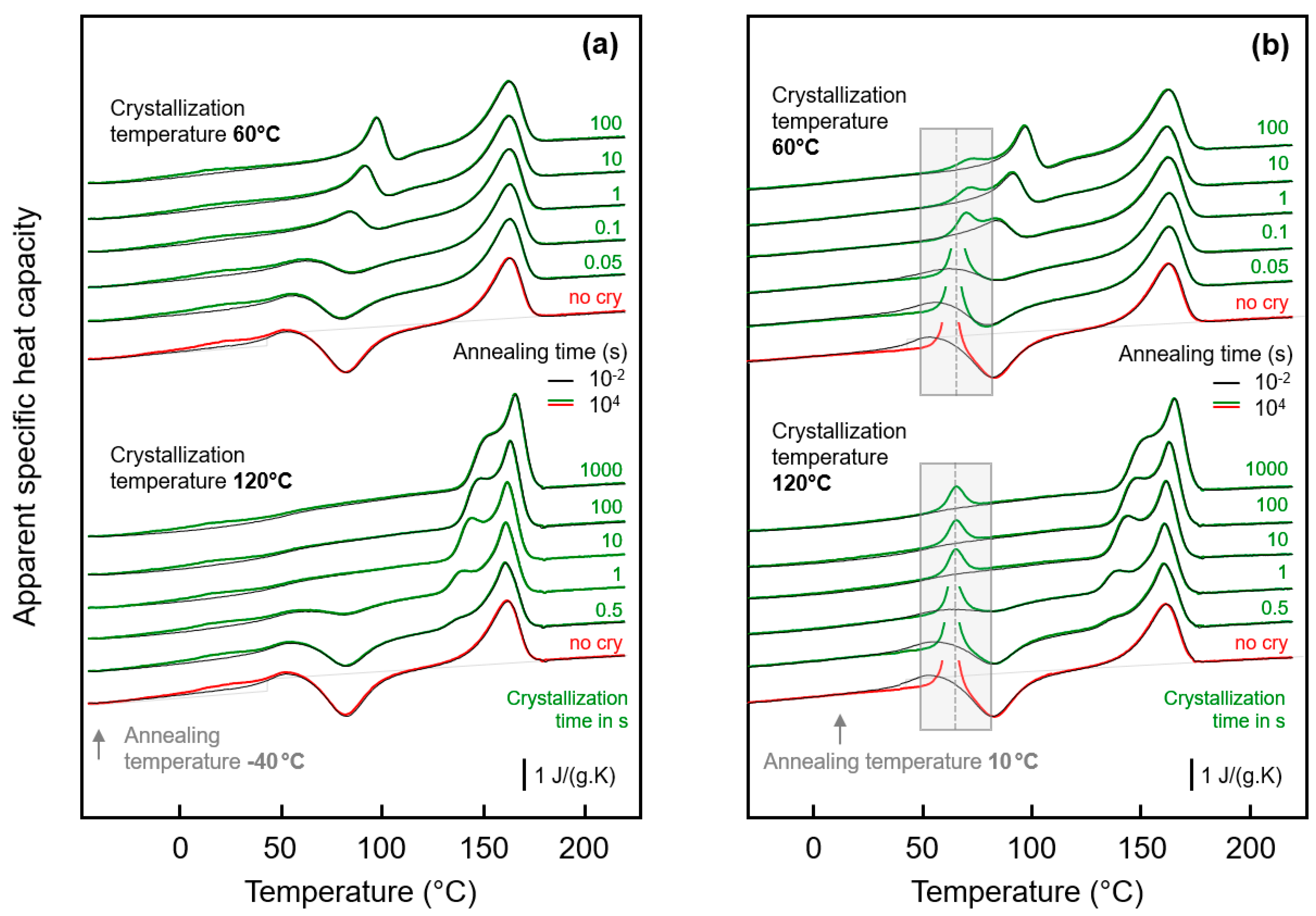
3.2. Glass Relaxation and/or Ordering in Semicrystalline PA 11
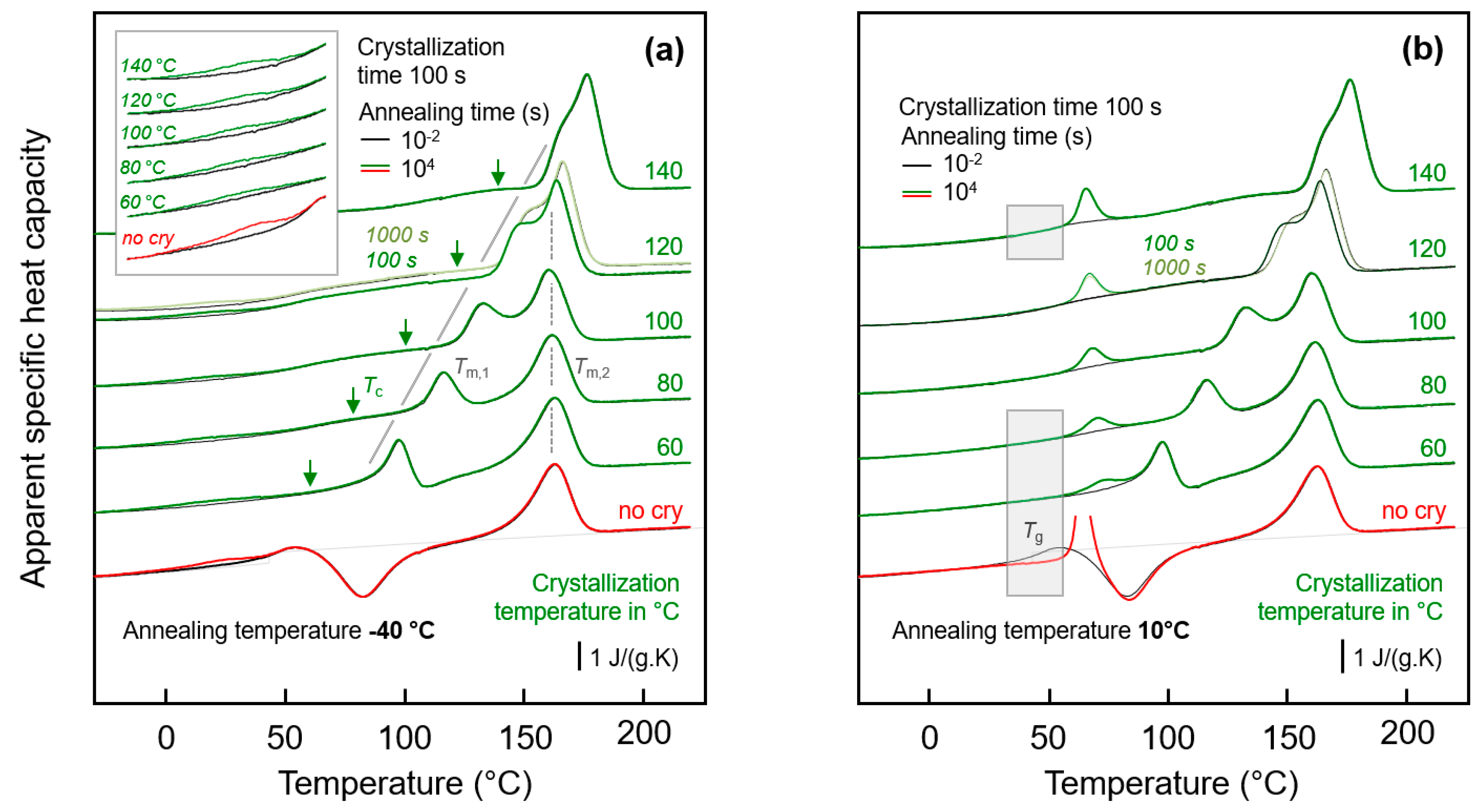
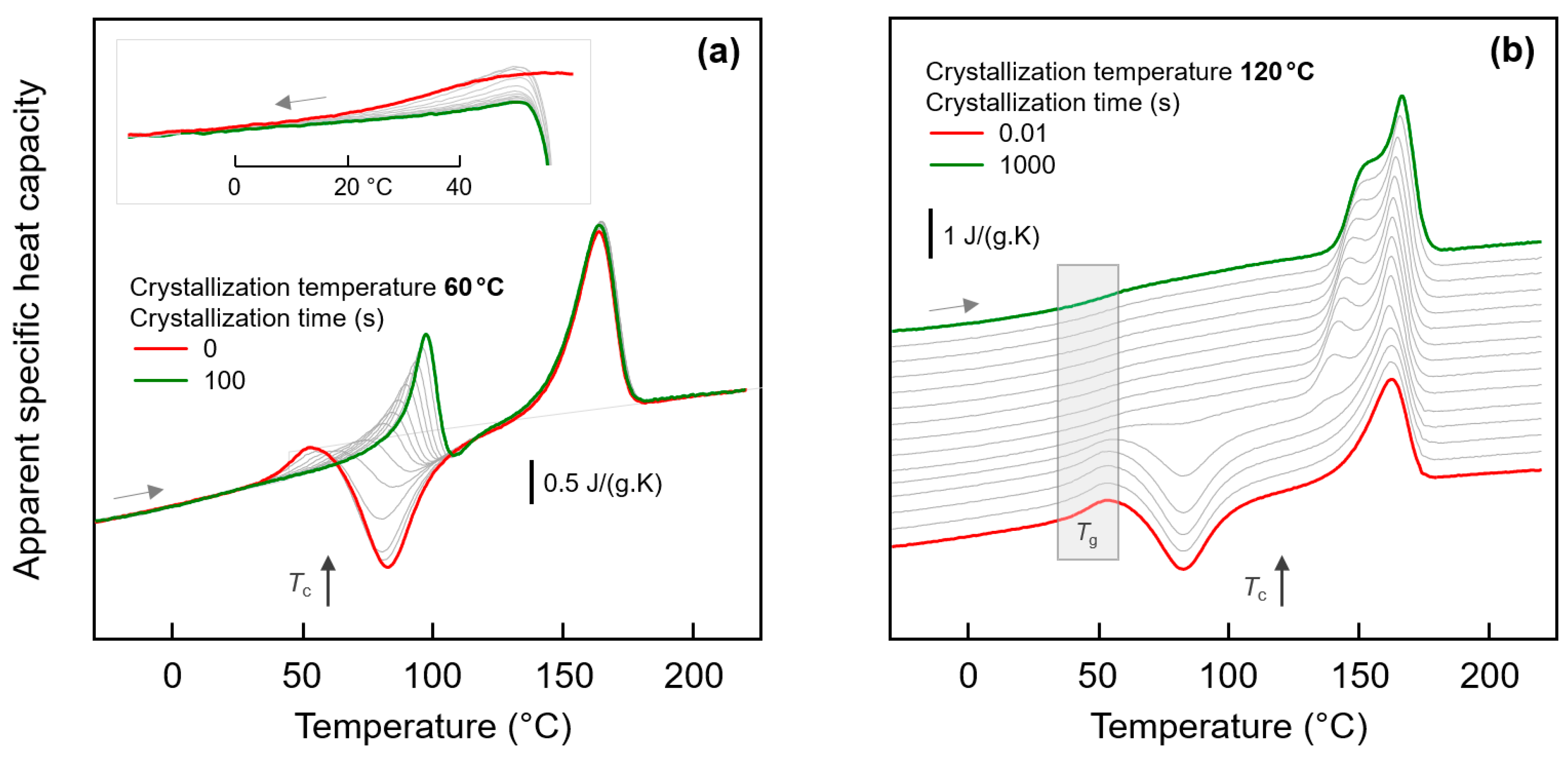
3.2.1. Effect of Crystallization Temperature
3.2.2. Effect of Crystallization Time
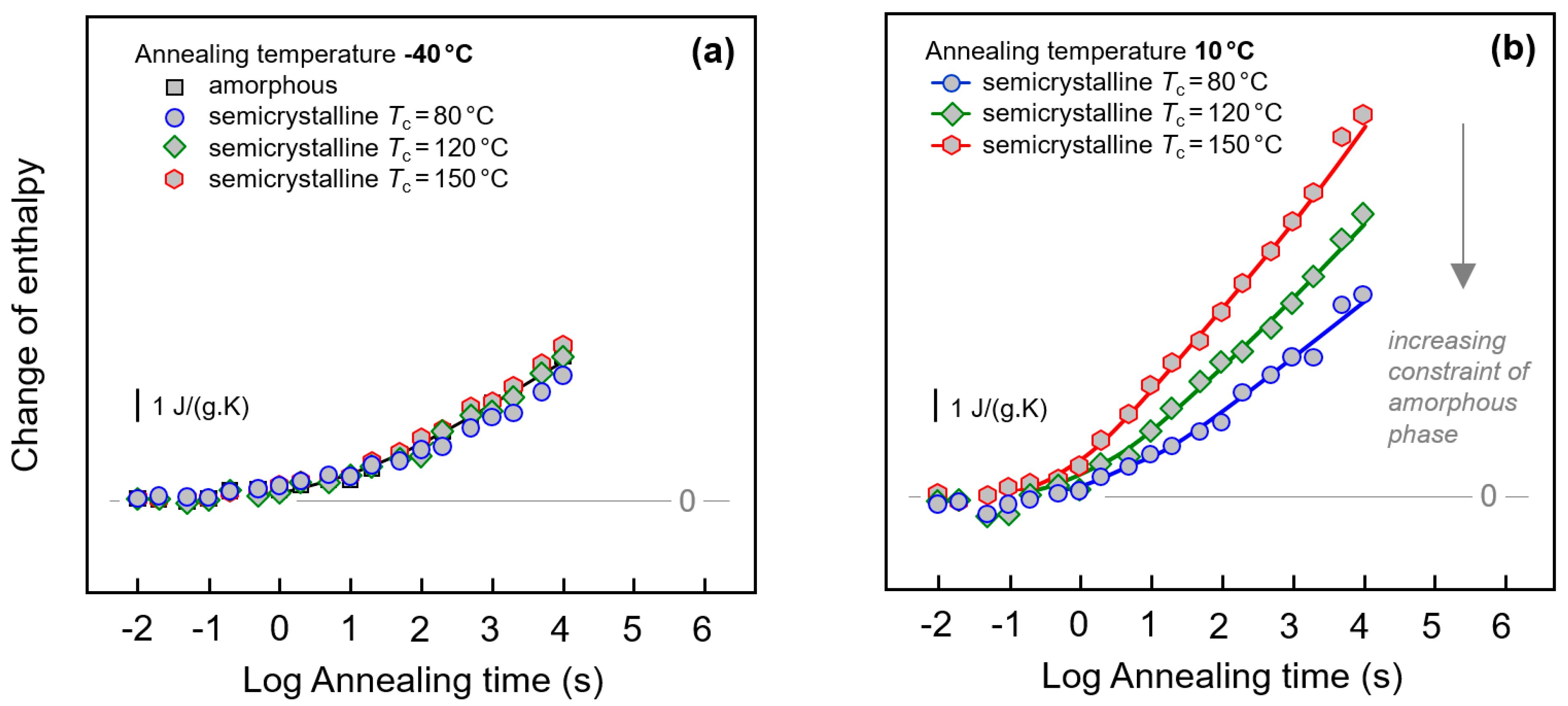
3.3. Enthalpy Relaxation versus Crystallization/Ordering
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arkema, Rilsan® Polyamide 11 Resins. Available online: www.extremematerials-arkema.com/en/product-families/rilsan-polyamide-11-family/ (accessed on 15 May 2019).
- Genas, M. Rilsan (Polyamid 11), Synthese und Eigenschaften. Angew. Chem. 1962, 74, 535–540. [Google Scholar] [CrossRef]
- Winnacker, M.; Rieger, B. Biobased polyamides: Recent advances in basic and applied research. Macromol. Rapid Comm. 2016, 37, 1391–1413. [Google Scholar] [CrossRef] [PubMed]
- Jariyavidyanont, K.; Focke, W.W.; Androsch, R. Thermal properties of biobased polyamide 11. Adv. Polym. Sci. 2019. [Google Scholar] [CrossRef]
- Mollova, A.; Androsch, R.; Mileva, D.; Schick, C.; Benhamida, A. Effect of supercooling on crystallization of polyamide 11. Macromolecules 2013, 46, 828–835. [Google Scholar] [CrossRef]
- Rhoades, A.M.; Wonderling, N.; Schick, C.; Androsch, R. Supercooling-controlled heterogeneous and homogenous crystal nucleation of polyamide 11 and its effect onto the crystal/mesophase polymorphism. Polymer 2016, 106, 29–34. [Google Scholar] [CrossRef]
- Jariyavidyanont, K.; Schick, C.; Androsch, R. Nucleation-controlled dual semicrystalline morphology of polyamide 11. Polym. Int. 2019, 68, 263–270. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C. Crystal nucleation of polymers at high supercooling of the melt. Adv. Polym. Sci. 2017, 276, 257–288. [Google Scholar]
- Toda, A.; Androsch, R.; Schick, C. Insights into polymer crystallization and melting from fast scanning chip calorimetry. Polymer 2016, 9, 239–263. [Google Scholar] [CrossRef]
- Schick, C.; Androsch, R. Nucleation-controlled semicrystalline morphology of bulk polymers. Polym. Cryst. 2018, 1, e10036. [Google Scholar] [CrossRef]
- Mileva, D.; Androsch, R.; Zhuravlev, E.; Schick, C. Morphology of mesophase and crystals of polyamide 6 prepared in a fast scanning chip calorimeter. Polymer 2012, 53, 3994–4001. [Google Scholar] [CrossRef]
- Mileva, D.; Kolesov, I.; Androsch, R. Morphology of cold-ordered polyamide 6. Coll. Polym. Sci. 2012, 290, 971–978. [Google Scholar] [CrossRef]
- Rhoades, A.M.; Williams, J.L.; Androsch, R. Crystallization of polyamide 66 at processing-relevant cooling conditions and at high supercooling. Thermochim. Acta 2015, 603, 103–109. [Google Scholar] [CrossRef]
- Fischer, C.; Seefried, A.; Drummer, D. Crystallization and component properties of PA 12 at processing-relevant cooling conditions. Polym. Eng. Sci. 2017, 57, 450–457. [Google Scholar] [CrossRef]
- Oliveira, M.J.; Botelho, G. Degradation of polyamide 11 in rotational moulding. Polym. Degrad. Stab. 2008, 93, 139–146. [Google Scholar] [CrossRef]
- Sherman, L.M. Additive manufacturing: Materials for ‘real-world’ parts. Plast. Techn. 2014, 60, 42–47. [Google Scholar]
- Bai, J.; Yuan, S.; Shen, F.; Zhang, B.; Chua, C.K.; Zhou, K.; Wei, J. Toughening of polyamide 11 with carbon nanotubes for additive manufacturing. Virtual Phys. Prototyp. 2017, 12, 235–240. [Google Scholar] [CrossRef]
- Jariyavidyanont, K.; Williams, J.L.; Rhoades, A.M.; Kühnert, I.; Focke, W.; Androsch, R. Crystallization of polyamide 11 during injection molding. Polym. Eng. Sci. 2018, 58, 1053–1061. [Google Scholar] [CrossRef]
- Nylon 11 (NYLON11) Heat Capacity, Enthalpy, Entropy, Gibbs Energy: Datasheet from “The Advanced THermal Analysis System (ATHAS) Databank – Polymer Thermodynamics” Release 2014 in SpringerMaterials. Available online: http://materials.springer.com/polymerthermodynamics/docs/athas_0022 (accessed on 10 October 2019).
- Zhang, Q.; Mo, Z.; Liu, S.; Zhang, H. Influence of annealing on structure of Nylon 11. Macromolecules 2000, 33, 5999–6005. [Google Scholar] [CrossRef]
- Larini, L.; Ottochian, A.; De Michele, C.; Leporini, D. Universal scaling between structural relaxation and vibrational dynamics in glass-forming liquids and polymers. Nat. Phys. 2008, 4, 42. [Google Scholar] [CrossRef]
- Ediger, M.D.; Angell, C.A.; Nagel, S.R. Supercooled liquids and glasses. J. Phys. Chem. 1996, 100, 13200–13212. [Google Scholar] [CrossRef]
- Zanotto, E.D.; Mauro, J.C. The glassy state of matter: Its definition and ultimate fate. J. Non-Cryst. Solids 2017, 471, 490–495. [Google Scholar] [CrossRef]
- Hodge, I.M. Enthalpy relaxation and recovery in amorphous materials. J. Non-Cryst. Solids 1994, 169, 211–266. [Google Scholar] [CrossRef]
- Angell, C.A.; Ngai, K.L.; McKenna, G.B.; McMillan, P.F.; Martin, S.W. Relaxation in glassforming liquids and amorphous solids. J. Appl. Phys. 2000, 88, 3113–3157. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Simon, S.L.; McKenna, G.B. Using 20-million-year-old amber to test the super-Arrhenius behaviour of glass-forming systems. Nat. Comm. 2013, 4, 1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donth, E. The size of cooperatively rearranging regions at the glass transition. J. Non-Cryst. Solids 1982, 53, 325–330. [Google Scholar] [CrossRef]
- Donth, E. Characteristic length of the glass transition. J. Polym. Sci. Polym. Phys. 1996, 34, 2881–2892. [Google Scholar] [CrossRef]
- Hodge, I.M. Physical aging in polymer glasses. Science 1995, 267, 1945–1947. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.M.; Smith, S.; Horne, B.; Gourlay, G.M. Physical aging of polycarbonate: enthalpy relaxation, creep response, and yielding behavior. Macromolecules 1999, 32, 5046–5061. [Google Scholar] [CrossRef]
- Iqbal, H.N.; Sungkapreecha, C.; Androsch, R. Enthalpy relaxation of the glass of poly (l-lactic acid) of different d-isomer content and its effect on mechanical properties. Polym. Bull. 2017, 74, 2565–2573. [Google Scholar] [CrossRef]
- Zhou, H.; Lofgren, E.A.; Jabarin, S.A. Effects of microcrystallinity and morphology on physical aging and its associated effects on tensile mechanical and environmental stress cracking properties of poly (ethylene terephthalate). J. Appl. Polym. Sci. 2009, 112, 2906–2917. [Google Scholar] [CrossRef]
- Aref-Azar, A.; Biddlestone, F.; Hay, J.N.; Haward, R.N. The effect of physical ageing on the properties of poly (ethylene terephthalate). Polymer 1983, 24, 1245–1251. [Google Scholar] [CrossRef]
- Schick, C.; Mathot, V. Fast Scanning Calorimetry; Springer: Basel, Switzerland, 2016. [Google Scholar]
- Zhuravlev, E.; Schmelzer, J.W.P.; Wunderlich, B.; Schick, C. Kinetics of nucleation and crystallization in poly(ε-caprolactone) (PCL). Polymer 2011, 52, 1983–1997. [Google Scholar] [CrossRef]
- Stolte, I.; Androsch, R.; Di Lorenzo, M.L.; Schick, C. Effect of aging the glass of isotactic polybutene-1 on form II nucleation and cold-crystallization. J. Phys. Chem. B 2013, 117, 15196–15203. [Google Scholar] [CrossRef] [PubMed]
- Androsch, R.; Schick, C.; Schmelzer, J.W.P. Sequence of enthalpy relaxation, homogeneous crystal nucleation and crystal growth in glassy Polyamide 6. Eur. Polym. J. 2014, 53, 100–108. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Zhuravlev, E.; Papageorgiou, G.Z.; Bikiaris, D.; Chrissafis, K.; Schick, C. Kinetics of nucleation and crystallization in poly(butylene succinate) nanocomposites. Polymer 2014, 55, 6725–6734. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C. Interplay between the relaxation of the glass of random l/d-lactide copolymers and homogeneous crystal nucleation: Evidence for segregation of chain defects. J. Phys. Chem. B 2016, 120, 4522–4528. [Google Scholar] [CrossRef]
- Androsch, R.; Zhuravlev, E.; Schmelzer, J.W.P.; Schick, C. Relaxation and crystal nucleation in polymer glasses. Eur. Polym. J. 2018, 102, 195–208. [Google Scholar]
- Angell, C.A.; Macfarlane, D.R.; Oguni, M. The Kauzmann paradox, metastable liquids, and ideal glasses: A summary. Ann. New York Acad. Sci. 1986, 484, 241–247. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S.; Fokin, V.M.; Schick, C.; Zanotto, E.D. Crystallization of glass-forming liquids: maxima of nucleation, growth, and overall crystallization rates. J. Non-Cryst. Solids 2015, 429, 24–32. [Google Scholar] [CrossRef]
- Androsch, R.; Di Lorenzo, M.L. Kinetics of crystal nucleation of poly(l-lactic acid). Polymer 2013, 54, 6882–6885. [Google Scholar] [CrossRef]
- Rybnikař, F. Secondary crystallization of polymers. J. Polym. Sci. 1960, 44, 517–522. [Google Scholar] [CrossRef]
- Strobl, G.R.; Engelke, T.; Meier, H.; Urban, G. Zum Mechanismus der Polymerkristallisation. Colloid Polym. Sci. 1982, 260, 394–403. [Google Scholar] [CrossRef]
- Statton, W.O.; Geil, P.H. Recrystallization of polyethylene during annealing. J. Appl. Polym. Sci. 1960, 3, 357–361. [Google Scholar] [CrossRef]
- Kawai, T. On the thickening of lamellar crystals during isothermal crystallization. Colloid Polym. Sci. 1969, 229, 116–124. [Google Scholar] [CrossRef]
- Hikosaka, M.; Amano, K.; Rastogi, S.; Keller, A. Lamellar thickening growth of an extended chain single crystal of polyethylene. 1. Pointers to a new crystallization mechanism of polymers. Macromolecules 1997, 30, 2067–2074. [Google Scholar] [CrossRef]
- Androsch, R.; Zhuravlev, E.; Schick, C. Solid-state reorganization, melting and melt-recrystallization of conformationally disordered crystals (α′-phase) of poly(l-lactic acid). Polymer 2014, 55, 4932–4941. [Google Scholar] [CrossRef]
- Jariyavidyanont, K.; Androsch, R.; Schick, C. Crystal reorganization of poly (butylene terephthalate). Polymer 2017, 124, 274–283. [Google Scholar] [CrossRef]
- RILSAN® PA11—Powerful By Nature. Available online: https://www.resinex.sk/en/products/rilsan.html (accessed on 28 July 2019).
- Perkins, W.G. Polymer toughness and impact resistance. Polym. Eng. Sci. 1999, 39, 2445–2460. [Google Scholar] [CrossRef]
- Starkweather, H.W.; Brooks, R.E. Effect of spherulites on the mechanical properties of nylon 66. J. Appl. Polym. Sci. 1959, 1, 236–239. [Google Scholar] [CrossRef] [Green Version]
- Way, J.L.; Atkinson, J.R.; Nutting, J. The effect of spherulite size on the fracture morphology of polypropylene. J. Mater. Sci. 1974, 9, 293–299. [Google Scholar] [CrossRef]
- Arkema, Materials Database. Available online: https://www.extremematerials-arkema.com/en/materials-database/products/datasheet/Rilsan%C2%AE%20BESNO%20TL (accessed on 28 July 2019).
- Fornes, T.D.; Paul, D.R. Structure and properties of nanocomposites based on nylon-11 and -12 compared with those based on nylon-6. Macromolecules 2004, 37, 7698–7709. [Google Scholar] [CrossRef]
- Poel, G.V.; Mathot, V.B.F. High performance differential scanning calorimetry (HPer DSC): A powerful analytical tool for the study of the metastability of polymers. Thermochim. Acta 2007, 461, 107–121. [Google Scholar] [CrossRef]
- Kolesov, I.S.; Androsch, R.; Radusch, H.J. Non-isothermal crystallization of polyethylenes as function of cooling rate and concentration of short chain branches. J. Therm. Anal. Cal. 2004, 78, 885–895. [Google Scholar] [CrossRef]
- Jariyavidyanont, K.; Abdelaziz, A.; Androsch, R.; Schick, C. Experimental analysis of lateral thermal inhomogeneity of a specific chip-calorimeter sensor. Thermochim. Acta 2019, 674, 95–99. [Google Scholar] [CrossRef]
- Koy, U.; Dehne, H.; Gnoth, M.; Schick, C. Glass transition in semicrystalline systems. Thermochim. Acta 1993, 229, 299–310. [Google Scholar] [CrossRef]
- Wunderlich, B. Reversible crystallization and the rigid–amorphous phase in semicrystalline macromolecules. Progr. Polym. Sci. 2003, 28, 383–450. [Google Scholar] [CrossRef]
- Righetti, M.C.; Tombari, E.; Di Lorenzo, M.L. Crystalline, mobile amorphous and rigid amorphous fractions in isotactic polystyrene. Eur. Polym. J. 2008, 44, 2659–2667. [Google Scholar] [CrossRef]
- Lee, Y.; Porter, R.S.; Lin, J.S. On the double-melting behavior of poly (ether ether ketone). Macromolecules 1989, 22, 1756–1760. [Google Scholar] [CrossRef]
- Minakov, A.A.; Mordvintsev, D.A.; Schick, C. Melting and reorganization of poly (ethylene terephthalate) on fast heating (1000 K/s). Polymer 2004, 45, 3755–3763. [Google Scholar] [CrossRef]
- Furushima, Y.; Schick, C.; Toda, A. Crystallization, recrystallization, and melting of polymer crystals on heating and cooling examined with fast scanning calorimetry. Polym. Cryst. 2018, 1, e10005. [Google Scholar] [CrossRef]
- Toda, A. Fast-scan and temperature-modulated calorimetry of recrystallization of poly(ethylene terephthalate). Thermochim. Acta 2019, 682, 178404. [Google Scholar] [CrossRef]
- Baeten, D.; Mathot, V.B.F.; Pijpers, T.F.J.; Verkinderen, O.; Portale, G.; Van Puyvelde, P.; Goderis, B. Simultaneous synchrotron WAXD and fast scanning (chip) calorimetry: On the (isothermal) crystallization of HDPE and PA 11 at high supercoolings and cooling rates up to 200 °C s−1. Macromol. Rap. Comm. 2015, 36, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Jariyavidyanont, K.; Janke, A.; Androsch, R. Crystal self-nucleation in polyamide 11 of different semicrystalline morphology. Polymer 2019, in press. [Google Scholar] [CrossRef]
- Jariyavidyanont, K.; Janke, A.; Androsch, R. Crystal self-nucleation in polyamide 11. Thermochim. Acta 2019, 677, 139–143. [Google Scholar] [CrossRef]
- Schawe, J.E. Mobile amorphous, rigid amorphous and crystalline fractions in isotactic polypropylene during fast cooling. J. Therm. Anal. Calor. 2017, 127, 931–937. [Google Scholar] [CrossRef]
- Androsch, R.; Wunderlich, B. The link between rigid amorphous fraction and crystal perfection in cold-crystallized poly (ethylene terephthalate). Polymer 2005, 46, 12556–12566. [Google Scholar] [CrossRef]
- Kolesov, I.; Androsch, R. The rigid amorphous fraction of cold-crystallized polyamide 6. Polymer 2012, 53, 4770–4777. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R.; Stolte, I. Tailoring the rigid amorphous fraction of isotactic polybutene-1 by ethylene chain defects. Polymer 2014, 55, 6132–6139. [Google Scholar] [CrossRef]
- Robertson, R.E. Segmental mobility in the equilibrium liquid below the glass transition. Macromolecules 1985, 18, 953–958. [Google Scholar] [CrossRef]
- Berens, A.R.; Hodge, I.M. Effects of annealing and prior history on enthalpy relaxation in glassy polymers. 1. Experimental study on poly (vinyl chloride). Macromolecules 1982, 15, 756–761. [Google Scholar] [CrossRef]
- Perez-De Eulate, N.G.; Cangialosi, D. The very long-term physical aging of glassy polymers. Phys. Chem. Chem. Phys. 2018, 20, 12356–12361. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Wang, T.T. Sub-sub Tg structural relaxation in glassy polymers. J. Appl. Phys. 1981, 52, 5898–5902. [Google Scholar] [CrossRef]
- Boucher, V.M.; Cangialosi, D.; Alegría, A.; Colmenero, J. Reaching the ideal glass transition by aging polymer films. Phys. Chem. Chem. Phys. 2017, 19, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Boucher, V.M.; Cangialosi, D.; Alegría, A.; Colmenero, J. Complex nonequilibrium dynamics of stacked polystyrene films deep in the glassy state. J. Chem. Phys. 2017, 146, 203312. [Google Scholar] [CrossRef] [PubMed]
- Cangialosi, D.; Boucher, V.M.; Alegría, A.; Colmenero, J. Direct evidence of two equilibration mechanisms in glassy polymers. Phys. Rev. Lett. 2013, 111, 095701. [Google Scholar] [CrossRef]
- Chen, H.S.; Inoue, A.; Masumoto, T. Two-stage enthalpy relaxation behaviour of (Fe0.5Ni0.5)83P17 and (Fe0.5Ni0.5)83B17 amorphous alloys upon annealing. J. Mater. Sci. 1985, 20, 2417–2438. [Google Scholar] [CrossRef]
- Aji, D.P.B.; Johari, G.P. Kinetic-freezing and unfreezing of local-region fluctuations in a glass structure observed by heat capacity hysteresis. J. Chem. Phys. 2015, 142, 214501. [Google Scholar]
- Louzguine-Luzgin, D.V.; Seki, I.; Yamamoto, T.; Kawaji, H.; Suryanarayana, C.; Inoue, A. Double-stage glass transition in a metallic glass. Phys. Rev. B 2010, 81, 144202. [Google Scholar] [CrossRef] [Green Version]
- Gallino, I.; Cangialosi, D.; Evenson, Z.; Schmitt, L.; Hechler, S.; Stolpe, M.; Ruta, B. Hierarchical aging pathways and reversible fragile-to-strong transition upon annealing of a metallic glass former. Acta Mater. 2018, 144, 400–410. [Google Scholar] [CrossRef]
- Song, L.; Xu, W.; Huo, J.; Wang, J.Q.; Wang, X.; Li, R. Two-step relaxations in metallic glasses during isothermal annealing. Intermetallics 2018, 93, 101–105. [Google Scholar] [CrossRef]
- Fan, J.; Cooper, E.I.; Angell, C.A. Glasses with strong calorimetric beta-glass transitions and the relation to the protein glass transition problem. J. Phys. Chem. 1994, 98, 9345–9349. [Google Scholar] [CrossRef]
- Tant, M.R.; Wilkes, G.L. Physical aging studies of semicrystalline poly (ethylene terephthalate). J. Appl. Polym. Sci. 1981, 26, 2813–2825. [Google Scholar] [CrossRef]
- Vigier, G.; Tatibouet, J. Physical ageing of amorphous and semicrystalline poly (ethylene terephthalate). Polymer 1993, 34, 4257–4266. [Google Scholar] [CrossRef]
- Hagege, R. Ageing behavior of pre-oriented PET yarns, followed by DSC. Text. Res. J. 1977, 47, 229–231. [Google Scholar] [CrossRef]
- Qian, R.; Shen, D.; Sun, F.; Wu, L. The effects of physical ageing on conformational changes of poly (ethylene terephthalate) in the glass transition region. Macromol. Chem. Phys. 1996, 197, 1485–1493. [Google Scholar] [CrossRef]
- Qian, R. The concept of cohesional entanglement. Macromol. Symp. 1977, 124, 15–26. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, D.; Qian, R. Subglass-transition-temperature annealing of poly (ethylene terephthalate) studied by FTIR. J. Polym. Sci. Part B: Polym. Phys. 1998, 36, 783–788. [Google Scholar] [CrossRef]
- Frubing, P.; Kremmer, A.; Neumann, W.; Gerhard-Multhaupt, R.; Guy, I.L. Dielectric relaxation in piezo-, pyro-and ferroelectric polyamide 11. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 271–279. [Google Scholar] [CrossRef]
- Frübing, P.; Kremmer, A.; Gerhard-Multhaupt, R.; Spanoudaki, A.; Pissis, P. Relaxation processes at the glass transition in polyamide 11: From rigidity to viscoelasticity. J. Chem. Phys. 2006, 125, 214701. [Google Scholar] [CrossRef] [Green Version]
- Neagu, R.M.; Neagu, E.; Kyritsis, A.; Pissis, P. Dielectric studies of dipolar relaxation processes in nylon 11. J. Phys. D Appl. Phys. 2000, 33, 1921–1931. [Google Scholar] [CrossRef]
- Wunderlich, B. Macromolecular Physics, Vol 2: Crystal Nucleation, Growth, Annealing; Academic Press: New York, NY, USA, 1973. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Androsch, R.; Jariyavidyanont, K.; Schick, C. Enthalpy Relaxation of Polyamide 11 of Different Morphology Far Below the Glass Transition Temperature. Entropy 2019, 21, 984. https://doi.org/10.3390/e21100984
Androsch R, Jariyavidyanont K, Schick C. Enthalpy Relaxation of Polyamide 11 of Different Morphology Far Below the Glass Transition Temperature. Entropy. 2019; 21(10):984. https://doi.org/10.3390/e21100984
Chicago/Turabian StyleAndrosch, René, Katalee Jariyavidyanont, and Christoph Schick. 2019. "Enthalpy Relaxation of Polyamide 11 of Different Morphology Far Below the Glass Transition Temperature" Entropy 21, no. 10: 984. https://doi.org/10.3390/e21100984
APA StyleAndrosch, R., Jariyavidyanont, K., & Schick, C. (2019). Enthalpy Relaxation of Polyamide 11 of Different Morphology Far Below the Glass Transition Temperature. Entropy, 21(10), 984. https://doi.org/10.3390/e21100984





