Multiscale Approximate Entropy for Gait Analysis in Patients with Neurodegenerative Diseases
Abstract
1. Introduction
2. Methods
2.1. Data Source and Study Protocol
2.2. Multiscale Approximate Entropy
- (1)
- Given the pressure signals on time series as {P1, P2, P3,……Pn}, consecutive coarse-grained on time series as {x(τ)} determined by the scale factor τ, which was constructed as our previous publication [15]. Briefly, the {x(τ)} was generated by averaging the data within a non-overlapping window with increasing length, τ. In this study, we calculated the complexity of scale factors (τ = 1, 2, …, 20) by using approximate entropy.
- (2)
- Define the series {x(τ)} with length N and the two parameters of m and r (where m = Embedded dimension of the vector; r = tolerance). Generally, these parameters are used by experience and conditioned by repeated tests. In this study, we used N = 3000, m = 2 and r = 0.2.
- (3)
- Form a sequence vector …, in with m-dimensional space by:
- (4)
- Then we used the sequence …, to construct:
- (5)
- In which was defined as:
- (6)
- Then we define:
- (7)
- Therefore, approximate entropy (AppEn) can be defined as:
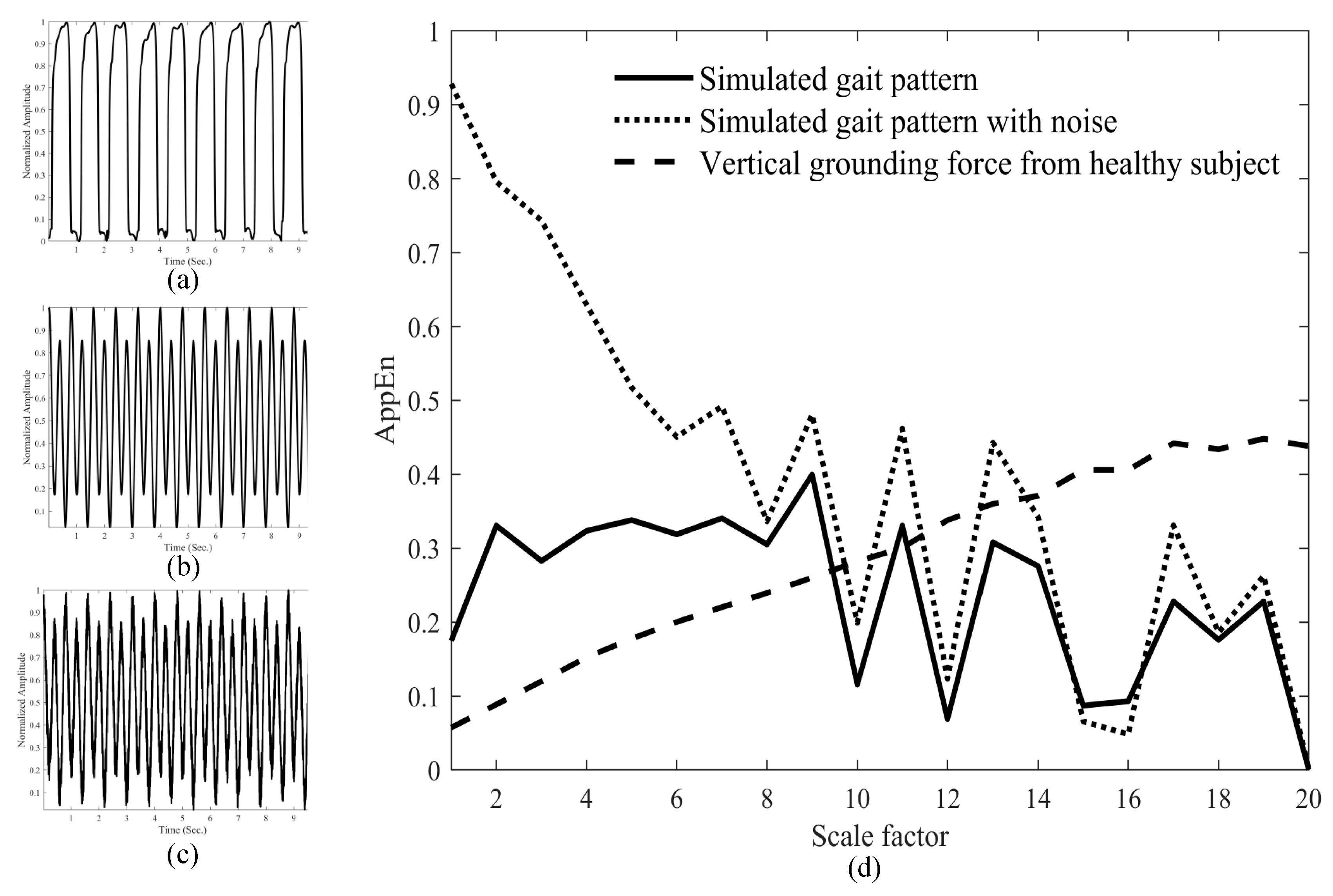
2.3. Generation and Analysis of Simulated Gait Signs
2.4. Statistical Analysis
3. Results
3.1. Age, Sex and Demographic Data of the Subjects
3.2. MAE Analyses of Normal Gait Signals and Simulated Signals
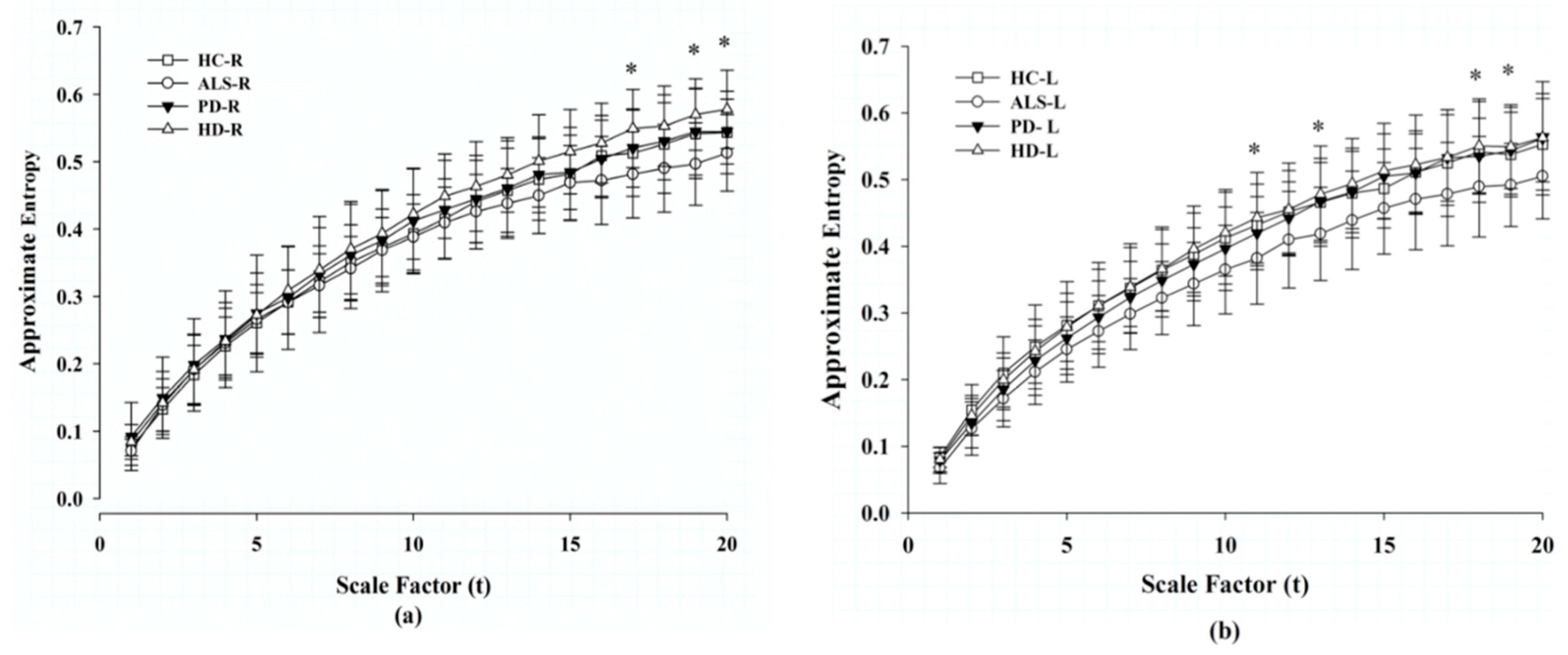
3.3. MAE Analysis of Gait Signals in Each Foot
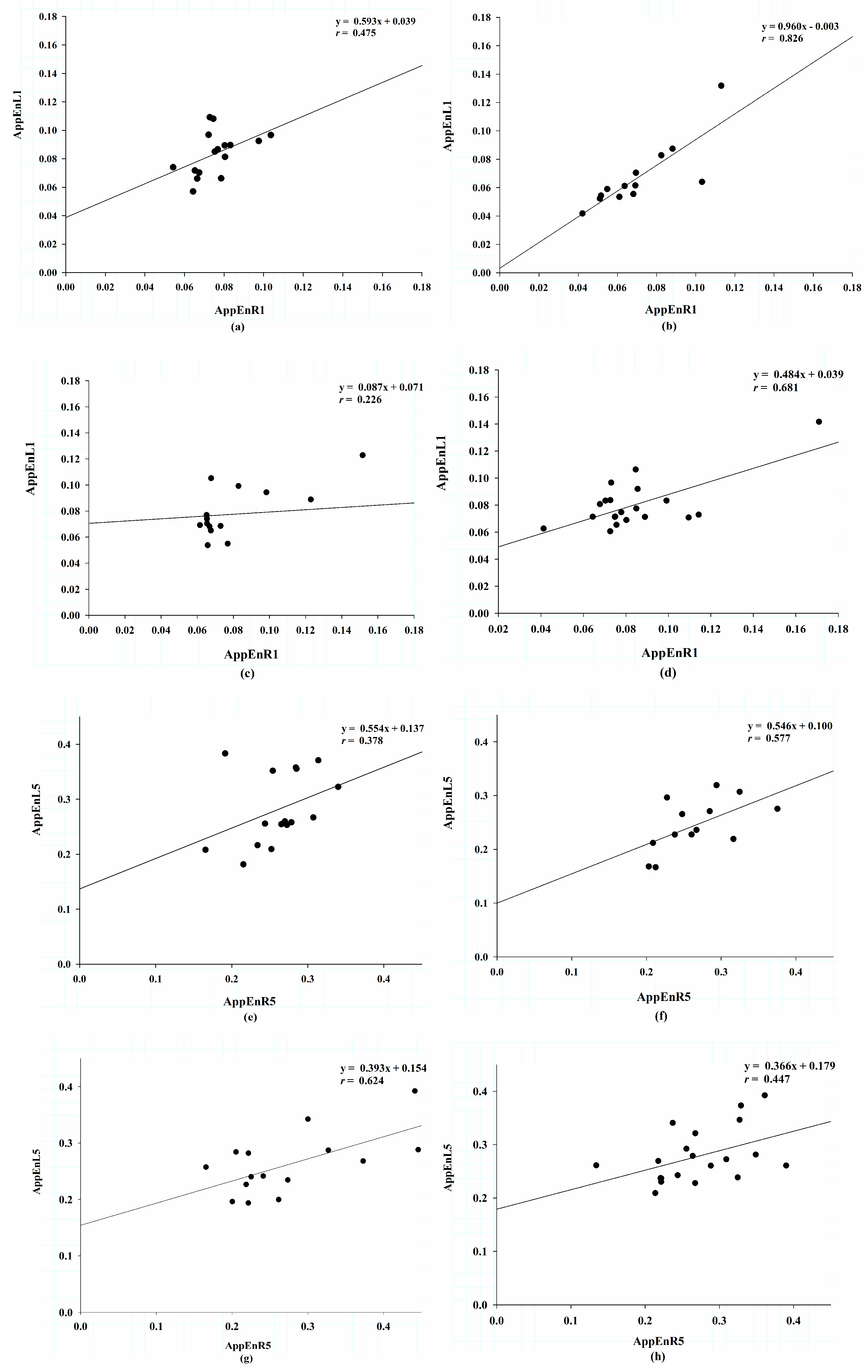
3.4. Correlation of Approximate Entropy Between the Right and Left Sides.
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snijders, A.H.; van de Warrenburg, B.P.; Giladi, N.; Bloem, B.R. Neurological gait disorders in elderly people: Clinical approach and classification. Lancet Neurol. 2007, 6, 63–74. [Google Scholar] [CrossRef]
- Pirker, W.; Katzenschlager, R. Gait disorders in adults and the elderly: A clinical guide. Wien. Klin. Wochenschr. 2017, 129, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Salzman, B. Gait and balance disorders in older adults. Am. Fam. Phys. 2010, 82, 61–68. [Google Scholar] [PubMed]
- Murray, M.P.; Kory, R.C.; Clarkson, B.H. Walking patterns in healthy old men. J. Gerontol. 1969, 24, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Sudarsky, L. Geriatrics: Gait disorders in the elderly. N. Engl. J. Med. 1990, 322, 1441–1446. [Google Scholar] [PubMed]
- Muro-De-La-Herran, A.; Garcia-Zapirain, B.; Mendez-Zorrilla, A. Gait analysis methods: An overview of wearable and non-wearable systems, highlighting clinical applications. Sensors 2014, 14, 3362–3394. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, 215–220. [Google Scholar] [CrossRef]
- Pham, T.D. Texture classification and visualization of time series of gait dynamics in patients with neuro-degenerative diseases. IEEE. Trans. Neural. Syst. Rehabil. Eng. 2018, 26, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Tang, S.; Fang, F.; Luo, L.; Xu, L.; Bringas-Vega, M.L.; Yao, D.; Kendrick, K.M.; Valdes-Sosa, P.A. Gait rhythm fluctuation analysis for neurodegenerative diseases by empirical mode decomposition. IEEE Trans. Biomed. Eng. 2017, 64, 52–60. [Google Scholar] [CrossRef]
- Buchman, T.G. The community of the self. Nature 2002, 420, 246–251. [Google Scholar] [CrossRef]
- Baker, R. Gait analysis methods in rehabilitation. J. Neuroeng. Rehabil. 2006, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002, 89, 068102. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy to distinguish physiologic and synthetic RR time series. Comput. Cardiol. 2002, 29, 137–140. [Google Scholar] [PubMed]
- Wu, H.T.; Hsu, P.C.; Lin, C.F.; Wang, H.J.; Sun, C.K.; Liu, A.B.; Lo, M.T.; Tang, C.J. Multiscale entropy analysis of pulse wave velocity for assessing atherosclerosis in the aged and diabetic. IEEE Trans. Biomed. Eng. 2011, 58, 2978–2981. [Google Scholar] [PubMed]
- Hausdorff, J.M. Gait dynamics, fractals and falls: Finding meaning in the stride-to-stride fluctuations of human walking. Hum. Mov. Sci. 2007, 26, 555–589. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Peng, C.-K.; Ary, L.; Goldbergera, A.L.; Hausdor, J.M. Multiscale entropy analysis of human gait dynamics. Physica 2003, 330, 53–60. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, P.; Luo, X.; Wu, X.; Liao, L.; Yang, S.; Rangayyan, R.M. Measuring signal fluctuations in gait rhythm time series of patientswith Parkinson’s disease using entropy parameters. Biomed. Signal. Process. Control 2017, 31, 265–271. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Ladin, Z.; Wei, J.Y. Footswitch system for measurement of the temporal parameters of gait. J. Biomech. 1995, 28, 347–351. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Cudkowicz, M.E.; Firtion, R.; Wei, J.Y.; Goldberger, A.L. Gait variability and basal ganglia disorders: Stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov. Disord. 1998, 13, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M.; Lertratanakul, A.; Cudkowicz, M.E.; Peterson, A.L.; Kaliton, D.; Goldberger, A.L. Dynamic markers of altered gait rhythm in amyotrophic lateral sclerosis. J. Appl. Physiol. 2000, 88, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, N.E.; Long, S.R.; Peng, C.K. On the trend, detrending, and variability of nonlinear and nonstationary time series. Proc. Natl. Acad. Sci. USA 2007, 104, 14889–14894. [Google Scholar] [CrossRef]
- Huang, N.E.; Shen, Z.; Long, S.R.; Wu, M.C.; Shih, H.H.; Zheng, Q.; Yen, N.C.; Tung, C.C.; Liu, H.H. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. A Math. Phys. Eng. Sci. 1998, 454, 903–995. [Google Scholar] [CrossRef]
- Van Emmerik, R.E.; Ducharme, S.W.; Amado, A.C.; Hamill, J. Comparing dynamical systems concepts and techniques for biomechanical analysis. J. Sport Health Sci. 2016, 5, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Moody, G.B.; Mark, R.G.; Goldberger, A.L. PhysioNet: Physiologic signals, time series and related open source software for basic, clinical, and applied research. In Proceedings of the 33rd Annual International Conference of the IEEE EMBS, Boston, MA, USA, 30 August–3 September 2011; pp. 8327–8330. [Google Scholar]
- Ren, P.; Zhao, W.; Zhao, Z.; Bringas-Vega, M.L.; Valdes-Sosa, P.A.; Kendrick, K.M. Analysis of Gait Rhythm Fluctuations for Neurodegenerative Diseases by Phase Synchronization and Conditional Entropy. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Qi, L.; Dong, J.; Yu, H. Dual channel LSTM based multi-feature extraction in gait for diagnosis of Neurodegenerative diseases. Knowl.-Based Syst. 2018, 145, 91–97. [Google Scholar] [CrossRef]
- Garcia Ruiz, P.J.; Hernandez, J.; Cantarero, S.; Bartolome, M.; Sanchez Bernardos, V.; Garcia de Yebenez, J. Bradykinesia in Huntington’s disease. A prospective, follow-up study. J. Neurol. 2002, 249, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Pupillo, E.; Bianchi, E.; Messina, P.; Chiveri, L.; Lunetta, C.; Corbo, M.; Filosto, M.; Lorusso, L.; Marin, B.; Mandrioli, J.; et al. Extrapyramidal and cognitive signs in amyotrophic lateral sclerosis: A population based cross-sectional study. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 324–330. [Google Scholar] [CrossRef]
- Beauchet, O.; Allali, G.; Sekhon, H.; Verghese, J.; Guilain, S.; Steinmetz, J.P.; Kressig, R.W.; Barden, J.M.; Szturm, T.; Launay, C.P.; et al. Guidelines for assessment of gait and reference values for spatiotemporal gait parameters in older adults: The biomathics and canadian gait consortiums initiative. Front. Hum. Neurosci. 2017, 11, 353. [Google Scholar] [CrossRef]
- Kaczmarczyk, K.; Wiszomirska, I.; Blazkiewicz, M.; Wychowanski, M.; Wit, A. First signs of elderly gait for women. Med. Pr. 2017, 68, 441–448. [Google Scholar] [CrossRef][Green Version]
- Ryan, S.M.; Goldberger, A.L.; Pincus, S.M.; Mietus, J.; Lipsitz, L.A. Gender- and age-related differences in heart rate dynamics: Are women more complex than men? J. Am. Coll. Cardiol. 1994, 24, 1700–1707. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Broken asymmetry of the human heartbeat: Loss of time irreversibility in aging and disease. Phys. Rev. 2005, 95, 198102. [Google Scholar]
- Lipsitz, L.A. Age-related changes in the “complexity” of cardiovascular dynamics: A potential marker of vulnerability to disease. Chaos 1995, 5, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Gao, Q.; Lu, Y.; Ye, Q. A novel approach for analysis of altered gait variability in amyotrophic lateral sclerosis. Med. Biol. Eng. Comput. 2016, 54, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Afsar, O.; Tirnakli, U.; Kurths, J. Entropy-based complexity measures for gait data of patients with Parkinson’s disease. Chaos 2016, 26, 023115. [Google Scholar] [CrossRef]
| HC (n = 16) | ALS (n = 13) | PD (n = 15) | HD (n = 20) | |
|---|---|---|---|---|
| Age (years) | 38.69 ± 18.73 | 55.62 ± 12.83 * | 67.20 ± 10.69 ** | 47.37 ± 12.51 |
| Sex (Male %) | 12.5% | 66.7% | 30.0% | 76.9% |
| Height (m) | 1.833 ± 0.087 | 1.7446 ± 0.950 | 1.87 ± 0.152 | 1.8437 ± 0.089 |
| Weight (kg) | 66.81 ± 11.08 | 77.12 ± 21.15 | 75.07 ± 16.90 | 73.47 ± 16.24 |
| BMI (kg/m2) | 19.87 ± 2.71 | 25.21 ± 5.35 * | 21.21 ± 2.64 | 21.55 ± 4.44 |
| Gait speed (m/s) | 1.354 ± 0.160 | 1.054 ± 0.218 * | 0.999 ± 0.202 ** | 1.15 ± 0.349 |
| HC | ALS | PD | HD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | Slope | p | r | Slope | p | r | Slope | p | r | Slope | p | |
| AppEnR1/L1 | 0.475 | 0.593 | 0.063 | 0.826 | 0.906 | 0.001 * | 0.226 | 0.087 | 0.417 | 0.681 | 0.484 | 0.001 * |
| AppEnR2/L2 | 0.392 | 0.458 | 0.133 | 0.793 | 0.812 | 0.001 * | 0.494 | 0.303 | 0.061 | 0.536 | 0.345 | 0.015 * |
| AppEnR3/L3 | 0.372 | 0.466 | 0.156 | 0.694 | 0.576 | 0.008 * | 0.53 | 0.363 | 0.042 * | 0.494 | 0.379 | 0.027 * |
| AppEnR4/L4 | 0.397 | 0.58 | 0.128 | 0.656 | 0.616 | 0.015 * | 0.544 | 0.395 | 0.036 * | 0.42 | 0.351 | 0.065 |
| AppEnR5/L5 | 0.378 | 0.554 | 0.149 | 0.577 | 0.546 | 0.039 * | 0.624 | 0.393 | 0.013 * | 0.447 | 0.366 | 0.048 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, A.-B.; Lin, C.-W. Multiscale Approximate Entropy for Gait Analysis in Patients with Neurodegenerative Diseases. Entropy 2019, 21, 934. https://doi.org/10.3390/e21100934
Liu A-B, Lin C-W. Multiscale Approximate Entropy for Gait Analysis in Patients with Neurodegenerative Diseases. Entropy. 2019; 21(10):934. https://doi.org/10.3390/e21100934
Chicago/Turabian StyleLiu, An-Bang, and Che-Wei Lin. 2019. "Multiscale Approximate Entropy for Gait Analysis in Patients with Neurodegenerative Diseases" Entropy 21, no. 10: 934. https://doi.org/10.3390/e21100934
APA StyleLiu, A.-B., & Lin, C.-W. (2019). Multiscale Approximate Entropy for Gait Analysis in Patients with Neurodegenerative Diseases. Entropy, 21(10), 934. https://doi.org/10.3390/e21100934





