Effects of Nitrogen Content on the Structure and Mechanical Properties of (Al0.5CrFeNiTi0.25)Nx High-Entropy Films by Reactive Sputtering
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
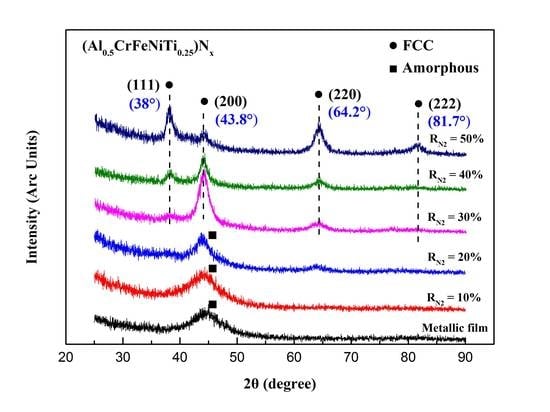
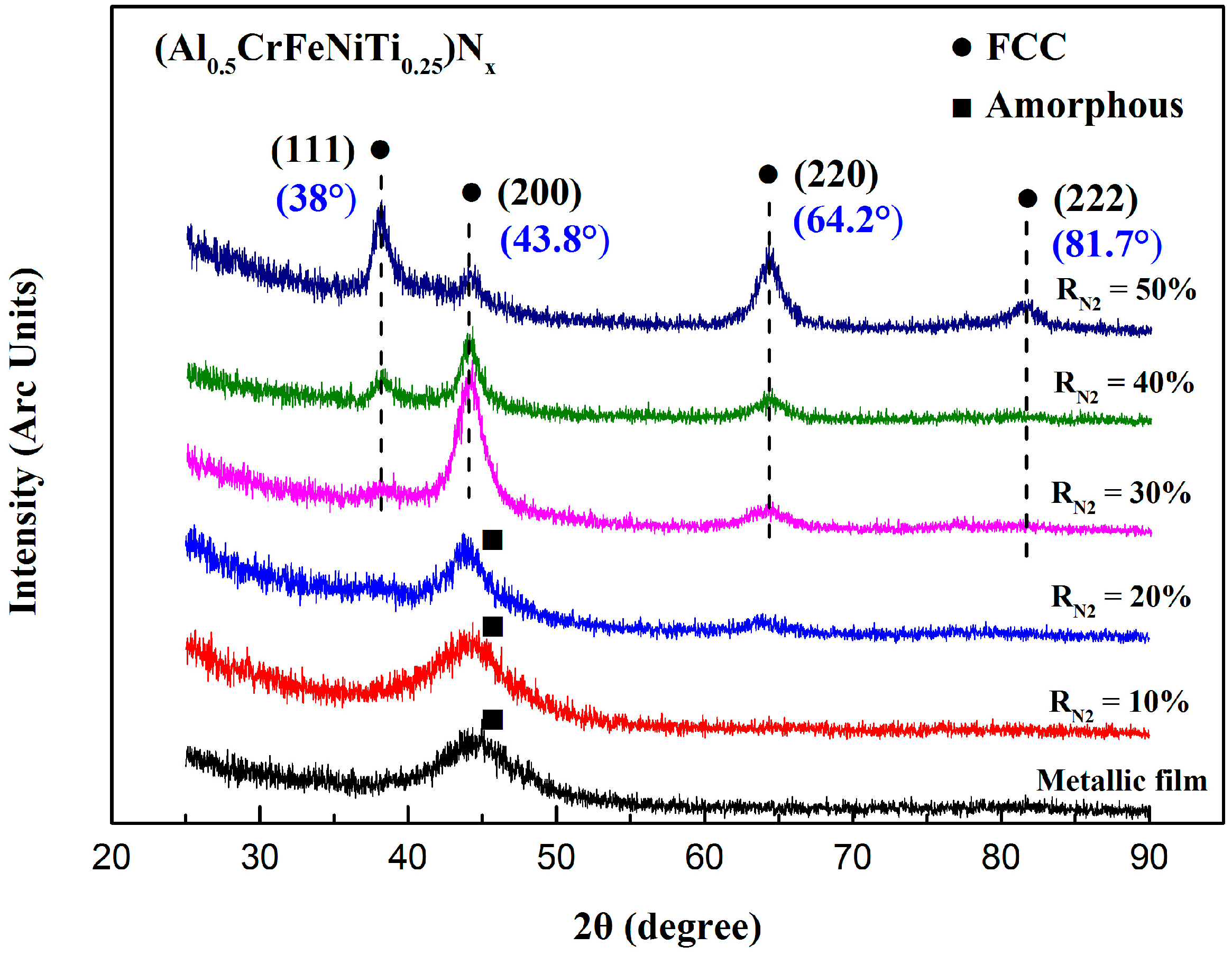
3.1. Phase Structures
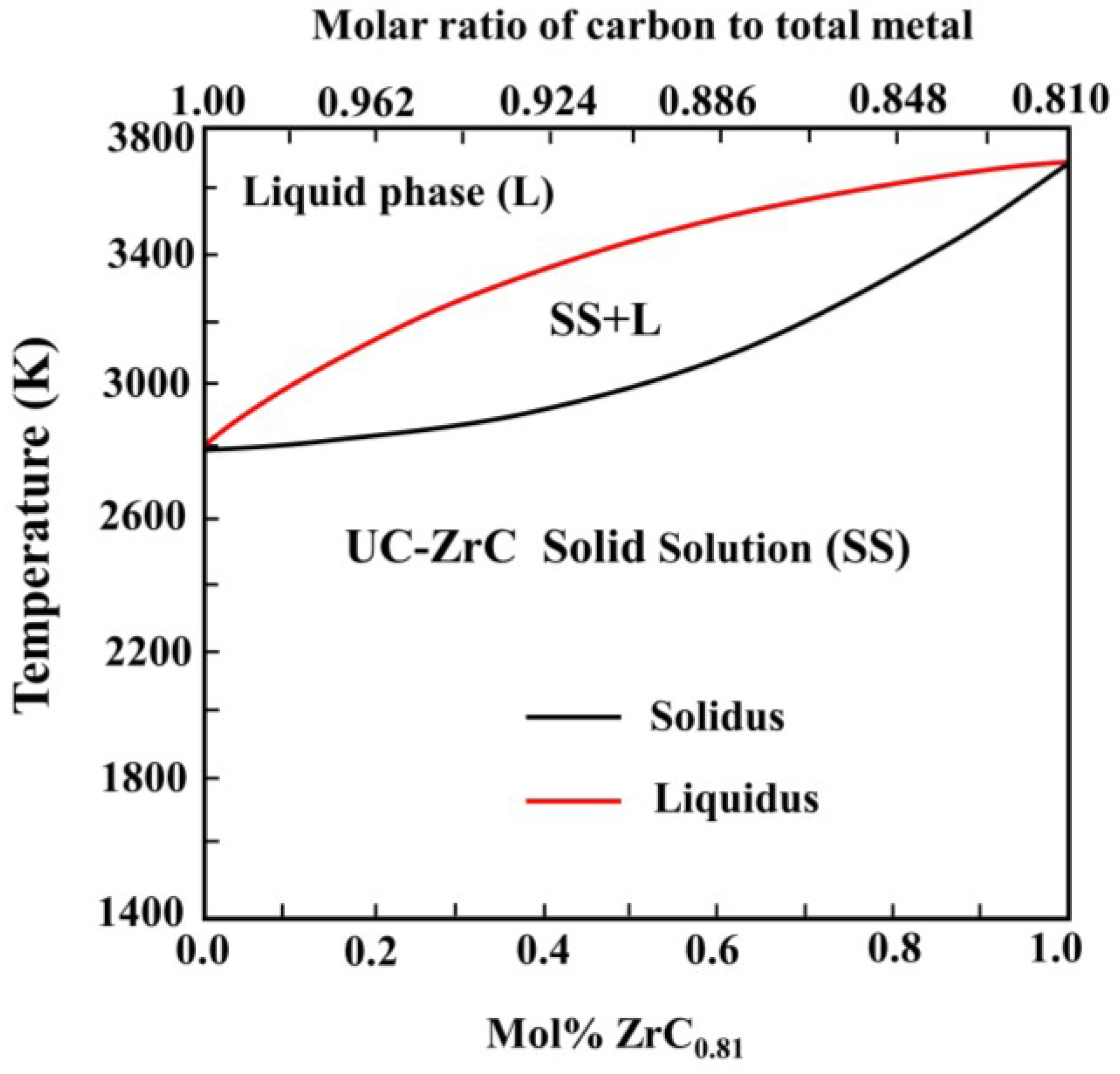
3.1.1. Phase Formation Mechanism Analysis
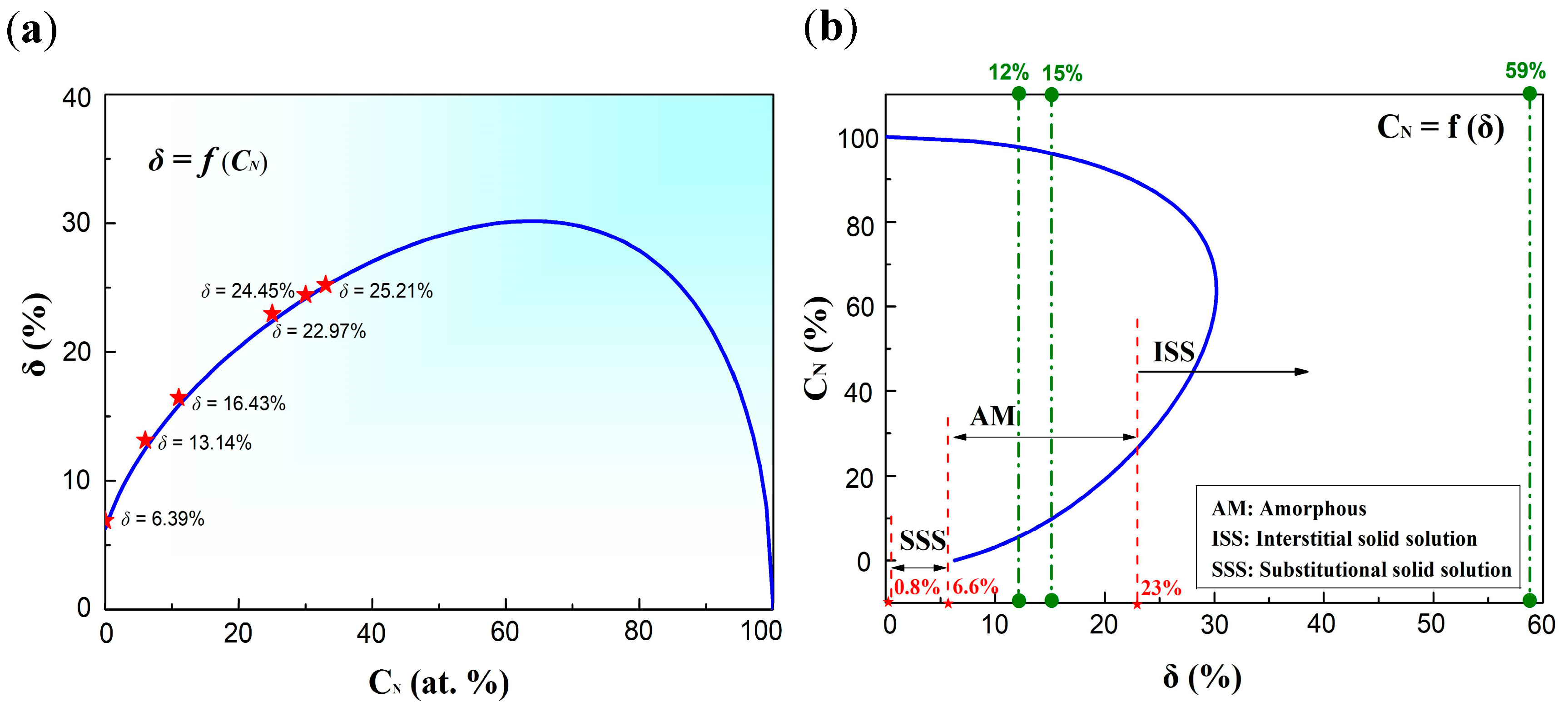
3.1.2. Solid Solution Formation Ability
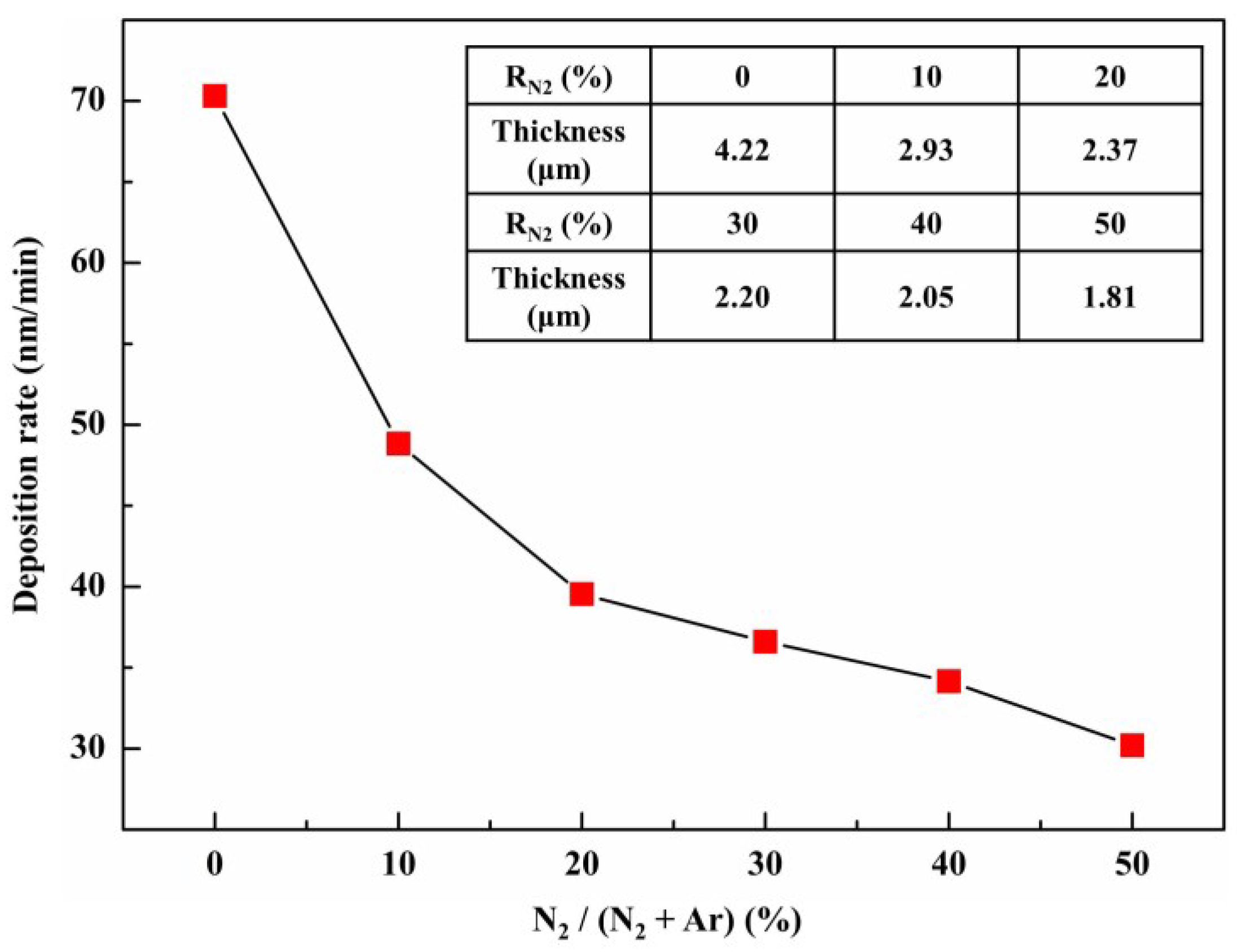
3.2. Deposition Rates
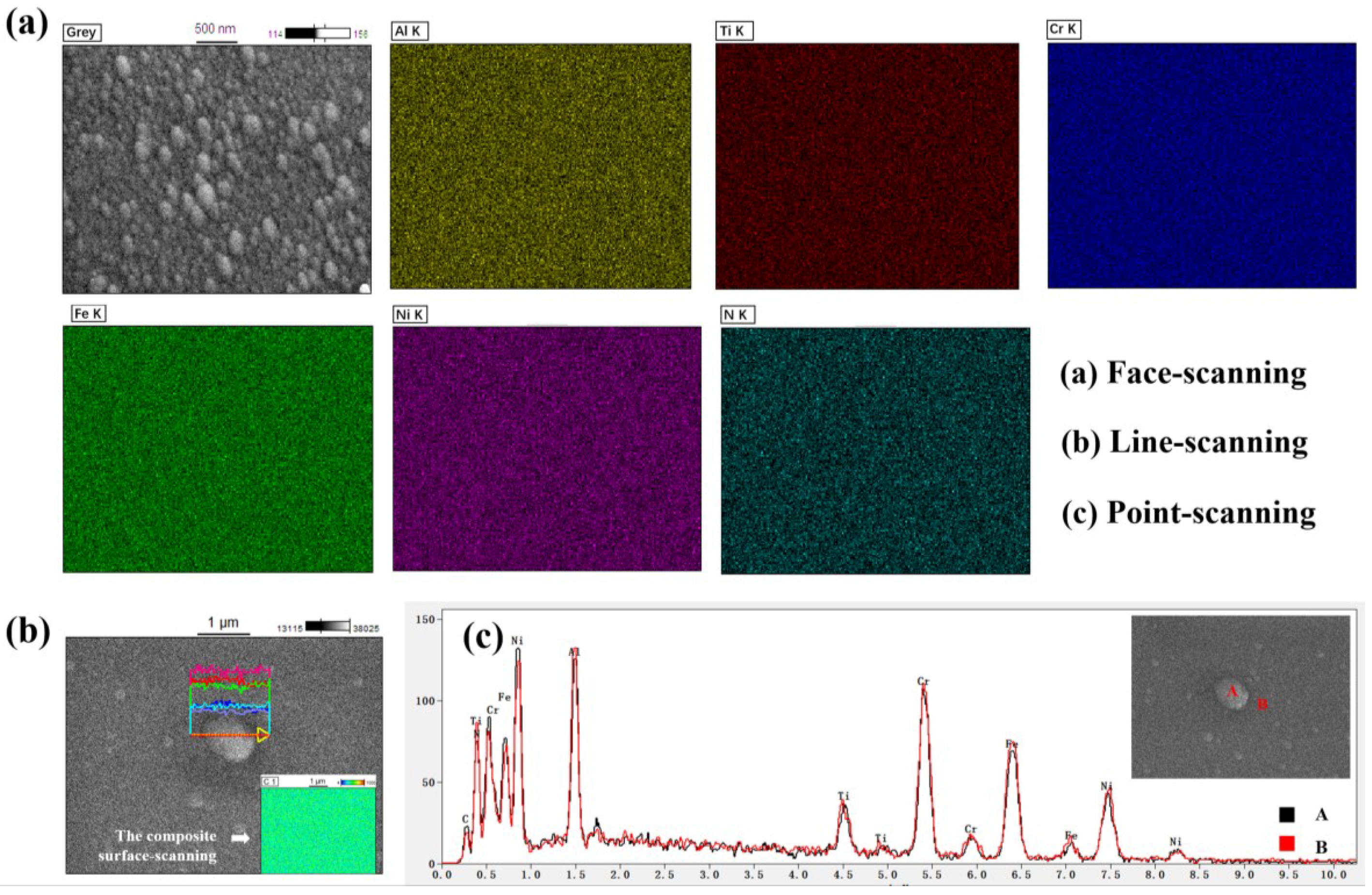
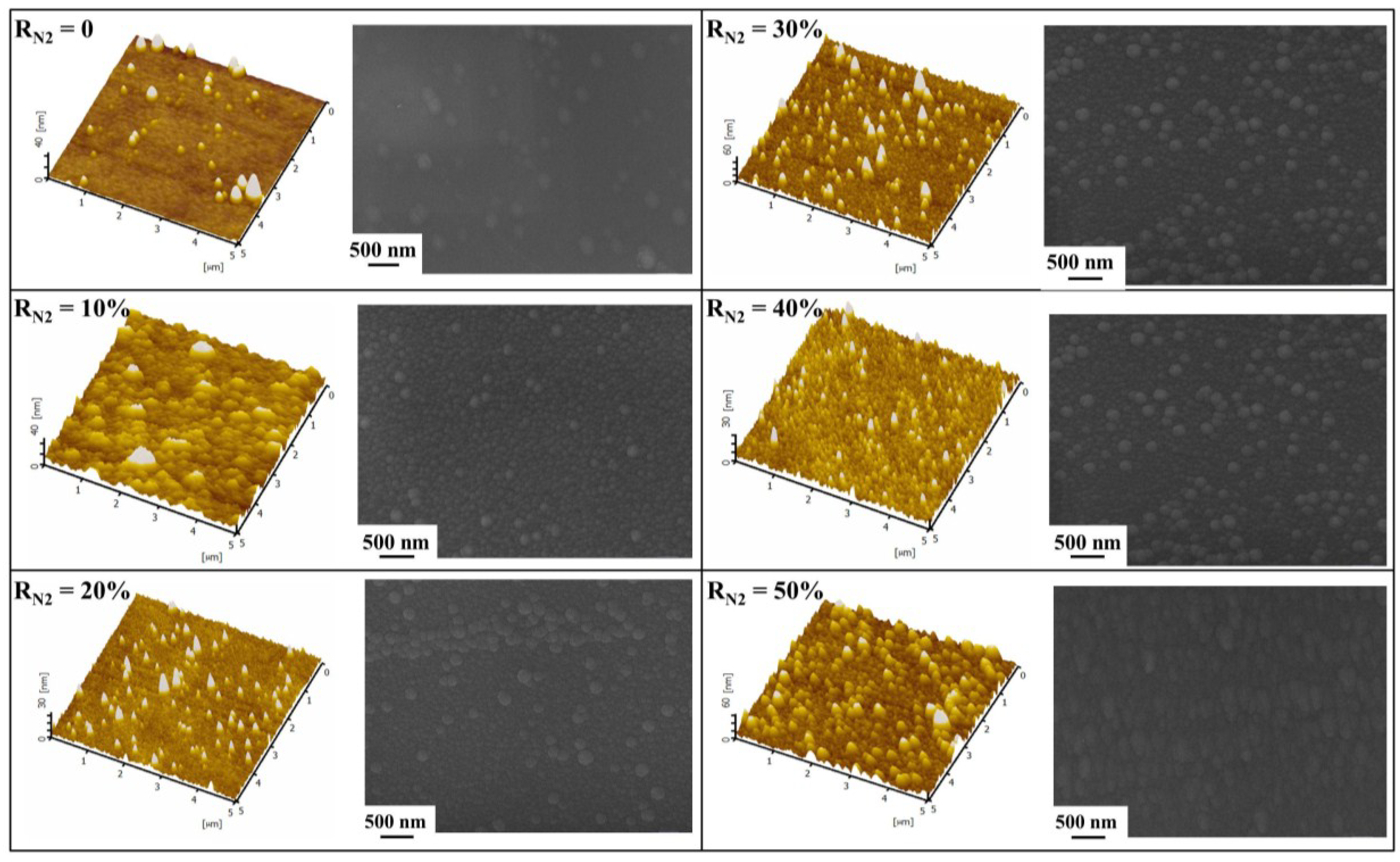
3.3. Surface Morphologies
3.4. Mechanical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef]
- Ma, D.; Grabowski, B.; Körmann, F.; Neugebauer, J.; Raabe, D. Ab initio thermodynamics of the CoCrFeMnNi high entropy alloy: Importance of entropy contributions beyond the configurational one. Acta Mater. 2015, 100, 90–97. [Google Scholar] [CrossRef]
- Wu, Y.D.; Cai, Y.H.; Chen, X.H.; Wang, T.; Si, J.J.; Wang, L.; Wang, Y.D.; Hui, X.D. Phase composition and solid solution strengthening effect in TiZrNbMoV high-entropy alloys. Mater. Des. 2015, 83, 651–660. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-Solution Phase Formation Rules for Multi-component Alloys. Adv. Eng. Mater. 2010, 10, 534–538. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Tsai, M.H.; Shen, W.J.; Yeh, J.W. Structure and properties of two Al–Cr–Nb–Si–Ti high-entropy nitride coatings. Surf. Coat. Technol. 2013, 221, 118–123. [Google Scholar] [CrossRef]
- Huang, P.K.; Yeh, J.W. Effects of substrate temperature and post-annealing on microstructure and properties of (AlCrNbSiTiV)N coatings. Thin Solid Films 2009, 518, 180–184. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, J.B.; Hou, C.; Li, J.C.; Jiang, Q. Dense and smooth amorphous films of multicomponent FeCoNiCuVZrAl high-entropy alloy deposited by direct current magnetron sputtering. Mater. Des. 2013, 46, 675–679. [Google Scholar] [CrossRef]
- Sheng, W.; Yang, X.; Wang, C.; Zhang, Y. Nano-Crystallization of High-Entropy Amorphous NbTiAlSiWxNy Films Prepared by Magnetron Sputtering. Entropy 2016, 18, 226. [Google Scholar] [CrossRef]
- Gao, L.; Song, J.; Jiao, Z.; Liao, W.; Luan, J.; Surjadi, J.U.; Li, J.; Zhang, H.; Sun, D.; Liu, C.T. High-Entropy Alloy (HEA)-Coated Nanolattice Structures and Their Mechanical Properties. Adv. Eng. Mater. 2017, 20. [Google Scholar] [CrossRef]
- Liao, W.; Lan, S.; Gao, L.; Zhang, H.; Xu, S.; Song, J.; Wang, X.; Lu, Y. Nanocrystalline high-entropy alloy (CoCrFeNiAl 0.3) thin-film coating by magnetron sputtering. Thin Solid Films 2017, 638, 383–388. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, N.; He, B.; Zhang, G.; Zhang, Y.; Li, D. Tribological properties of FeCoCrNiAlB x high-entropy alloys coating prepared by laser cladding. J. Iron Steel Res. Int. 2017, 24, 184–189. [Google Scholar] [CrossRef]
- Pogrebnjak, A.; Yakushchenko, I.; Bagdasaryan, A.; Bondar, O.; Krause-Rehberg, R.; Abadias, G.; Chartier, P.; Oyoshi, K.; Takeda, Y.; Beresnev, V. Microstructure, physical and chemical properties of nanostructured (Ti–Hf–Zr–V–Nb) N coatings under different deposition conditions. Mater. Chem. Phys. 2014, 147, 1079–1091. [Google Scholar] [CrossRef]
- Ye, Q.; Feng, K.; Li, Z.; Lu, F.; Li, R.; Huang, J.; Wu, Y. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2016, 396, 1420–1426. [Google Scholar] [CrossRef]
- Hsueh, H.T.; Shen, W.J.; Tsai, M.H.; Yeh, J.W. Effect of nitrogen content and substrate bias on mechanical and corrosion properties of high-entropy films (AlCrSiTiZr)100−xNx. Surf. Coat. Technol. 2012, 206, 4106–4112. [Google Scholar] [CrossRef]
- Shon, Y.; Joshi, S.S.; Katakam, S.; Rajamure, R.S.; Dahotre, N.B. Laser additive synthesis of high entropy alloy coating on aluminum: Corrosion behavior. Mater. Lett. 2015, 142, 122–125. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W.; Gan, J.Y. Diffusion barrier properties of AlMoNbSiTaTiVZr high-entropy alloy layer between copper and silicon. Thin Solid Films 2008, 516, 5527–5530. [Google Scholar] [CrossRef]
- Chang, S.Y.; Li, C.E.; Chiang, S.C.; Huang, Y.C. 4-nm thick multilayer structure of multi-component (AlCrRuTaTiZr)Nx as robust diffusion barrier for Cu interconnects. J. Alloys Compd. 2012, 515, 4–7. [Google Scholar] [CrossRef]
- Chang, S.Y.; Chen, D.S. 10-nm-thick quinary (AlCrTaTiZr)N film as effective diffusion barrier for Cu interconnects at 900 °C. Appl. Phys. Lett. 2009, 94, 222. [Google Scholar] [CrossRef]
- Sheng, W.J.; Yang, X.; Zhu, J.; Wang, C.; Zhang, Y. Amorphous phase stability of NbTiAlSiNX high-entropy films. Rare Metals 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Feng, X.; Fu, W.; Zhang, J.; Zhao, J.; Li, J.; Wu, K.; Liu, G.; Sun, J. Effects of nanotwins on the mechanical properties of Alx CoCrFeNi high entropy alloy thin films. Scripta Mater. 2017, 139, 71–76. [Google Scholar] [CrossRef]
- Feng, X.B.; Zhang, J.Y.; Wang, Y.Q.; Hou, Z.Q.; Wu, K.; Liu, G.; Sun, J. Size effects on the mechanical properties of nanocrystalline NbMoTaW refractory high entropy alloy thin films. Int. J. Plasticity 2017, 95, 264–277. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Yeh, J.W. High thermal stability of the amorphous structure of GexNbTaTiZr (x = 0.5, 1) high-entropy alloys. Mater. Lett. 2016, 181, 223–226. [Google Scholar] [CrossRef]
- Feng, X.; Tang, G.; Gu, L.; Ma, X.; Sun, M.; Wang, L. Preparation and characterization of TaNbTiW multi-element alloy films. Appl. Surf. Sci. 2012, 261, 447–453. [Google Scholar] [CrossRef]
- Lin, P.-C.; Cheng, C.-Y.; Yeh, J.-W.; Chin, T.-S. Soft Magnetic Properties of High-Entropy Fe-Co-Ni-Cr-Al-Si Thin Films. Entropy 2016, 18, 308. [Google Scholar] [CrossRef]
- Liu, S.; Gao, M.C.; Liaw, P.K.; Zhang, Y. Microstructures and mechanical properties of Alx CrFeNiTi 0.25 alloys. J. Alloys Compd. 2015, 619, 610–615. [Google Scholar] [CrossRef]
- Liang, S.C.; Tsai, D.C.; Chang, Z.C.; Sung, H.S.; Lin, Y.C.; Yeh, Y.J.; Deng, M.J.; Shieu, F.S. Structural and mechanical properties of multi-element (TiVCrZrHf)N coatings by reactive magnetron sputtering. Appl. Surf. Sci. 2011, 258, 399–403. [Google Scholar] [CrossRef]
- Lai, C.H.; Lin, S.J.; Yeh, J.W.; Chang, S.Y. Preparation and characterization of AlCrTaTiZr multi-element nitride coatings. Surf. Coat. Technol. 2006, 201, 3275–3280. [Google Scholar] [CrossRef]
- Lin, C.H.; Duh, J.G.; Yeh, J.W. Multi-component nitride coatings derived from Ti–Al–Cr–Si–V target in RF magnetron sputter. Surf. Coat. Technol. 2007, 201, 6304–6308. [Google Scholar] [CrossRef]
- Tsai, D.C.; Huang, Y.L.; Lin, S.R.; Liang, S.C.; Shieu, F.S. Effect of nitrogen flow ratios on the structure and mechanical properties of (TiVCrZrY)N coatings prepared by reactive magnetron sputtering. Appl. Surf. Sci. 2010, 257, 1361–1367. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, J.P.; Chen, G.L.; Yang, J.X.; Ren, Y.G.; Zhang, S.Z. Uranium carbide powder metallurgy. Powder Met. Technol. 1995, 4, 303–306. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, M.; Makino, Y.; Nose, M.; Nogi, K. Phase transition and properties of Ti–Al–N thin films prepared by rf-plasma assisted magnetron sputtering. Thin Solid Films 1999, 339, 203–208. [Google Scholar] [CrossRef]
- Kimura, A.; Kawate, M.; Hasegawa, H.; Suzuki, T. Anisotropic lattice expansion and shrinkage of hexagonal TiAlN and CrAlN films. Surf. Coat. Technol. 2003, 169, 367–370. [Google Scholar] [CrossRef]
- Hörling, A.; Sjölén, J.; Willmann, H.; Larsson, T.; Odén, M.; Hultman, L. Thermal stability, microstructure and mechanical properties of Ti1−xZrxN thin films. Thin Solid Films. 2008, 516, 6421–6431. [Google Scholar] [CrossRef]
- Vetter, J.; Scholl, H.; Knotek, O. (TiCr) N coatings deposited by cathodic vacuum arc evaporation. Surf. Coat. Technol. 1995, 74, 286–291. [Google Scholar] [CrossRef]
- Carvalho, S.; Rebouta, L.; Ribeiro, E.; Vaz, F.; Denannot, M.; Pacaud, J.; Rivière, J.; Paumier, F.; Gaboriaud, R.; Alves, E. Microstructure of (Ti, Si, Al) N nanocomposite coatings. Surf. Coat. Technol. 2004, 177, 369–375. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Z.P.; Ma, S.G.; Liaw, P.K.; Tang, Z.; Gao, M.C. Guidelines in predicting phase formation of high-entropy alloys. Mrs Commun. 2014, 4, 57–62. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Lu, C.Y.; Diyatmika, W.; Lou, B.S.; Lu, Y.C.; Duh, J.G.; Lee, J.W. Influences of target poisoning on the mechanical properties of TiCrBN thin films grown by a superimposed high power impulse and medium-frequency magnetron sputtering. Surf. Coat. Technol. 2017, 332, 86–95. [Google Scholar] [CrossRef]
- Arif, M.; Eisenmenger-Sittner, C. In situ assessment of target poisoning evolution in magnetron sputtering. Surf. Coat. Technol. 2017, 324, 345–352. [Google Scholar] [CrossRef]
- Pelleg, J. Mechanical Properties of Materials; Springer: New York, NY, USA, 2008; pp. 236–239. ISBN 978-94-007-4341-0.SG. [Google Scholar]
- Mizutani, U. The Hume-Rothery rules for structurally complex alloy phases. In Surface Properties and Engineering of Complex Intermetallics; World Scientific: Singapore, 2010; pp. 323–399. [Google Scholar] [CrossRef]
- Barrett, C.S. Structure of Metals; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1943. [Google Scholar]



| Element | N | Al | Ti | Cr | Fe | Ni |
|---|---|---|---|---|---|---|
| Radius (nm) | 0.071 | 0.1434 | 0.1445 | 0.1249 | 0.1241 | 0.1246 |
| RN2 (%) | 0 | 10 | 20 | 30 | 40 | 50 |
|---|---|---|---|---|---|---|
| Nitrogen Content (at %) | 0 | 5.94 | 10.97 | 25.10 | 29.55 | 32.21 |
| Ω | 1.60 | 1.31 | 0.44 | 0.23 | 0.20 | 0.19 |
| δ | 6.39% | 13.14% | 16.43% | 22.97% | 24.45% | 25.21% |
| Ra (nm) | 1.375 | 1.574 | 3.780 | 4.542 | 4.490 | 5.574 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yan, X.-H.; Liao, W.-B.; Zhao, K. Effects of Nitrogen Content on the Structure and Mechanical Properties of (Al0.5CrFeNiTi0.25)Nx High-Entropy Films by Reactive Sputtering. Entropy 2018, 20, 624. https://doi.org/10.3390/e20090624
Zhang Y, Yan X-H, Liao W-B, Zhao K. Effects of Nitrogen Content on the Structure and Mechanical Properties of (Al0.5CrFeNiTi0.25)Nx High-Entropy Films by Reactive Sputtering. Entropy. 2018; 20(9):624. https://doi.org/10.3390/e20090624
Chicago/Turabian StyleZhang, Yong, Xue-Hui Yan, Wei-Bing Liao, and Kun Zhao. 2018. "Effects of Nitrogen Content on the Structure and Mechanical Properties of (Al0.5CrFeNiTi0.25)Nx High-Entropy Films by Reactive Sputtering" Entropy 20, no. 9: 624. https://doi.org/10.3390/e20090624
APA StyleZhang, Y., Yan, X.-H., Liao, W.-B., & Zhao, K. (2018). Effects of Nitrogen Content on the Structure and Mechanical Properties of (Al0.5CrFeNiTi0.25)Nx High-Entropy Films by Reactive Sputtering. Entropy, 20(9), 624. https://doi.org/10.3390/e20090624