Formation of Photo-Responsive Liquid Crystalline Emulsion by Using Microfluidics Device
Abstract
:1. Introduction
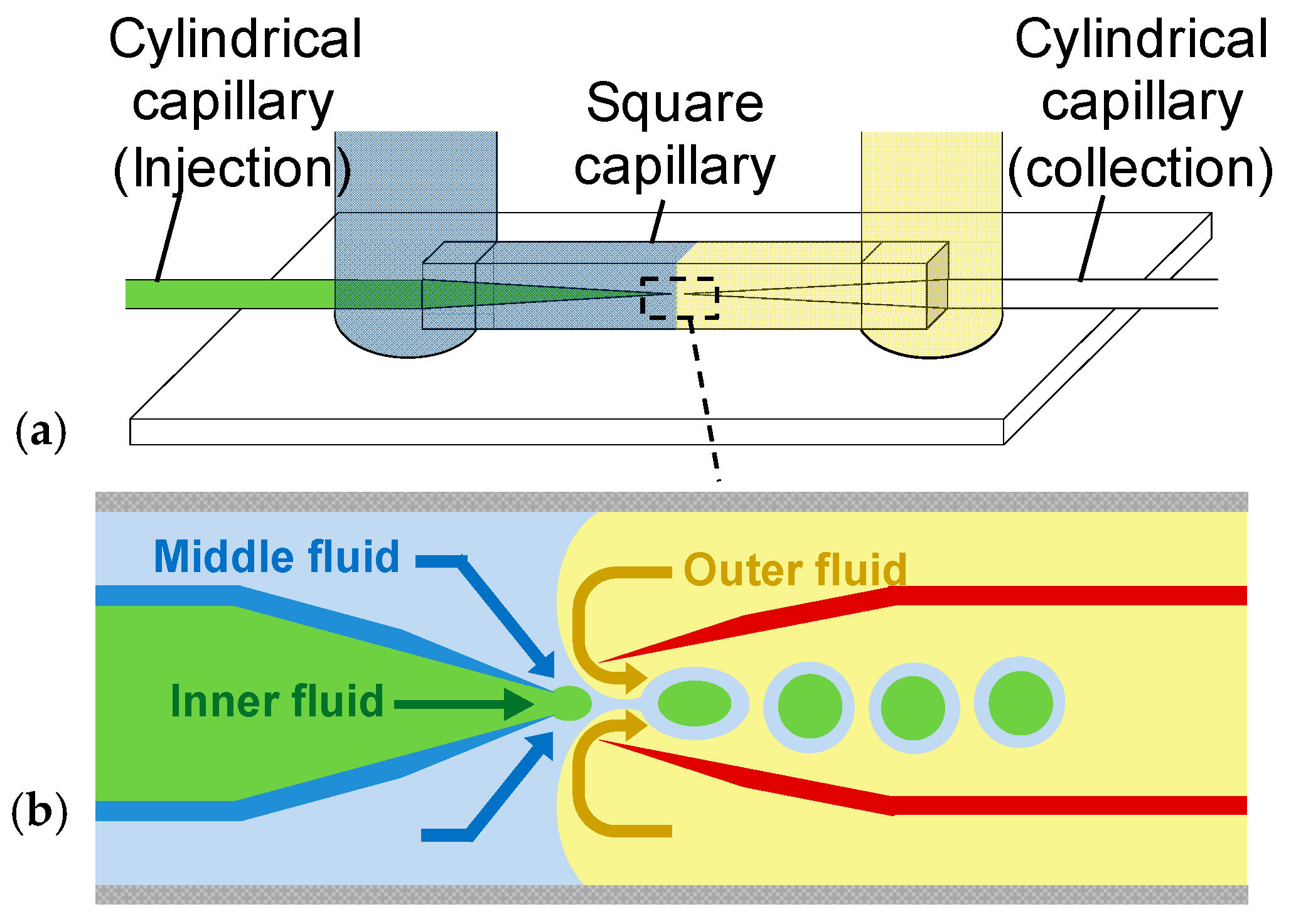
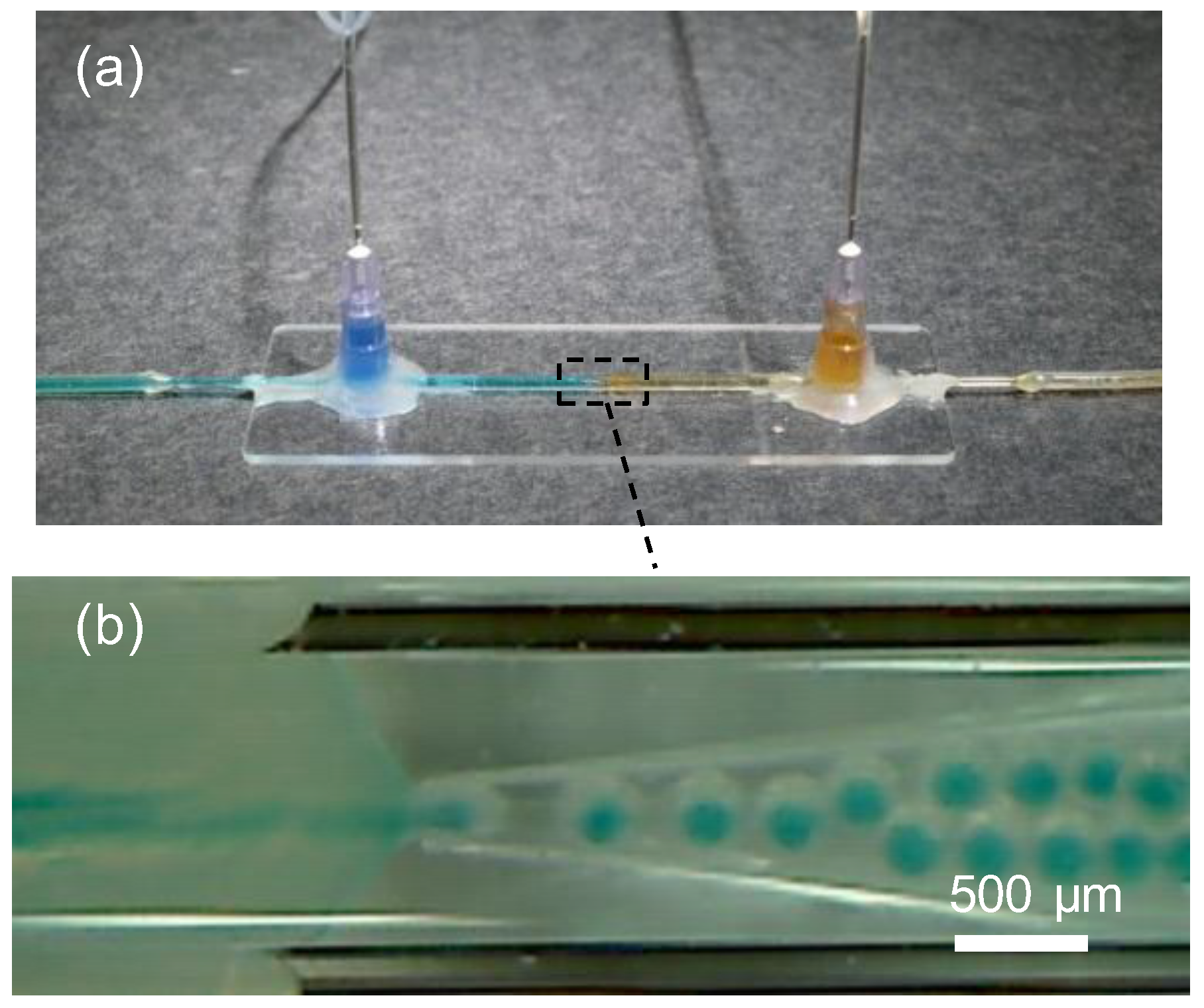
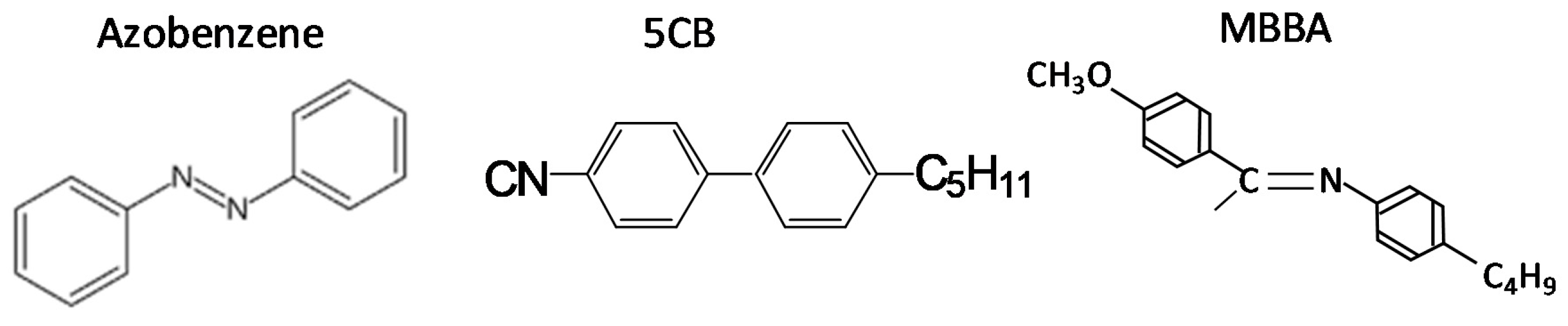
2. Materials and Methods
3. Results
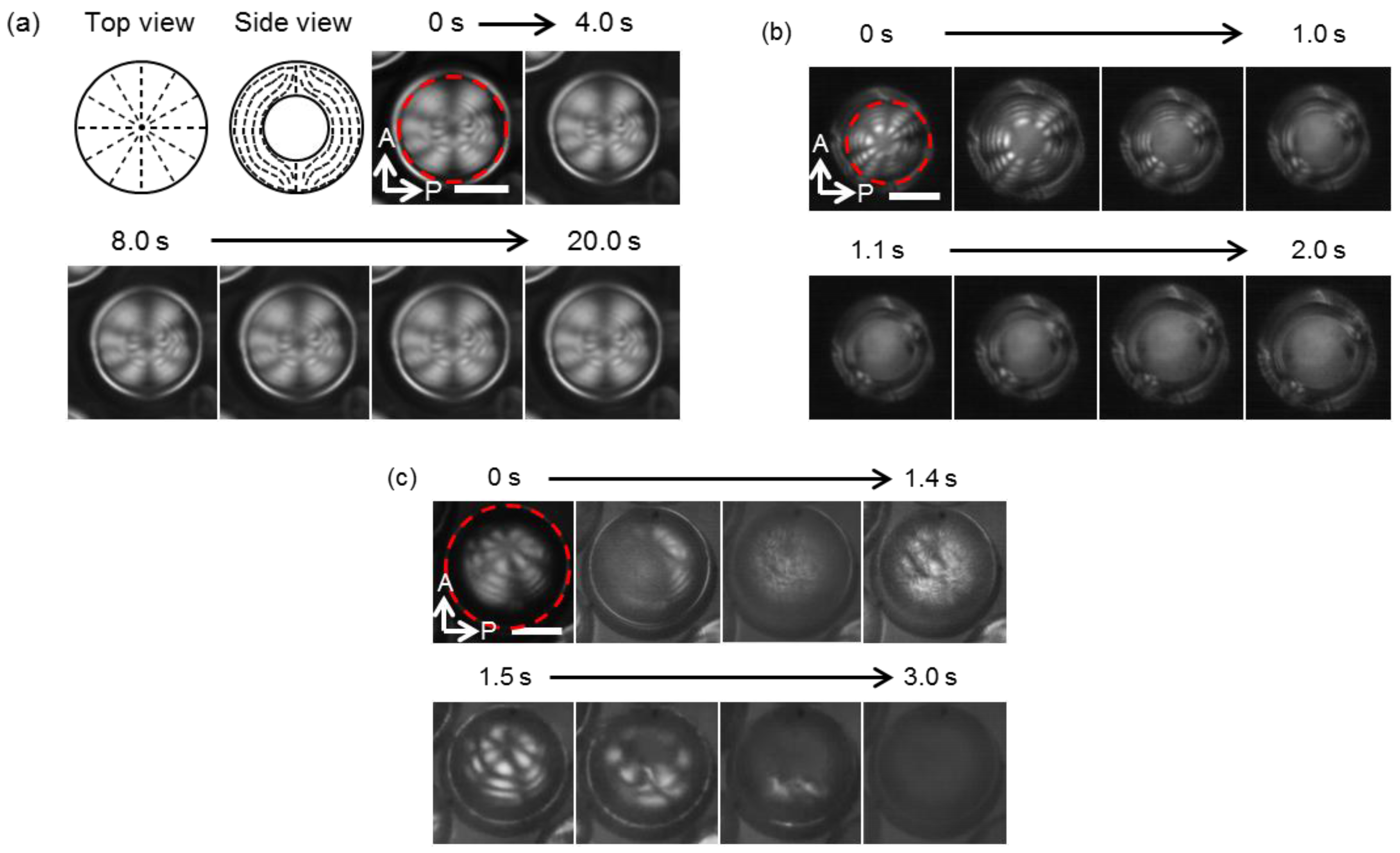
3.1. Observation of Photo-Response
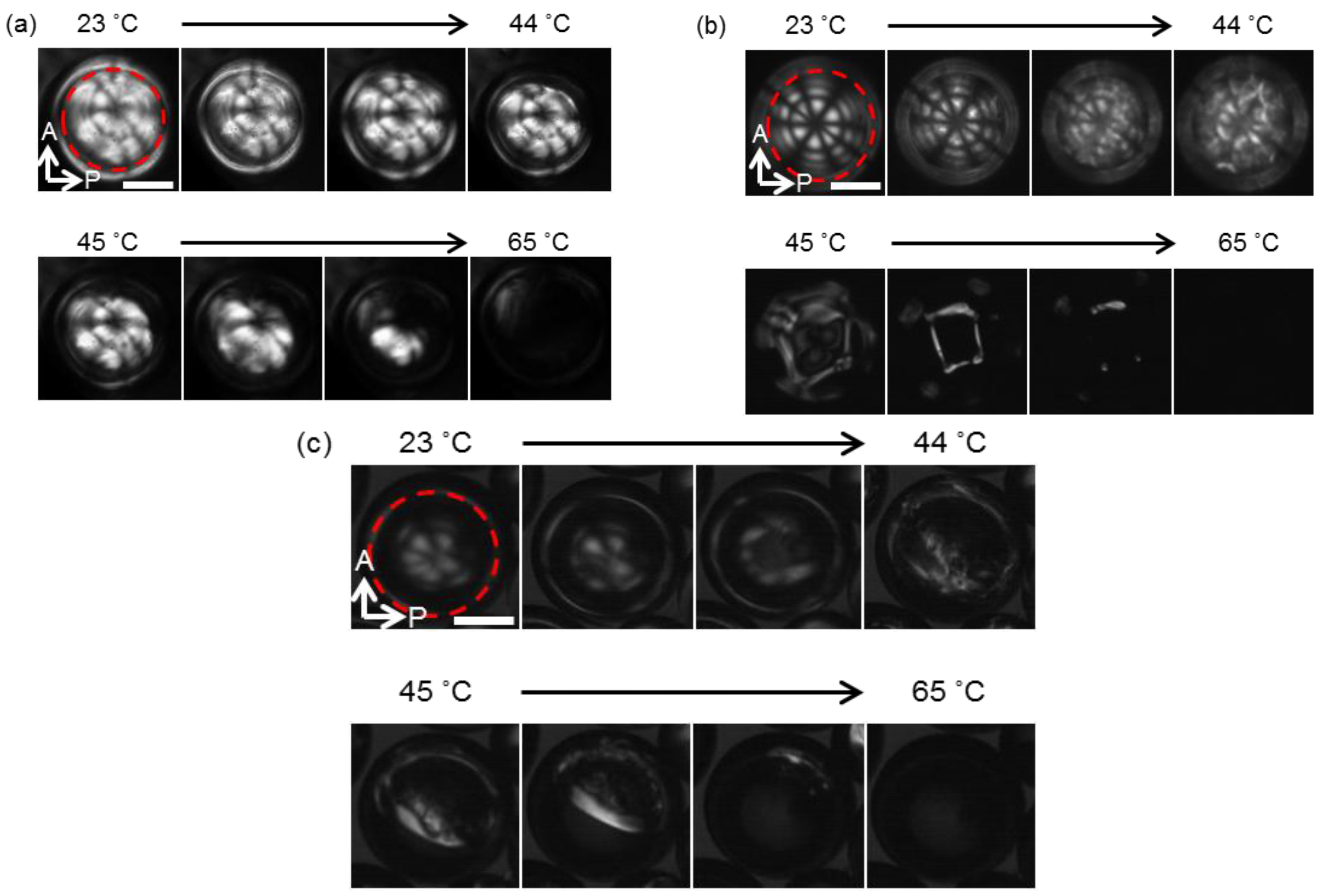
3.2. Observation of Thermal Response
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sánchez, S.; Soler, L.; Katuri, J. Chemically powered micro- and nanomotors. Angew. Chem. Int. Ed. 2015, 54, 1414–1444. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Müller, A.H.E. Janus particles: Synthesis, self-assembly, physical properties, and applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, Q.; Tripathy, M.; Luijten, E.; Schweizer, K.S.; Granick, S. Janus particle synthesis and assembly. Adv. Mater. 2010, 22, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Eremin, A.; Hirankittiwong, P.; Chattham, N.; Nádasi, H.; Stannarius, R.; Limtrakul, J.; Haba, O.; Yonetake, K.; Takezoe, H. Optically driven translational and rotational motions of microrod particles in a nematic liquid crystal. Proc. Natl. Acad. Sci. USA 2015, 112, 1716–1720. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sokolov, A.; Lavrentovich, O.D.; Aranson, I.S. Living liquid crystals. Proc. Natl. Acad. Sci. USA 2014, 111, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Turiv, T.; Guo, Y.; Wei, Q.-H.; Lavrentovich, O.D. Command of active matter by topological defects and patterns. Science 2016, 354, 882. [Google Scholar] [CrossRef] [PubMed]
- Keber, F.C.; Loiseau, E.; Sanchez, T.; DeCamp, S.J.; Giomi, L.; Bowick, M.J.; Marchetti, M.C.; Dogic, Z.; Bausch, A.R. Topology and dynamics of active nematic vesicles. Science 2014, 345, 1135. [Google Scholar] [CrossRef] [PubMed]
- Lavrentovich, O.D.; Lazo, I.; Pishnyak, O.P. Nonlinear electrophoresis of dielectric and metal spheres in a nematic liquid crystal. Nature 2010, 467, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Lazo, I.; Lavrentovich, O.D. Liquid-crystal-enabled electrophoresis of spheres in a nematic medium with negative dielectric anisotropy. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2013, 371, 20120255. [Google Scholar] [CrossRef] [PubMed]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse double emulsions generated from a microcapillary device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Nieves, A.; Vitelli, V.; Utada, A.S.; Link, D.R.; Márquez, M.; Nelson, D.R.; Weitz, D.A. Novel defect structures in nematic liquid crystal shells. Phys. Rev. Lett. 2007, 99, 157801. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Q. Stimuli-directing self-organized 3D liquid-crystalline nanostructures: From materials design to photonic applications. Adv. Funct. Mater. 2016, 26, 10–28. [Google Scholar] [CrossRef]
- Wang, L.; Chen, D.; Gutierrez-Cuevas, K.G.; Krishna Bisoyi, H.; Fan, J.; Zola, R.S.; Li, G.; Urbas, A.M.; Bunning, T.J.; Weitz, D.A.; et al. Optically reconfigurable chiral microspheres of self-organized helical superstructures with handedness inversion. Mater. Horiz. 2017, 4, 1190–1195. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Fan, J.; Bisoyi, H.K.; Weitz, D.A.; Li, Q. Photoresponsive monodisperse cholesteric liquid crystalline microshells for tunable omnidirectional lasing enabled by a visible light-driven chiral molecular switch. Adv. Opt. Mater. 2014, 2, 845–848. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, S.K.; Won, J.C.; Kim, Y.H.; Kim, S.-H. Reconfigurable photonic capsules containing cholesteric liquid crystals with planar alignment. Angew. Chem. Int. Ed. 2015, 54, 15266–15270. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.; Kim, S.H.; Lee, I.; Kim, S.K.; Cho, M.; Kim, H. Dynamics of nematic MBBA film induced by transient grating under a strong absorption condition. J. Phys. Chem. B 1998, 102, 7705–7713. [Google Scholar] [CrossRef]
- Ikeda, T.; Miyamoto, T.; Kurihara, S.; Tsukada, M.; Tazuke, S. Effect of structure of photoresponsive molecules on photochemical phase-transition of liquid-crystals. I, Synthesis and thermotropic properties of photochromic azobenzene derivatives. Mol. Cryst. Liq. Cryst. 1990, 182, 357–371. [Google Scholar] [CrossRef]
- Kurihara, S.; Ikeda, T.; Sasaki, T.; Kim, H.B.; Tazuke, S. Time-resolved observation of isothermal phase-transition of liquid-crystals induced by photoisomerization of azobenzene dopant. Mol. Cryst. Liq. Cryst. 1991, 195, 251–263. [Google Scholar] [CrossRef]
- Statman, D.; Jánossy, I. Study of photoisomerization of azo dyes in liquid crystals. J. Chem. Phys. 2003, 118, 3222–3232. [Google Scholar] [CrossRef]
- Lopez-Leon, T.; Fernandez-Nieves, A. Drops and shells of liquid crystal. Colloid Polym. Sci. 2011, 289, 345–359. [Google Scholar] [CrossRef]
- Ramos-Garcia, R.; Lazo-Martínez, I.; Guizar-Iturbide, I.; Sanchez-Castillo, A.; Boffety, M.; Rück, P. Colossal nonlinear optical effect in dye-doped liquid crystals. Mol. Cryst. Liq. Cryst. 2006, 454, 179/[581]–185/[587]. [Google Scholar] [CrossRef]
- Lucchetti, L.; Di Fabrizio, M.; Francescangeli, O.; Simoni, F. Colossal optical nonlinearity in dye doped liquid crystals. Opt. Commun. 2004, 233, 417–424. [Google Scholar] [CrossRef]
- Simoni, F.; Lucchetti, L.; Lucchetta, D.E.; Francescangeli, O. On the origin of the huge nonlinear response of dye-doped liquid crystals. Opt. Express 2001, 9, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, L.; Gentili, M.; Simoni, F. Pretransitional enhancement of the optical nonlinearity of thin dye-doped liquid crystals in the nematic phase. Appl. Phys. Lett. 2005, 86, 151117. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dogishi, Y.; Endo, S.; Sohn, W.Y.; Katayama, K. Formation of Photo-Responsive Liquid Crystalline Emulsion by Using Microfluidics Device. Entropy 2017, 19, 669. https://doi.org/10.3390/e19120669
Dogishi Y, Endo S, Sohn WY, Katayama K. Formation of Photo-Responsive Liquid Crystalline Emulsion by Using Microfluidics Device. Entropy. 2017; 19(12):669. https://doi.org/10.3390/e19120669
Chicago/Turabian StyleDogishi, Yoshiharu, Shun Endo, Woon Yong Sohn, and Kenji Katayama. 2017. "Formation of Photo-Responsive Liquid Crystalline Emulsion by Using Microfluidics Device" Entropy 19, no. 12: 669. https://doi.org/10.3390/e19120669
APA StyleDogishi, Y., Endo, S., Sohn, W. Y., & Katayama, K. (2017). Formation of Photo-Responsive Liquid Crystalline Emulsion by Using Microfluidics Device. Entropy, 19(12), 669. https://doi.org/10.3390/e19120669





