Optimal Thermodynamics—New Upperbounds
Abstract
:1. Introduction
1.1. CARNOT’s pioneering work
1.2. Onsager’s work
1.3. Finite Time Thermodynamics (F.T.T.)
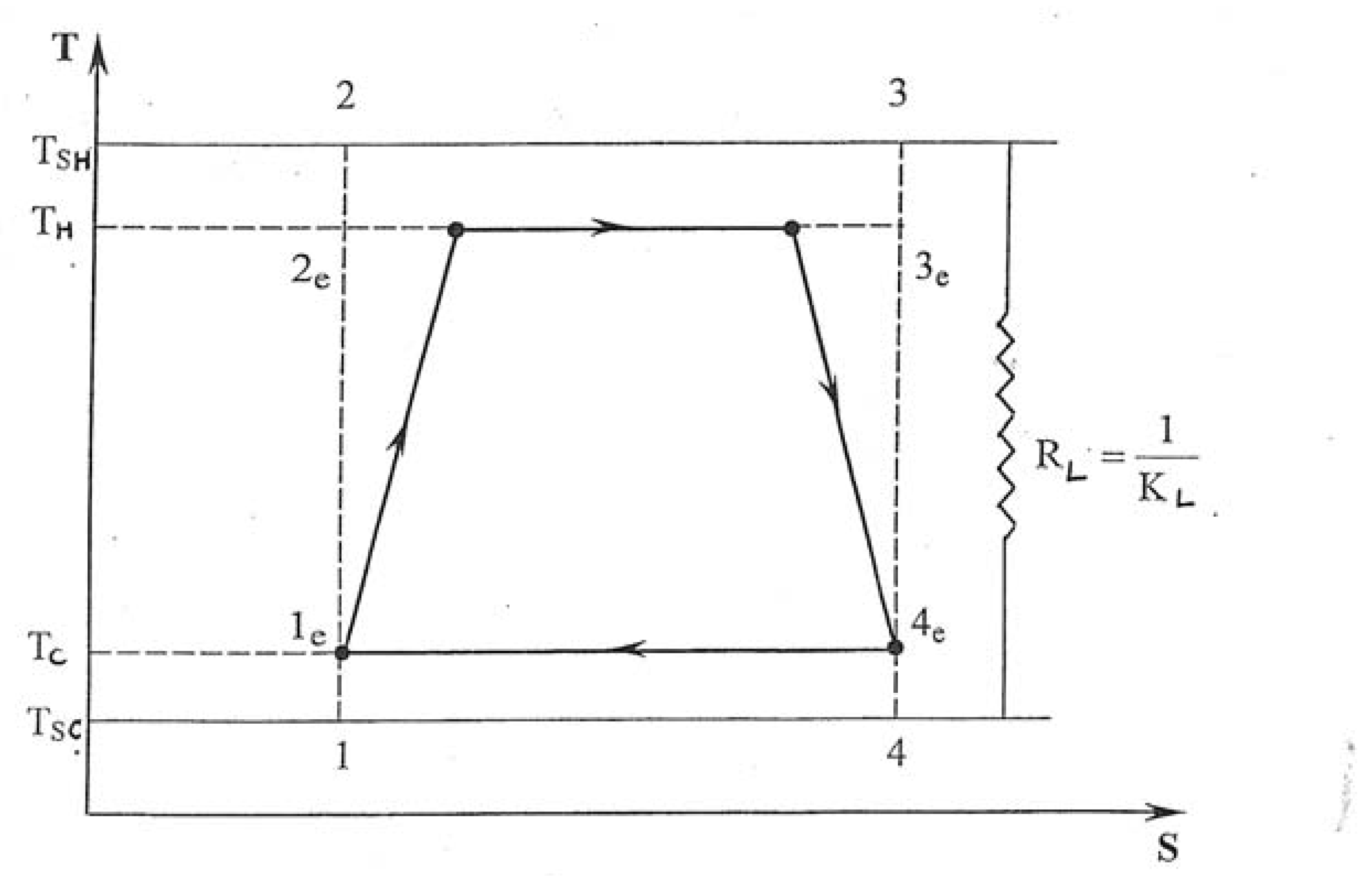
1.4. The aims of the present paper
2. The Model: from the Carnot Cycle to the Carnot Heat Engine
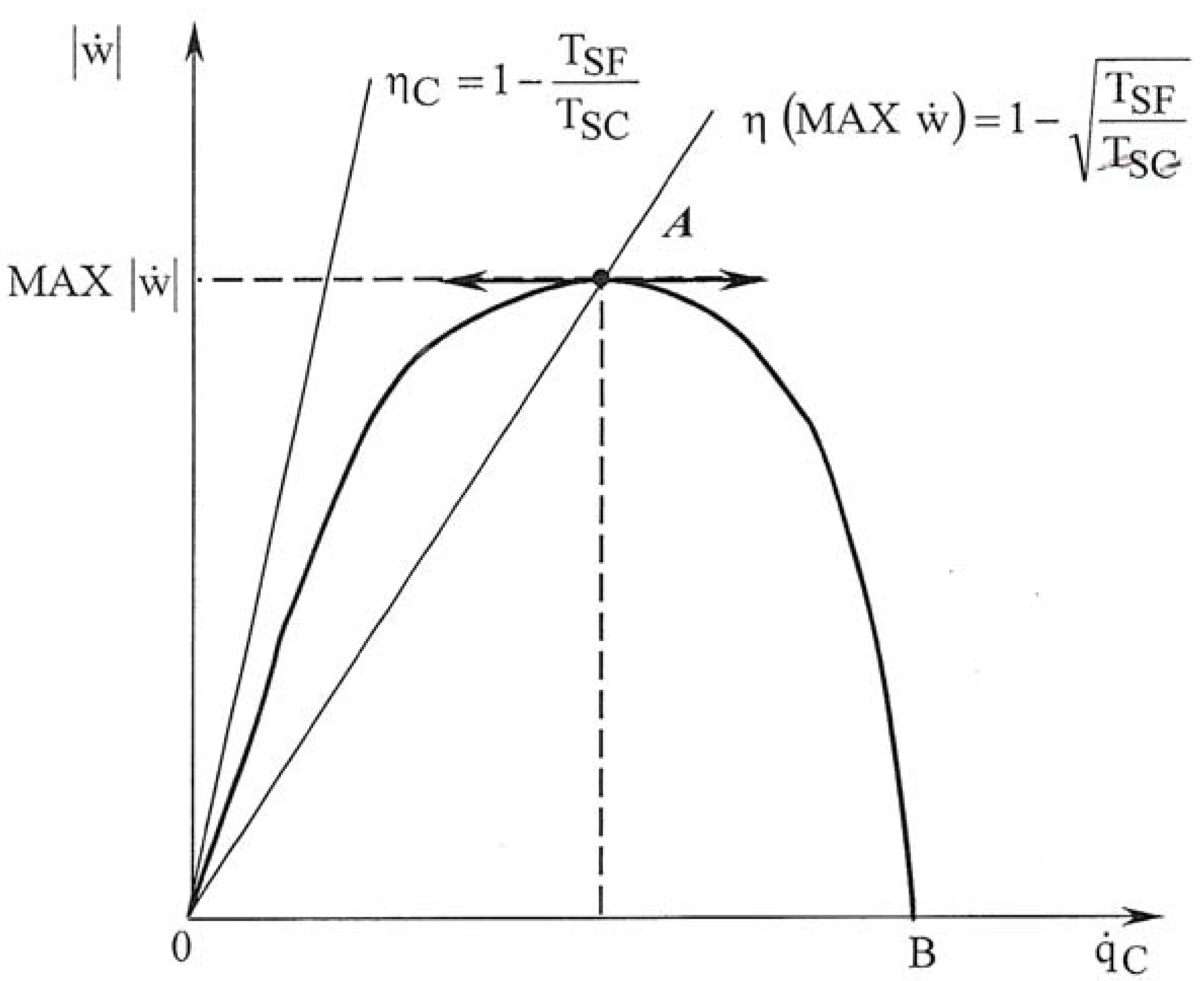

3. Entropy Generated versus Irreversibility Ratio
3.1. Irreversibility ratio method
3.2. Generated entropy flux method
3.3. A partial conclusion
4. Various Optimization Objectives
5. Engine Optimization with Constraints
5.1. Model equations
5.2. Added constraints
6. The Case of a Combined Heat and Power System (C.H.P.)
6.1. Comparison of the two irreversibility methods
6.2. Extension of these results to cases with an extra constraint
| O.F. | MAX () | |
| Added constraint | ||
| R = R0 | ||
| without | ||
7. The Case of the Internal Combustion Engine (I.C.E.)
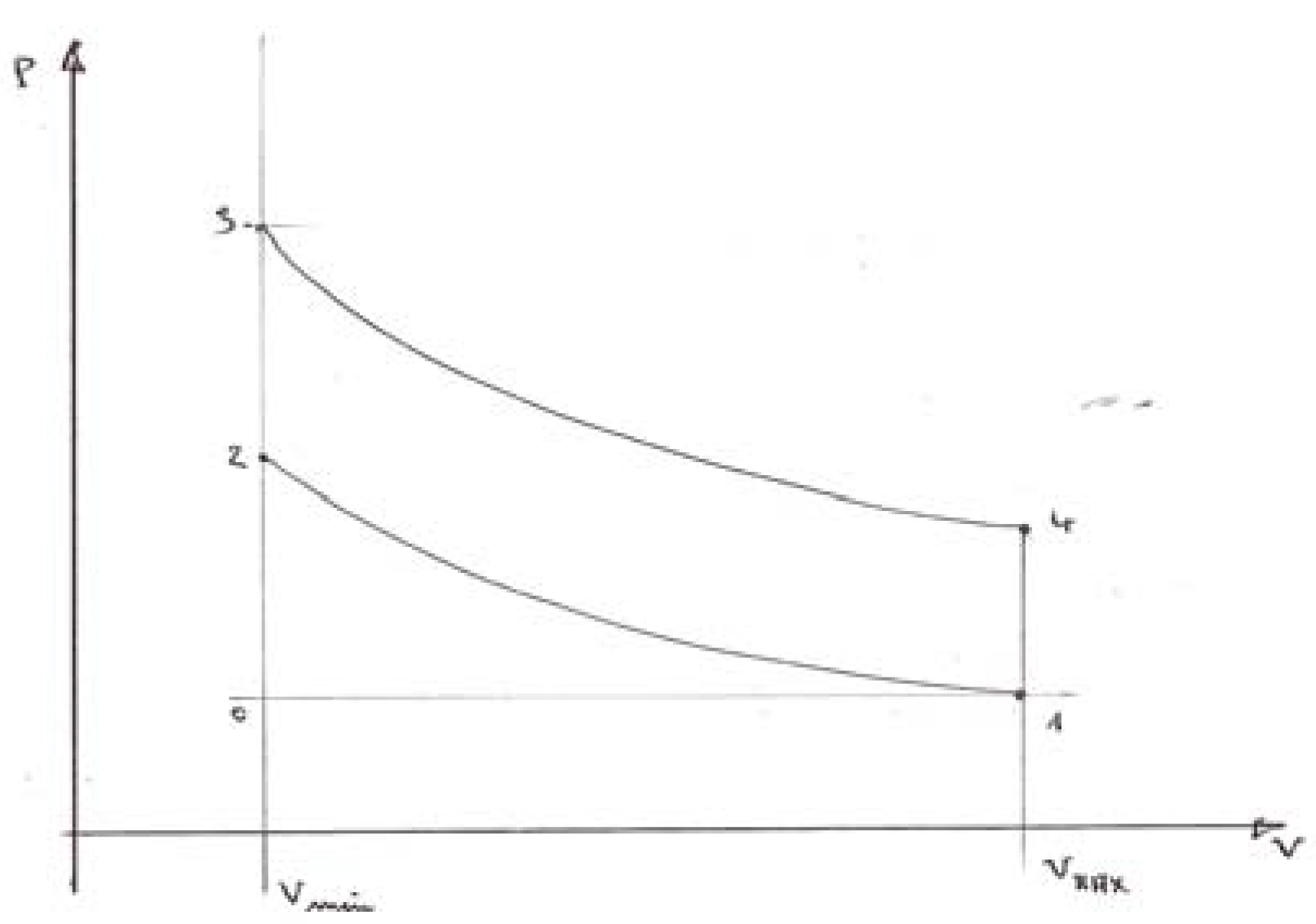
7.1. The incoming energy flux (fuel consumption) is imposed
7.2. An engine with imposed maximum temperature TMAX
7.3. Further details and results
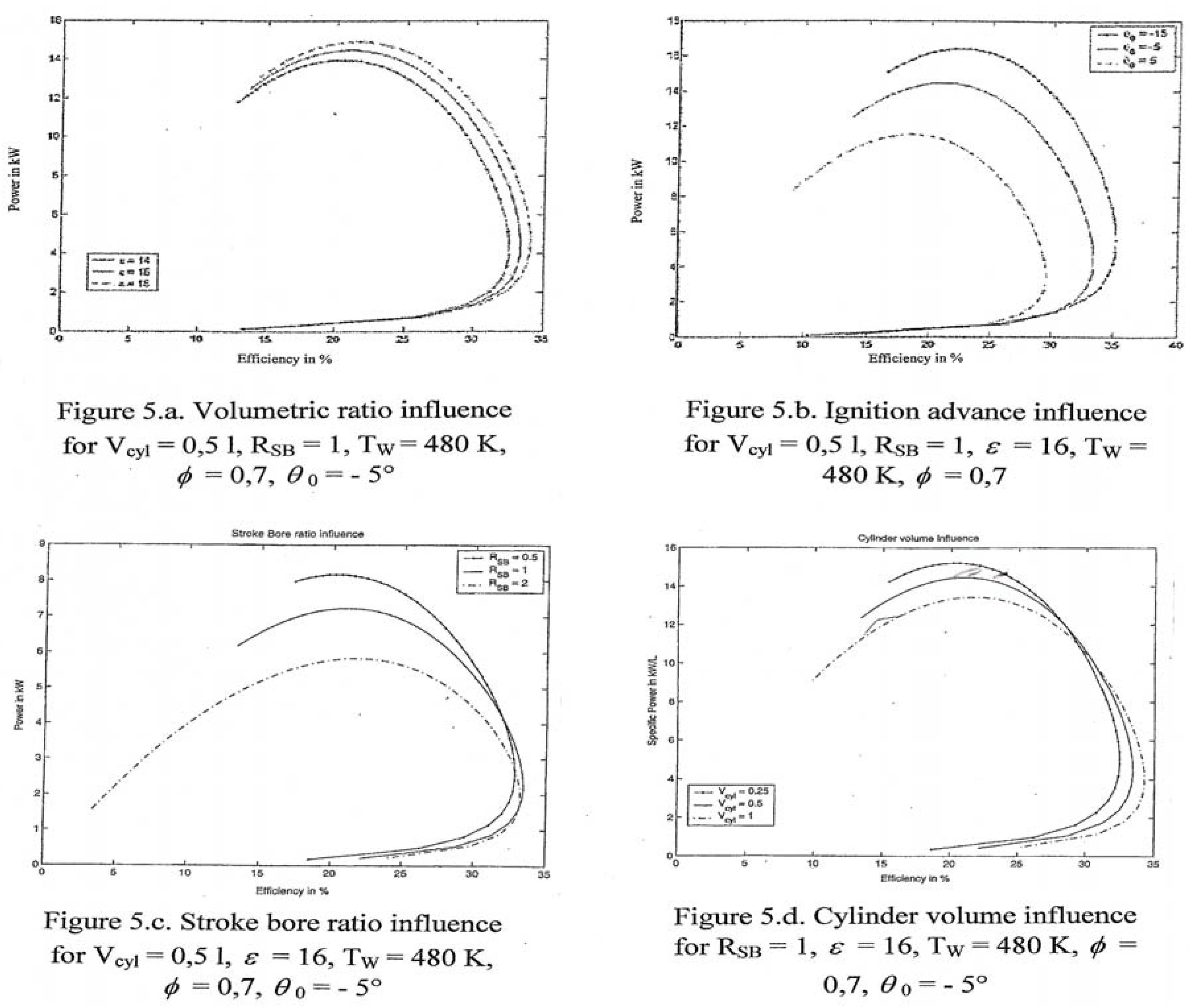
8. Conclusions
8.1. The present paper features a short overview of thermodynamics from Carnot to the present
8.2. Comparison between the irreversibility ratio and the entropy flux method
8.3. New objective functions (energy comsumption, environmental pollution etc)
8.4. New constraints (imposed energy consumption, power, efficiency etc.)
8.5. Enlarged concept of F.D.O.T.
References
- Maury, J.P. Carnot et la machine à vapeur; P.U.F.: Paris, France, 1987. [Google Scholar]
- Onsager, L. Reciprocal relations in irreversible processes I. Phys. Rev. 1931, 37, 405. [Google Scholar]Reciprocal relations in irreversible processes II. Phys. Rev. 1931, 38, 2265. [CrossRef]
- Curzon, F.L.; Ahlborn, B. Efficiency of a Carnot engine at maximum power output. Am. J. Phys. 1975, 1, 22–24. [Google Scholar] [CrossRef]
- Feidt, M. Thermodynamique et optimisation énergétique des systèmes et procédés; TEC et DOC: 2ème édition Paris, France, 1996; p. 343. [Google Scholar]
- Chambadal, P. Les centrales nucléaires; A. Colin: Paris, France, 1957. [Google Scholar]Le choix du cycle thermique dans une usine génératrice nucléaire; Revue Générale d'Electricité, 1958.Evolution et applications du concept d'entropie; Dunod: Paris, France, 1963; p. 84.
- Novikov, I. The efficiency of atomic power stations (a review). Atomaya Energiya 1957, 3–11. [Google Scholar] [CrossRef]
- Kondepudi, D.; Prigogine, I. Modern Thermodynamics; Wiley: Chicester, UK, 1998. [Google Scholar]
- Bejan, A. Entropy generation minimization : the new thermodynamics of finite size and finite time processes. J. Appl. Phys. 1997, 79, 1191–1218. [Google Scholar] [CrossRef]
- Chen, L.; Wu, C.; Sun, F. Finite time thermodynamic optimization or entropy generation minimization of energy system. J. Non Equil. Thermodyn. 1999, 24, 327–359. [Google Scholar] [CrossRef]
- Ourmayaz, A.; Sogut, S.O.; Sahin, B.; Yavuz, H. Optimization of thermal systems based on finite time thermodynamics and thermoeconomics. Prog. Energ. Combust. Sci. 2004, 30, 175–217. [Google Scholar] [CrossRef]
- Wu, C.; Kiang, R.L.; Lopardo, V.J.; Karpouzian, G.N. Finite time thermodynamics and endoreversible heat engines. Int. J. Mech. Eng. Edu. 1993, 21, 337–346. [Google Scholar] [CrossRef]
- Salomon, P.; Nitzan, A. Finite time optimization of a newton's law Carnot cycle. J. Chem. Phys 1981, 74, 3546–3560. [Google Scholar] [CrossRef]
- Salomon, P.; Andresen, B.; Berry, R.S. Thermodynamics in finite time II. Potentials for finite time processes. Phys. Rev. A 1977, 15, 2094–2102. [Google Scholar] [CrossRef]
- Feidt, M. Optimal use of energy systems and processes. Int. J. Exergy. (in press). [CrossRef]
- Gutkowicz-Krusin, D.; Procaccia, I.; Ross, J. On the efficiency of rate processes. Power and efficiency of heat engines. J. Chem. Phys. 1978, 69, 3898–3906. [Google Scholar] [CrossRef]
- Stitou, D.; Feidt, M. Nouveaux critères pour l'optimisation et la caractérisation des procédés thermiques de conversion énergétique. Int. J. Therm. Sci. 2005, 44, 1142–1153. [Google Scholar] [CrossRef]
- Andresen, B.; Salamon, R.; Berry, R.S. Thermodynamics in finite time : extremals for imperfect heat engines. J. Chem. Phys. 1977, 66, 1571–1577. [Google Scholar] [CrossRef]
- Patria, R.K.; Nulton, J.D.; Salamon, P. Carnot like processes in finite time II. Applications to model cycles. Am. J. Phys. 1993, 61, 916–924. [Google Scholar] [CrossRef]
- Gordon, J.M.; Huleilil, M. General performance characteristics of real heat engines. J. Appl. Phys. 1992, 7, 829–837. [Google Scholar] [CrossRef]
- Petre, C.; Costea, M.; Petre, C.; Petrescu, S. Optimization of the direct Carnot cycle. Appl. Therm. Eng. 2007, 27, 829–839. [Google Scholar]
- Wu, C.; Kiang, R.L. Finite time thermodynamic analysis of a Carnot engine with internal irreversibility. Energy 1992, 1, 1173–1178. [Google Scholar] [CrossRef]
- Feidt, M. Thermodynamics and optimization of reverse cycle machines, in Thermodynamics Optimization of Complex Energy Systems; Kluwer Academic Publishers: Morwell, MA, USA, 1999. [Google Scholar]
- Feidt, M.; Philippi, I. Basis of a general approach for finite time Thermodynamics applied to two heat reservoirs machine. In Int. Symposium ECOS'92, Zaragosa, Spain; 1992. [Google Scholar]
- Feidt, M. Thermodynamique en temps fini des cycles moteurs ou générateur de froid ou de chaud. In Invited Lecture at the Second Conference of Romanian Society of Thermotechnics, Bucuresti, Romania; 1993. [Google Scholar]
- Feidt, M.; Ramany-Bala, P.; Benelmir, R. Synthesis, unification and generalization of preceding finite time studies devoted to Carnot cycles; Flowers: Firenze, Italy, 1994. [Google Scholar]
- Goth, Y.; Feidt, M. Recherches des conditions optimales de fonctionnement des pompes à chaleur ou machines à froid associées à un cycle de Carnot endoreversible. C.R. Acad. Sc.: Paris, France, 1986; 303 Série II–1; pp. 113–122. [Google Scholar]
- Feidt, M. Optimization of thermal systems and processes, applications to heat pump. In ICIAM'87, Paris, France; 1987. [Google Scholar]
- Feidt, M.; Petrescu, S.; Costéa, M. Du rendement de Carnot à la Thermodynamique en Temps Fini.; CFGP: Nancy, France, 2001; pp. 125–133. [Google Scholar]
- Feidt, M. La Thermodynamique en Temps Fini, Histoire et Avenir. In Conférence invitée séminairede la Chaire de Thermotechnique, Université Polytechnique de Bucarest, Romania; 2003. [Google Scholar]
- Chen, J.; Yan, Z.; Lin, G.; Andresen, B. On the Curzon-Ahlborn efficiency and its connexion with efficiencies of real engines. Energ. Conv. Manage. 2001, 42, 173–181. [Google Scholar] [CrossRef]
- Ibrahim, O.M.; Klein, S.A.; Mitchell, J.W. Optimum heat power cycles for specified boundary conditions. J. Eng. Gas Turb. Power 1991, 113, 514. [Google Scholar] [CrossRef]
- Tondeur, D.; Kvaalen, E. Equipartition of entropy production. An optimally criterion for transfer and separation processes. Ind. Chem. Res. 1986, 26, 2827–2833. [Google Scholar]
- Feidt, M.; Lang, S. Conception optimale de systèmes combinés à génération de puissance, chaleur et froid. Entropie 2002, 242, 2–11. [Google Scholar]
- Descieux, D.; Feidt, M. Analysis of the non adiabatic Dual cycle at maximum imposed temperature, application to C.H.P. system. In Proceedings ECOS'07, Padova, Italy; 2007; pp. 885–892. [Google Scholar]
- Feidt, M.; Costea, M.; Postelnicu, V. Comparaison entre le cycle simple de Brayton avec apport thermique imposé et avec contrainte de température maximale. OGST Revue de l'IFP 2006, 61, 237–247. [Google Scholar] [CrossRef]
- Descieux, D. Modélisations et comparaison énergétique de systèmes de cogénération. Ph.D. Thesis, University Henri Poincaré, Nancy, France, 2007. [Google Scholar]
- Descieux, D.; Feidt, M. One zone thermodynamic model simulation of an ignition compression engine. Appl. Therm. Eng. 2007, 27, 1457–1466. [Google Scholar] [CrossRef]
- Feidt, M. Thermodynamics and optimization of reverse cycle machines : refrigeration, heat pump, air conditioning, cryogenics. In thermodynamic optimization of complex energy systems; Kluwer Academic Publishers: Morwell, MA, USA, 1998; pp. 403–410. [Google Scholar]
- Feidt, M. Advanced Thermodynamics of reverse cycle machine in low temperature and cryogenic refrigeration; Kluwer Academic Publishers: Morwell, MA, USA, 2003; pp. 39–82. [Google Scholar]
- Petre, C. L'utilisation de la thermodynamique à vitesse finie pour l'étude et l'optimisation de cycle de CARNOT et des machines de STIRLING. Ph.D. Thesis, Universities of Nancy, Nancy, France, 2007. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Feidt, M. Optimal Thermodynamics—New Upperbounds. Entropy 2009, 11, 529-547. https://doi.org/10.3390/e11040529
Feidt M. Optimal Thermodynamics—New Upperbounds. Entropy. 2009; 11(4):529-547. https://doi.org/10.3390/e11040529
Chicago/Turabian StyleFeidt, Michel. 2009. "Optimal Thermodynamics—New Upperbounds" Entropy 11, no. 4: 529-547. https://doi.org/10.3390/e11040529
APA StyleFeidt, M. (2009). Optimal Thermodynamics—New Upperbounds. Entropy, 11(4), 529-547. https://doi.org/10.3390/e11040529




