Electrical Explosion Synthesis, Oxidation and Sintering Behavior of Ti-Al Intermetallide Powders
Abstract
1. Introduction
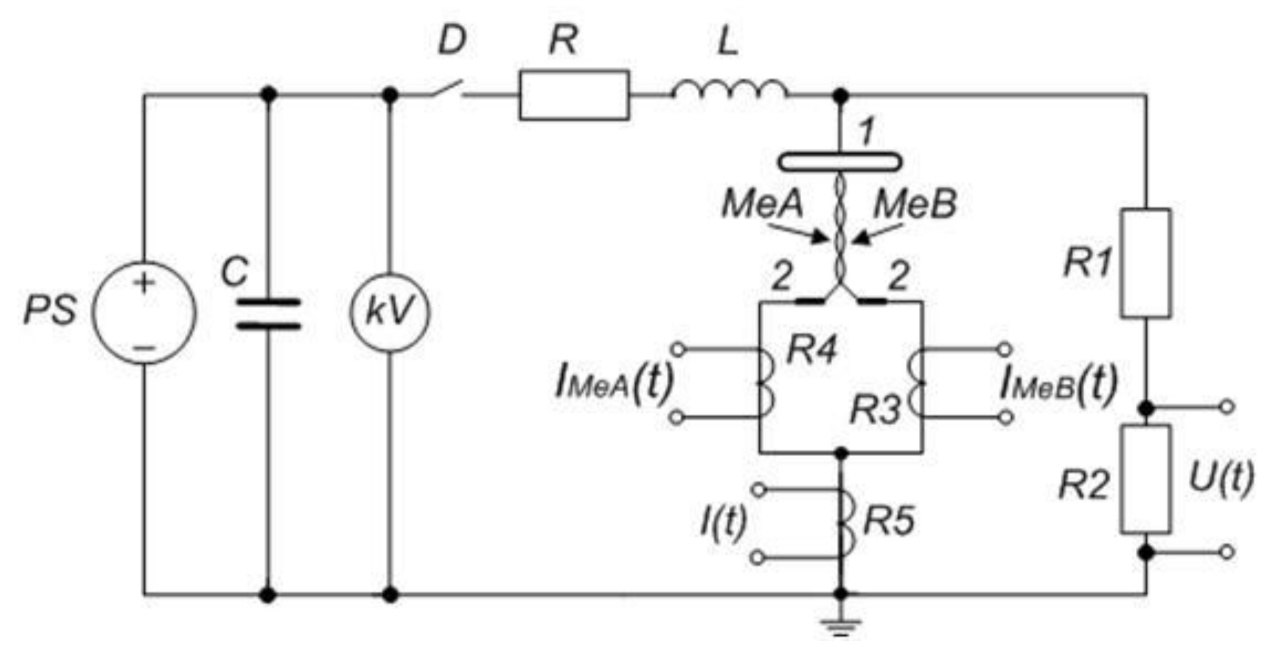
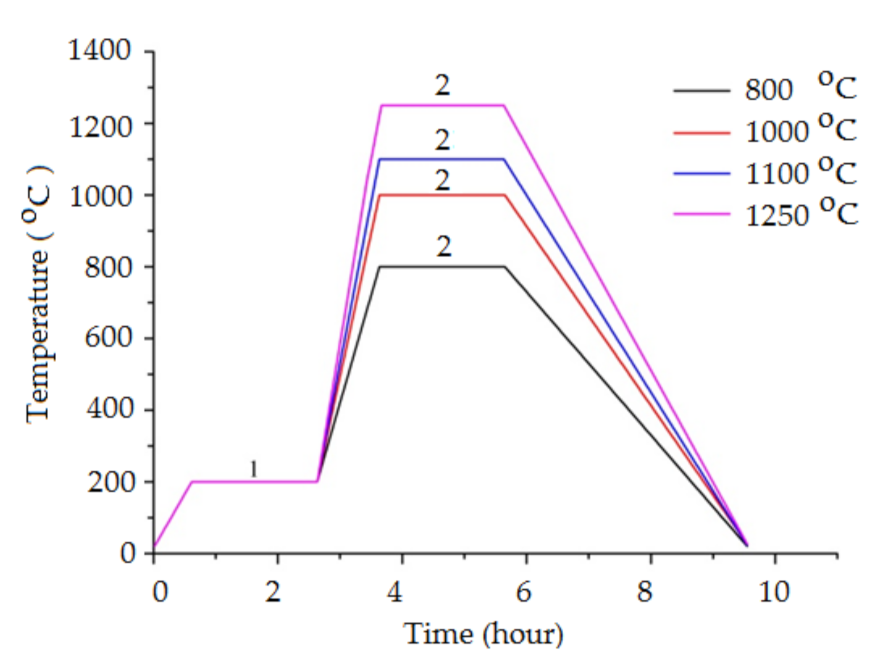
2. Materials and Methods
3. Results and Discussion
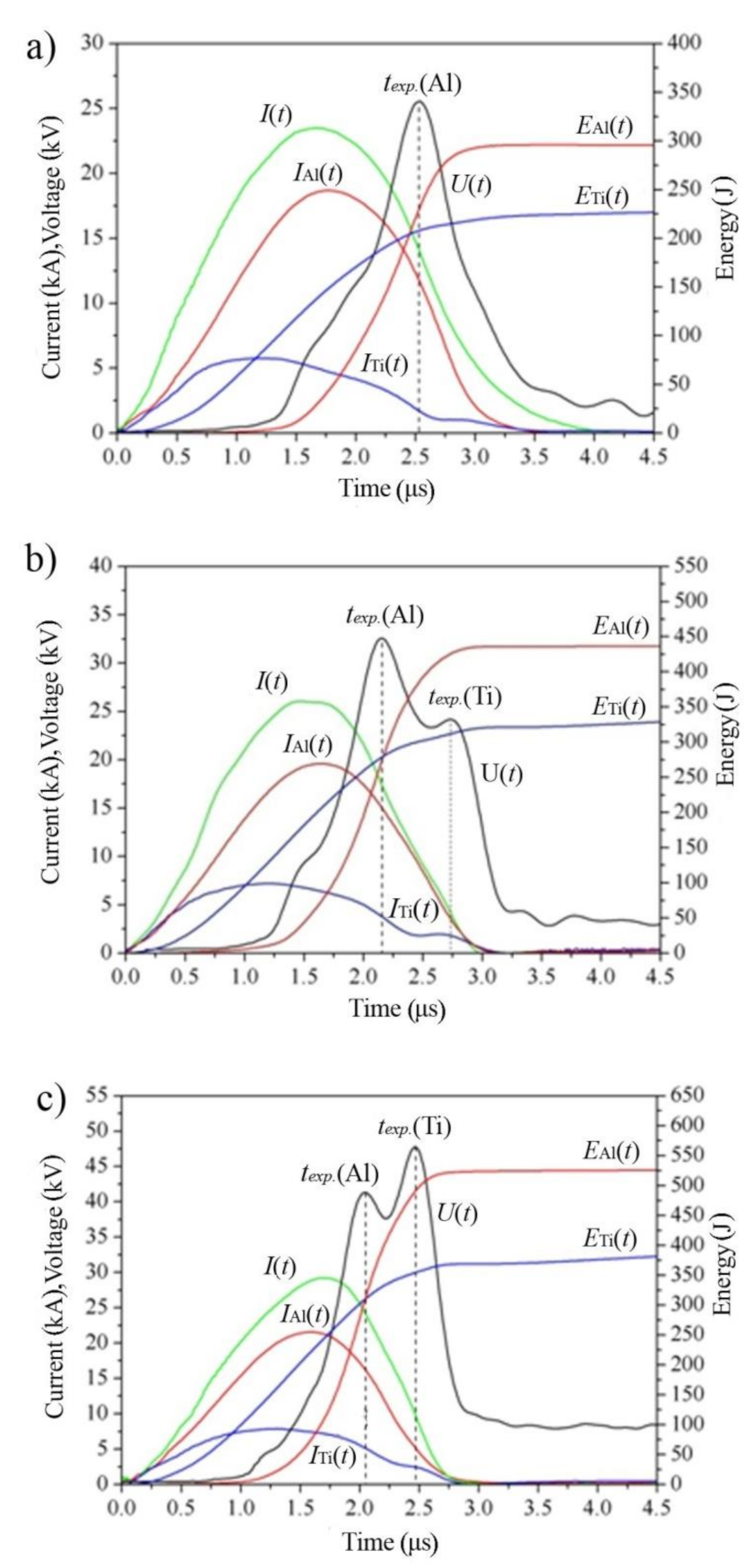
3.1. Electrical Explosion Phenomenon
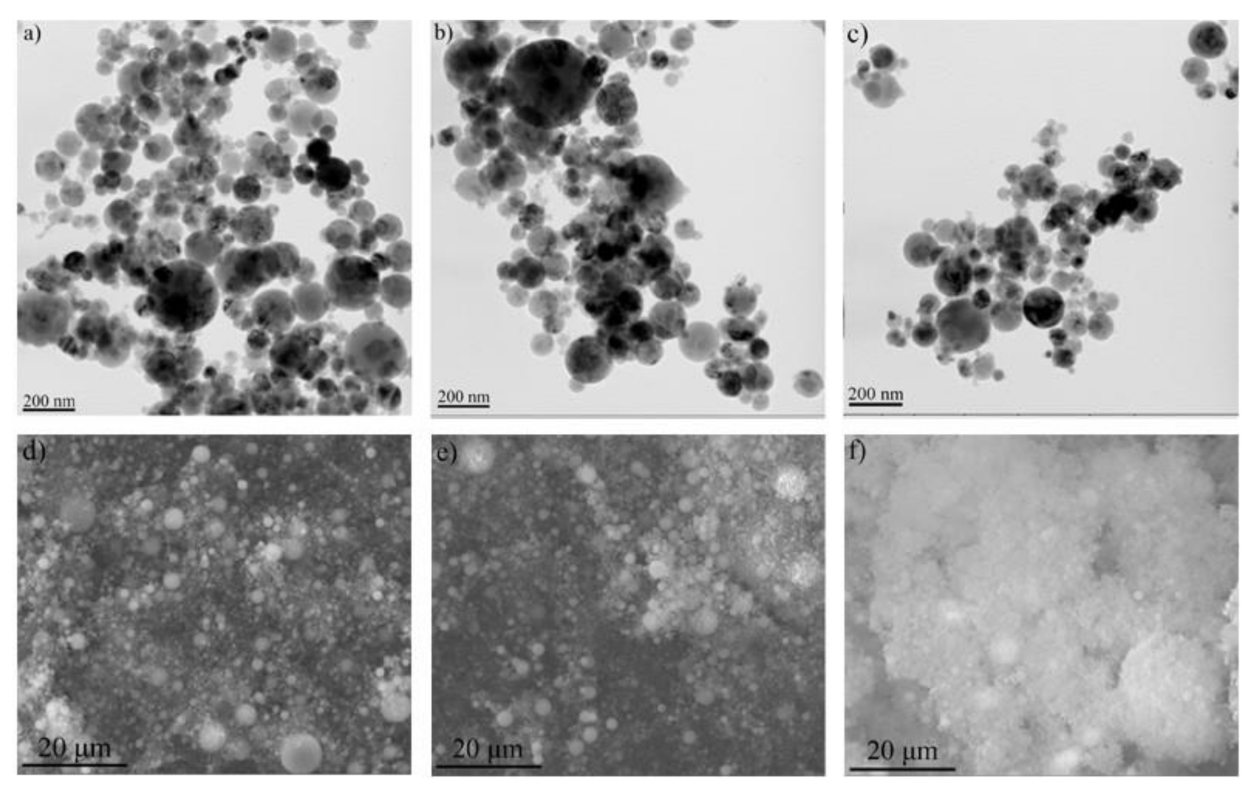
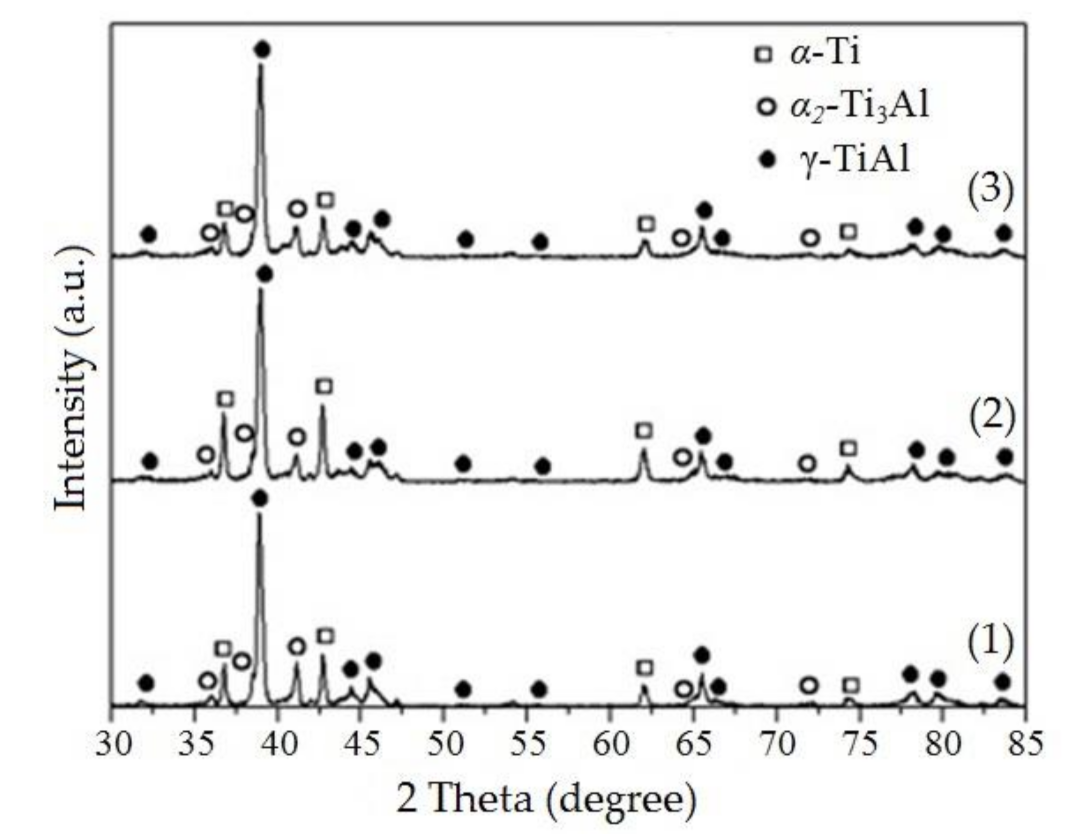
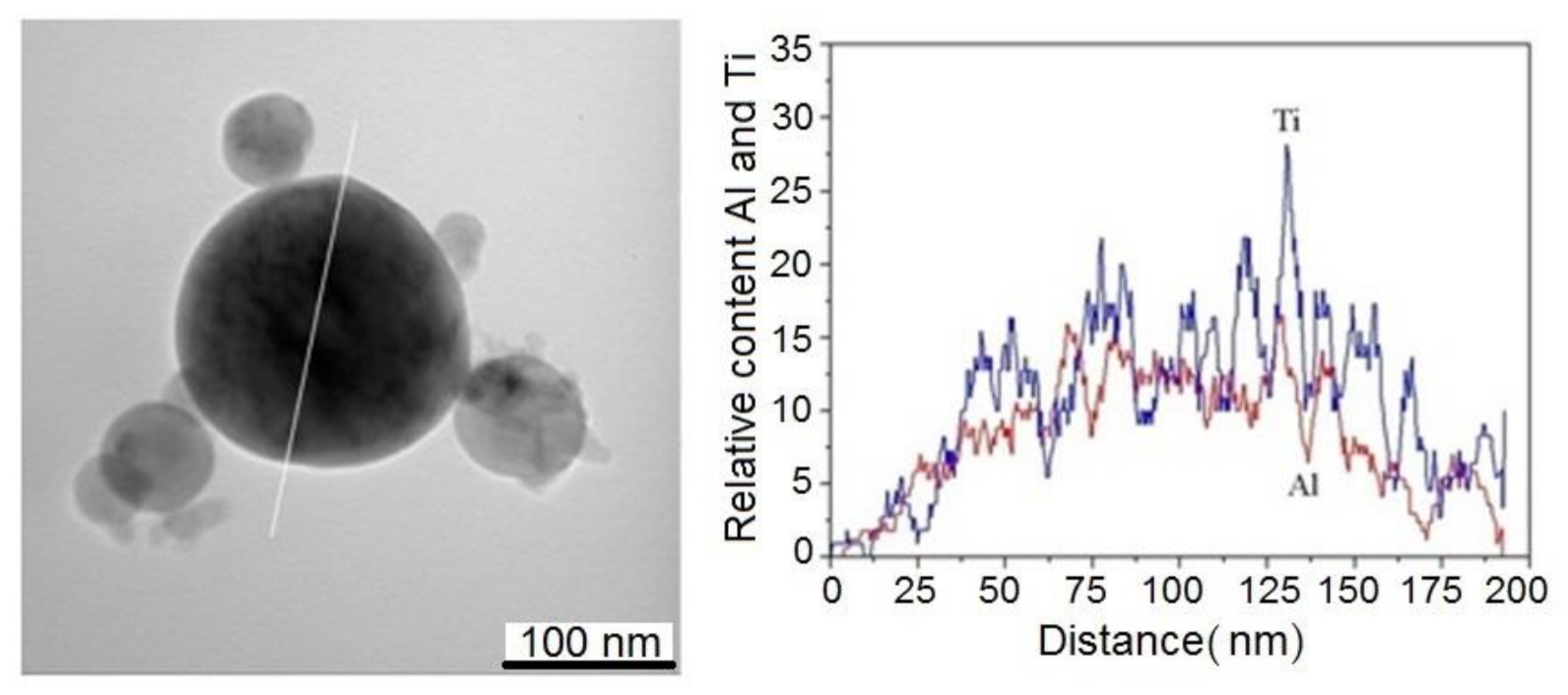
3.2. Ti-Al Particle Characterization
3.3. Ti-Al Particle Formation
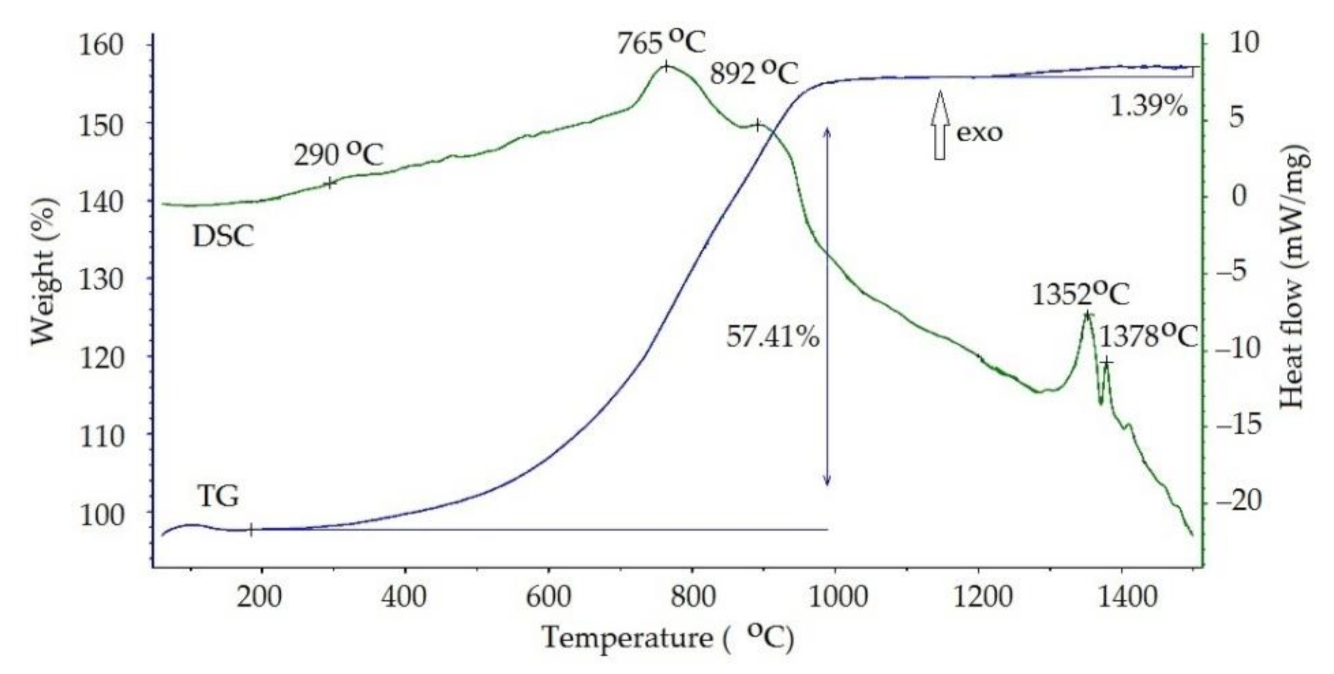
3.4. Ti-Al Particle Oxidation
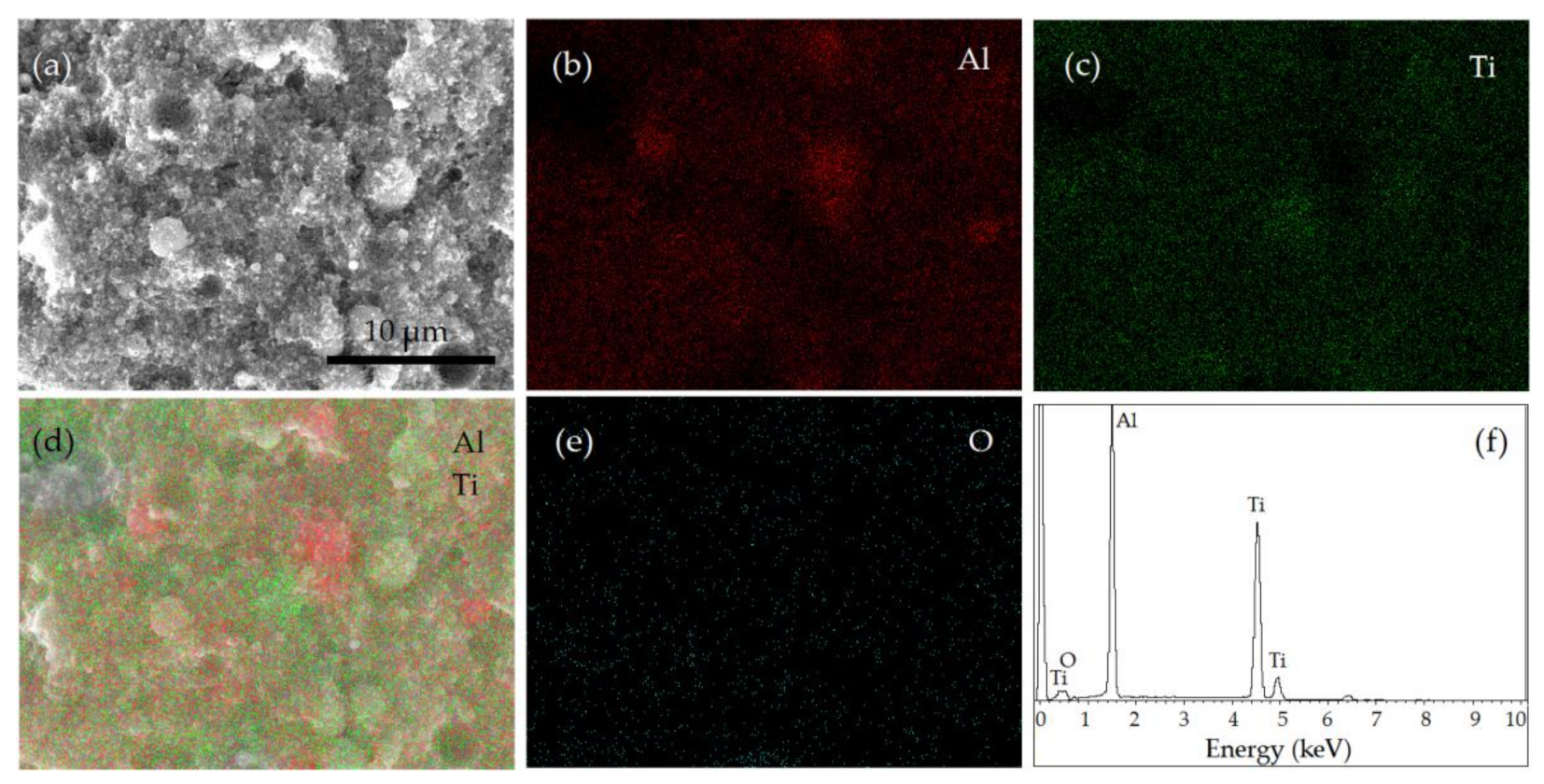
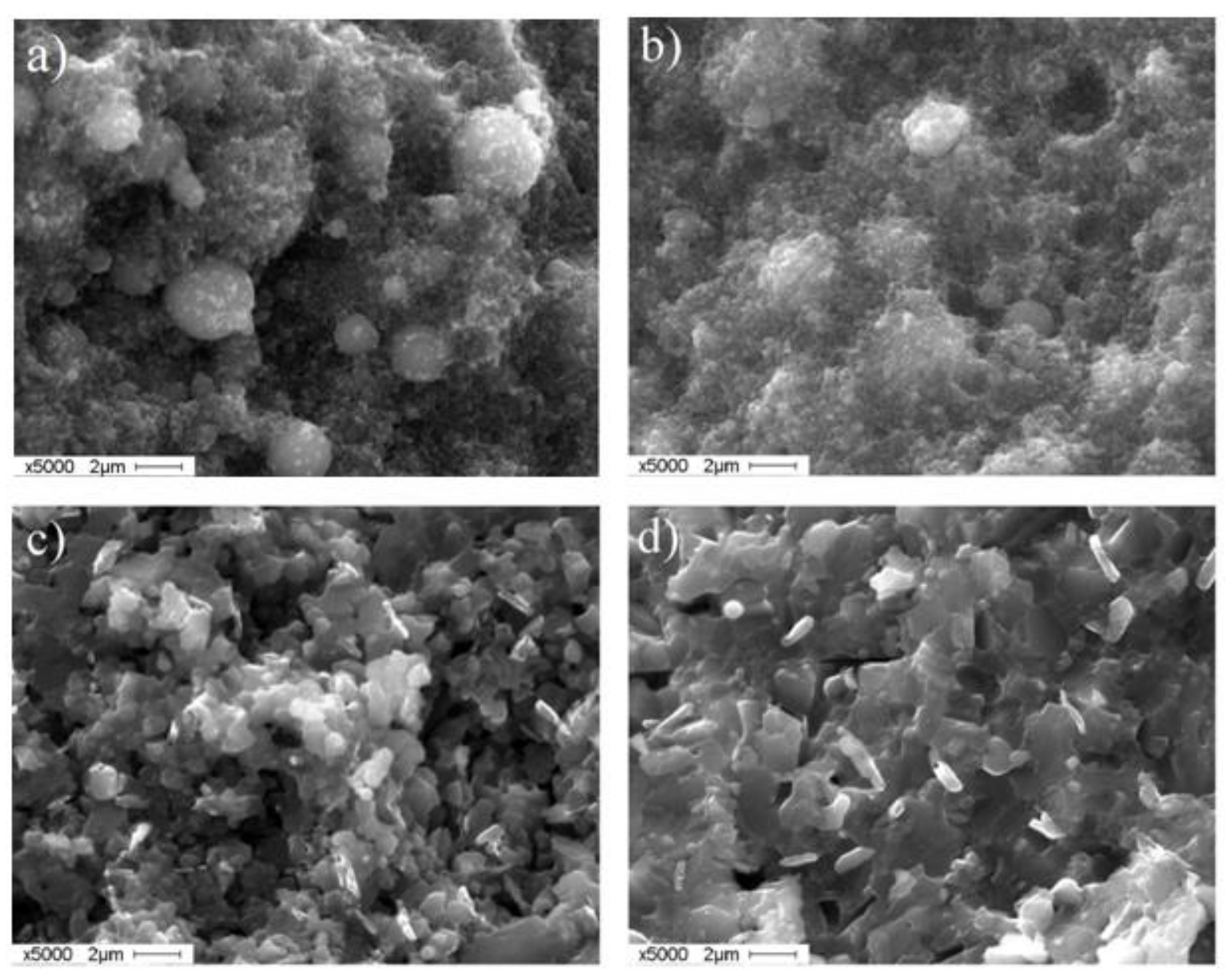
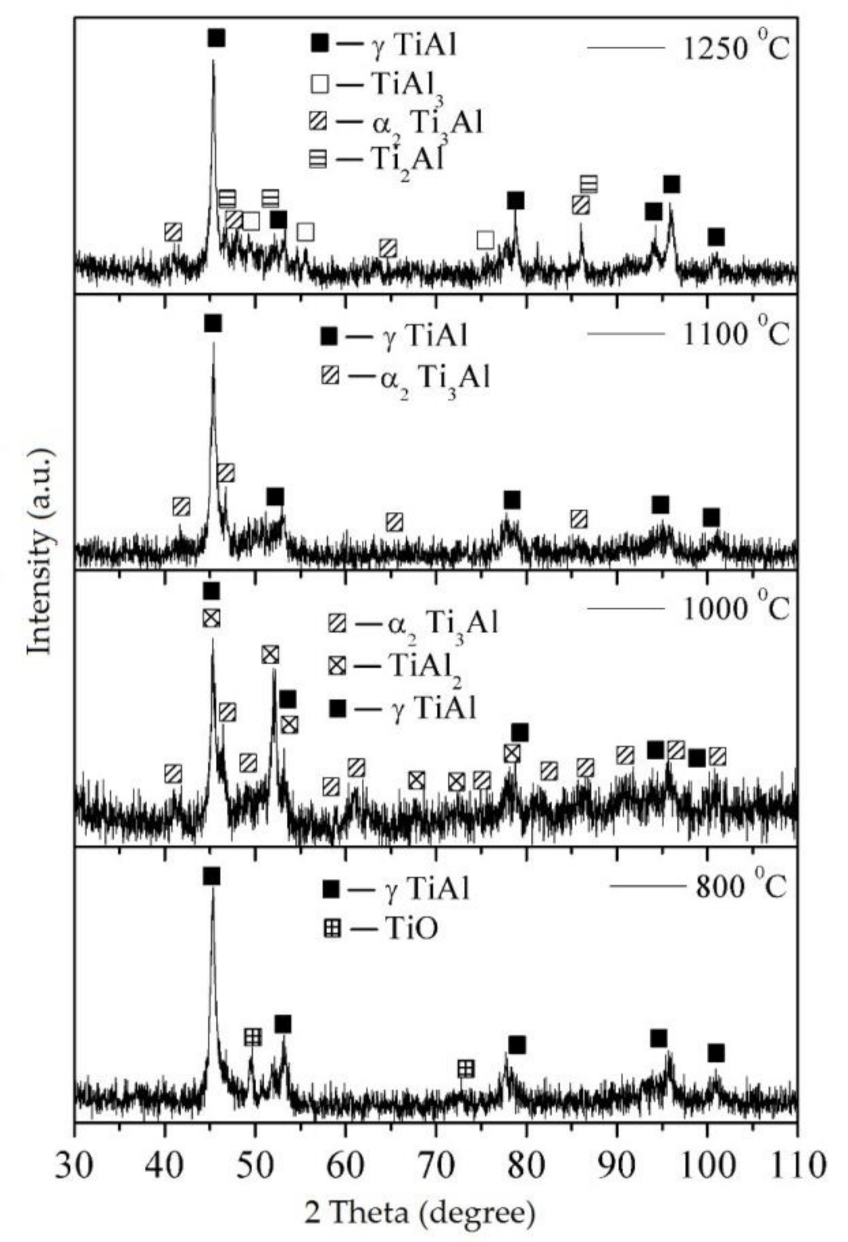
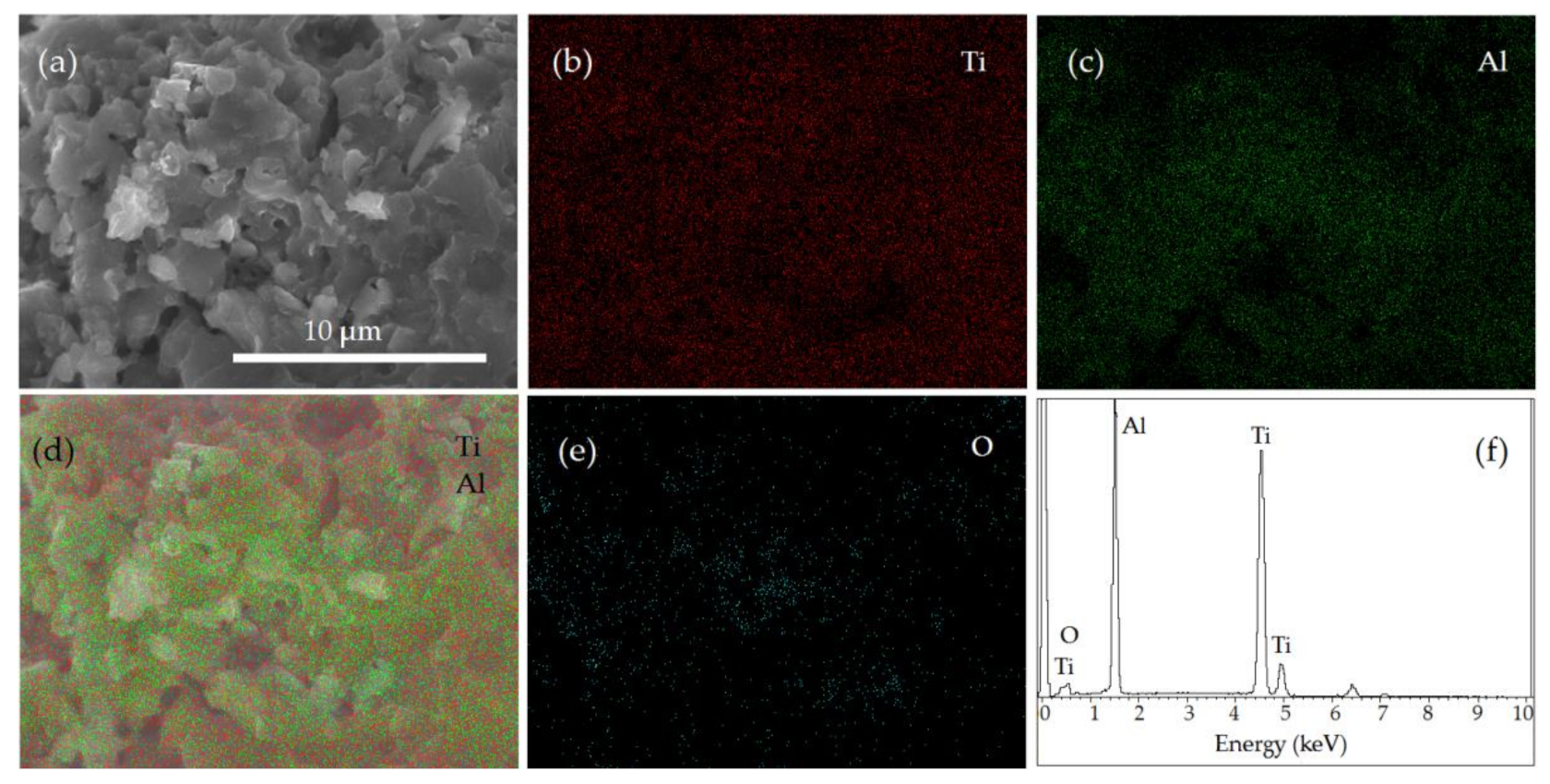
3.5. Characterization of the Sintered Ti-Al Structures
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cinca, N.; Lima, C.R.C.; Guilemany, J.M. An overview of intermetallics research and application: Status of thermal spray coatings. J. Mater. Res. Technol. 2013, 2, 75–86. [Google Scholar] [CrossRef]
- Brotzu, A.; Felli, F.; Marra, F.; Pilone, D.; Pulci, G. Mechanical properties of a TiAl-based alloy at room and high temperatures. Mater. Sci. Technol. 2018, 34, 1847–1853. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Intermetallic titanium aluminides in aerospace applications–processing, microstructure and properties. Mater. High Temp. 2016, 33, 560–570. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl alloys in commercial aircraft engines. Mater. High Temp. 2016, 33, 549–559. [Google Scholar] [CrossRef]
- Wang, G.-X.; Dahms, M. Synthesizing gamma-TiAl alloys by reactive powder processing. JOM 1993, 45, 52–56. [Google Scholar] [CrossRef]
- Lee-Sullivan, P. HIP processing of Ti-Al intermetallic using blended elemental powder. J. Mater. Process. Technol. 1993, 38, 1–13. [Google Scholar] [CrossRef]
- Wang, G.-X.; Dahms, M. An effective method for reducing porosity in the titanium aluminide alloy Ti52Al48 prepared by elemental powder metallurgy. Scr. Metall. Mater. 1992, 26, 1469–1474. [Google Scholar] [CrossRef]
- Dahms, M.; Schmelzer, F.; Seeger, J.; Wildhagen, B. Microstructure and Mechanical Properties of γ Base Titanium Aluminide Produced from Extruded Elemental Powders. Mater. Sci. Technol. 1992, 8, 359–362. [Google Scholar] [CrossRef]
- Kennedy, S.; Rao, S.K.T.S. Densification of γ-TiAl Powders by Spark Plasma Sintering. Mater. Sci. Forum 2012, 710, 303–307. [Google Scholar] [CrossRef]
- Mogale, N.F.; Matizamhuka, W.R. Spark Plasma Sintering of Titanium Aluminides: A Progress Review on Processing, Structure-Property Relations, Alloy Development and Challenges. Metals 2020, 10, 1080. [Google Scholar] [CrossRef]
- Xiao, S.; Tian, J.; Xu, L.; Chen, Y.; Yu, H.; Han, J. Microstructures and mechanical properties of TiAl alloy prepared by spark plasma sintering. Trans. Nonferrous Met. Soc. China 2009, 19, 1423–1427. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W. Mechanical alloying and spark plasma sintering of the intermetallic compound Ti50Al50. J. Alloys Compd. 2007, 440, 154–157. [Google Scholar] [CrossRef]
- Gerling, R.; Clemens, H.; Schimansky, F.P. Powder metallurgical processing of intermetallic gamma titanium aluminides. Adv. Eng. Mater. 2004, 6, 23–38. [Google Scholar] [CrossRef]
- Li, X.B.; Zhang, J.B.; Ji, X.C.; Wu, X.Q.; Luo, J.S.; Tang, Y.J. A Small Patch Production of γ-TiAl Alloys Nanoparticles via Physical Vapor-phase Method. Integr. Ferroelectr. 2013, 147, 146–153. [Google Scholar] [CrossRef]
- Froes, F.H.; Senkov, O.N.; Baburaj, E.G. Synthesis of nanocrystalline materials. Mater. Sci. Eng. A 2001, 301, 44–53. [Google Scholar] [CrossRef]
- Wu, X. Review of alloy and process development of TiAl alloys. Intermetallics 2006, 14, 1114–1122. [Google Scholar] [CrossRef]
- Calderon, H.A.; Garibay-Febles, V.; Umemoto, M.; Yamaguchi, M. Mechanical properties of nanocrystalline Ti–Al–X alloys. Mater. Sci. Eng. A 2002, 329–331, 196–205. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, T.; Zhu, M.; Qin, C. Synthesis and structures of Al–Ti nanoparticles by hydrogen plasma-metal reaction. J. Nanopart. Res. 2012, 14, 738. [Google Scholar] [CrossRef]
- Kotov, Y.A. Electric Explosion of Wires as a Method for Preparation of Nanopowders. J. Nanopart. Res. 2003, 5, 539–550. [Google Scholar] [CrossRef]
- Pervikov, A.V.; Kazantsev, S.O.; Lozhkomoev, A.S.; Lerner, M.I. Bimetallic Al/Ag, Al/Cu and Al/Zn nanoparticles with controllable phase compositions prepared by the electrical explosion of two wires. Powder Technol. 2020, 372, 136–147. [Google Scholar] [CrossRef]
- Pervikov, A.V.; Suliz, K.V.; Lerner, M.I. Nanoalloying of clusters of immiscible metals and the formation of bimetallic nanoparticles in the conditions of non-synchronous explosion of two wires. Powder Technol. 2020, 360, 855–862. [Google Scholar] [CrossRef]
- Lerner, M.I.; Pervikov, A.V.; Glazkova, E.A.; Svarovskaya, N.V.; Lozhkomoev, A.S.; Psakhie, S.G. Structures of binary metallic nanoparticles produced by electrical explosion of two wires from immiscible elements. Powder Technol. 2016, 288, 371–378. [Google Scholar] [CrossRef]
- Sarkisov, G.S.; Sasorov, P.V.; Struve, K.W.; McDaniel, D.H. State of the metal core in nanosecond exploding wires and related phenomena. J. Appl. Phys. 2004, 96, 1674–1686. [Google Scholar] [CrossRef]
- Pervikov, A.; Glazkova, E.; Lerner, M. Energy characteristics of the electrical explosion of two intertwined wires made of dissimilar metals. Phys. Plasmas 2018, 25, 070701. [Google Scholar] [CrossRef]
- Kattner, U.R.; Lin, J.C.; Chang, Y.A. Thermodynamic Assessment and Calculation of the Ti-Al system. Metall. Trans. A 1992, 23, 2081–2090. [Google Scholar] [CrossRef]
- Romanova, V.M.; Ivanenkov, G.V.; Mingaleev, A.R.; Ter-Oganesyan, A.E.; Tilikin, I.N.; Shelkovenko, T.A.; Pikuz, S.A. On the phase state of thin silver wire cores during a fast electric explosion. Phys. Plasmas 2018, 25, 112704. [Google Scholar] [CrossRef]
- Pervikov, A.; Lerner, M. Mechanism of the formation of the structure and phase state of binary metallic nanoparticles obtained by the electric explosion of two wires made of different metals. Curr. Appl. Phys. 2017, 17, 1494–1500. [Google Scholar] [CrossRef]
- Ishihara, S.; Koishi, T.; Orikawa, T.; Suematsu, H.; Nakayama, T.; Suzuki, T.; Niihara, K. Synthesis of intermetallic NiAl compound nanoparticles by pulsed wire discharge of twisted Ni and Al wires. Intermetallics 2012, 23, 134–142. [Google Scholar] [CrossRef]
- Abraham, A.; Nie, H.; Schoenitz, M.; Vorozhtsov, A.; Lerner, M.; Pervikov, A.; Rodkevich, N.; Dreizin, E. Bimetal Al–Ni nano-powders for energetic formulations. Combust. Flame 2016, 173, 179–186. [Google Scholar] [CrossRef]
- Bora, B.; Kausik, S.S.; Wong, C.S.; Chin, O.H.; Yap, S.L.; Soto, L. Observation of the partial reheating of the metallic vapor during the wire explosion process for nanoparticle synthesis. Appl. Phys. Lett. 2014, 104, 223108. [Google Scholar] [CrossRef]
- Vorozhtsov, A.B.; Lerner, M.; Rodkevich, N.; Nie, H.; Abraham, A.; Schoenitz, M.; Dreizin, E.L. Oxidation of nano-sized aluminum powders. Thermochim. Acta 2016, 636, 48–56. [Google Scholar] [CrossRef]
- Ouyang, P.; Mi, G.; Li, P.; He, L.; Cao, J.; Huang, X. Non-Isothermal Oxidation Behaviors and Mechanisms of Ti-Al Intermetallic Compounds. Materials 2019, 12, 2114. [Google Scholar] [CrossRef]
- Vorozhtsov, A.B.; Rodkevich, N.G.; Bondarchuk, I.S.; Lerner, M.I.; Zhukov, A.S.; Glazkova, E.A.; Bondarchuk, S.S. Thermokinetic investigation of the aluminum nanoparticles oxidation. Int. J. Energetic Mater. Chem. Propuls. 2017, 16, 309–320. [Google Scholar] [CrossRef]
- Bondarchuk, I.S.; Bondarchuk, S.S.; Borisov, B.V. Identification of kinetic triplets by results of derivatographic analysis. MATEC Web Conf. 2018, 194, 01010. [Google Scholar] [CrossRef]
- Jones, S.A.; Kaufman, M.J. Phase equlibria and transformations in intermediate titanium–aluminum alloys. Acta Metall. Mater. 1993, 41, 387–398. [Google Scholar] [CrossRef]
- Bahador, A.; Umeda, J.; Yamanoglu, R.; Bakar, T.A.A.; Kondoh, K. Strengthening evaluation and high-temperature behavior of Ti–Fe–O–Cu–Si alloy. Mater. Sci. Eng. A 2021, 800, 140324. [Google Scholar] [CrossRef]
- Bahador, A.; Umeda, J.; Ghandvar, H.; Bakar, T.A.A.; Yamanoglu, R.; Issariyapat, A.; Kondoh, K. Microstructure globularization of high oxygen concentration dual-phase extruded Ti alloys via powder metallurgy route. Mater. Charact. 2021, 172, 110855. [Google Scholar] [CrossRef]
- Wan, Q.; Li, F.; Wang, W.; Hou, J.; Cui, W.; Li, Y. Study on In-Situ Synthesis Process of Ti–Al Intermetallic Compound-Reinforced Al Matrix Composites. Materials 2019, 12, 1967. [Google Scholar] [CrossRef]
- Thiyaneshwaran, N.; Sivaprasad, K.; Ravisankar, B. Nucleation and growth of TiAl3 intermetallic phase in diffusion bonded Ti/Al Metal Intermetallic Laminate. Sci. Rep. 2018, 8, 16797. [Google Scholar] [CrossRef]
- Xu, L.; Cui, Y.Y.; Hao, Y.L.; Yang, R. Growth of intermetallic layer in multi-laminated Ti/Al difusion couples. Mater. Sci. Eng. A 2006, 435–436, 638–647. [Google Scholar] [CrossRef]
| Wire | Wire Diameter, mm | Wire Length, mm | Sublimation Enthalpy, kJ/g | Sublimation Heat (), J |
|---|---|---|---|---|
| Ti | 0.32 | 100 | 8.95 | 326 |
| Al | 0.35 | 100 | 11 | 285 |
| Sample | U0, kV | Average Particle Size, nm | Phase Composition, wt.% | ||
|---|---|---|---|---|---|
| α-Ti | α2-Ti3Al | γ-TiAl | |||
| 1 | 22 | 240 ± 21 | 19.4 ± 2.4 | 8.1 ± 1.1 | 72.5 ± 7.1 |
| 2 | 25 | 178 ± 16 | 22.3 ± 2.7 | 9.2 ± 1.3 | 68.3 ± 8.2 |
| 3 | 29 | 133 ± 13 | 14.2 ± 1.4 | 7.8 ± 1.1 | 78.0 ± 8.7 |
| Stage | Mass Gain, % | T0, °C | Range, °C | ΔT, °C |
|---|---|---|---|---|
| I | 19,3 | 614 | 326–903 | 576 |
| II | 26,8 | 780 | 634–925 | 290 |
| III | 12,2 | 903 | 824–983 | 159 |
| IV | 1,5 | 1288 | 1159–1417 | 257 |
| Sintering Temperature, °C | Phase Composition, wt.% | |||||
|---|---|---|---|---|---|---|
| TiO | Ti2Al | α2-Ti3Al | γ-TiAl | TiAl2 | TiAl3 | |
| 800 | 17.3 ± 1.5 | — | — | 82.7 ± 8.1 | — | — |
| 1000 | — | — | 15.5 ± 1.2 | 42.4 ± 4.3 | 42.1 ± 4.3 | — |
| 1100 | — | — | 12.4 ± 1.0 | 87.6 ± 8.6 | — | — |
| 1250 | — | 12.1 ± 1.1 | 25.6 ± 2.3 | 47.0 ± 5.5 | — | 15.3 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lerner, M.; Pervikov, A.; Glazkova, E.; Rodkevich, N.; Toropkov, N. Electrical Explosion Synthesis, Oxidation and Sintering Behavior of Ti-Al Intermetallide Powders. Metals 2021, 11, 760. https://doi.org/10.3390/met11050760
Lerner M, Pervikov A, Glazkova E, Rodkevich N, Toropkov N. Electrical Explosion Synthesis, Oxidation and Sintering Behavior of Ti-Al Intermetallide Powders. Metals. 2021; 11(5):760. https://doi.org/10.3390/met11050760
Chicago/Turabian StyleLerner, Marat, Alexandr Pervikov, Elena Glazkova, Nikolay Rodkevich, and Nikita Toropkov. 2021. "Electrical Explosion Synthesis, Oxidation and Sintering Behavior of Ti-Al Intermetallide Powders" Metals 11, no. 5: 760. https://doi.org/10.3390/met11050760
APA StyleLerner, M., Pervikov, A., Glazkova, E., Rodkevich, N., & Toropkov, N. (2021). Electrical Explosion Synthesis, Oxidation and Sintering Behavior of Ti-Al Intermetallide Powders. Metals, 11(5), 760. https://doi.org/10.3390/met11050760






