Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells
Abstract
:1. Introduction
2. Criteria for the Selection of Experimental Papers
3. Characteristics and Function of Phenolic Compounds—Crucial Plant Secondary Metabolites with Antioxidative Properties
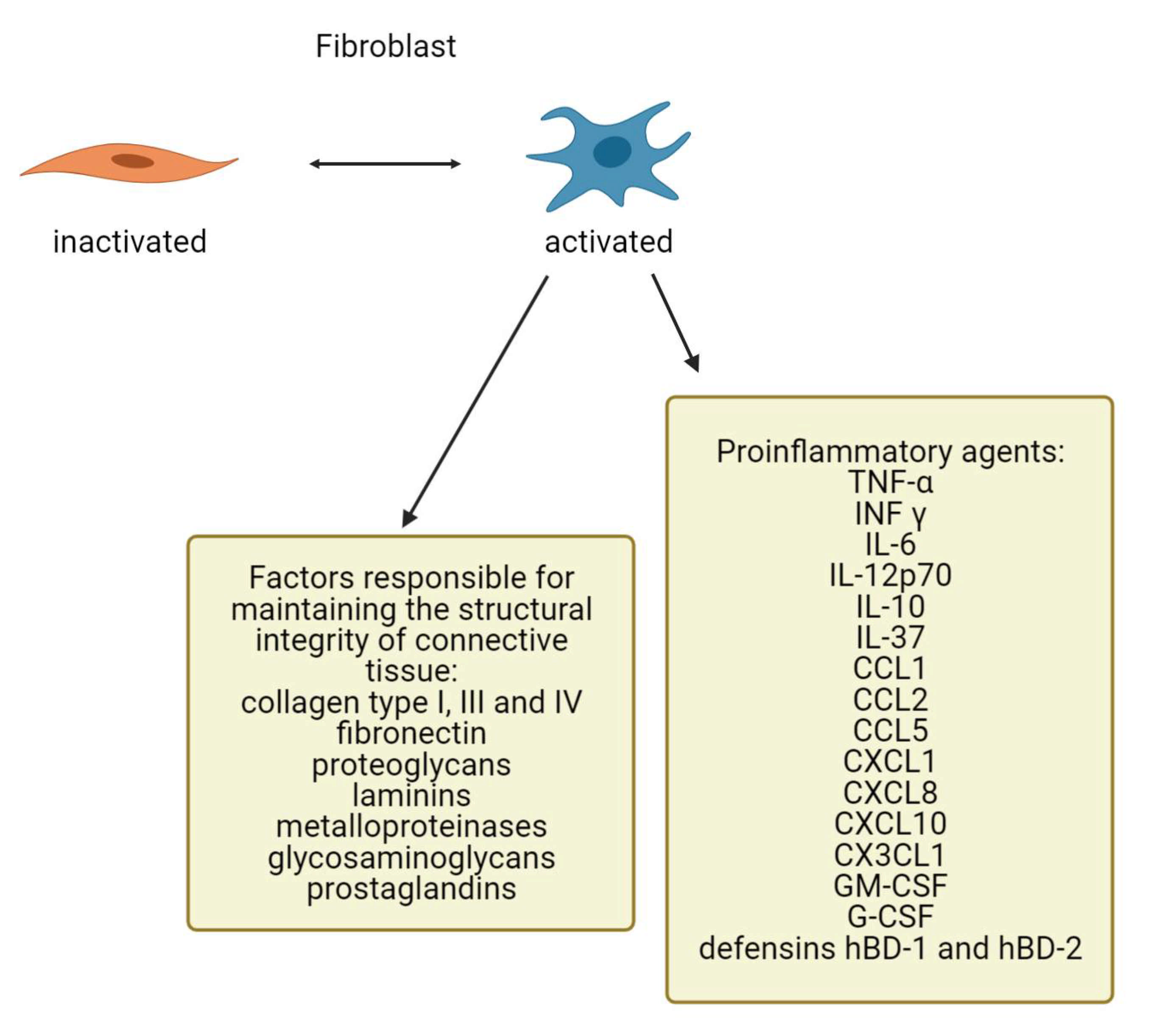
4. Fibroblasts as Important Dermis Resident Cells and Their Characterization
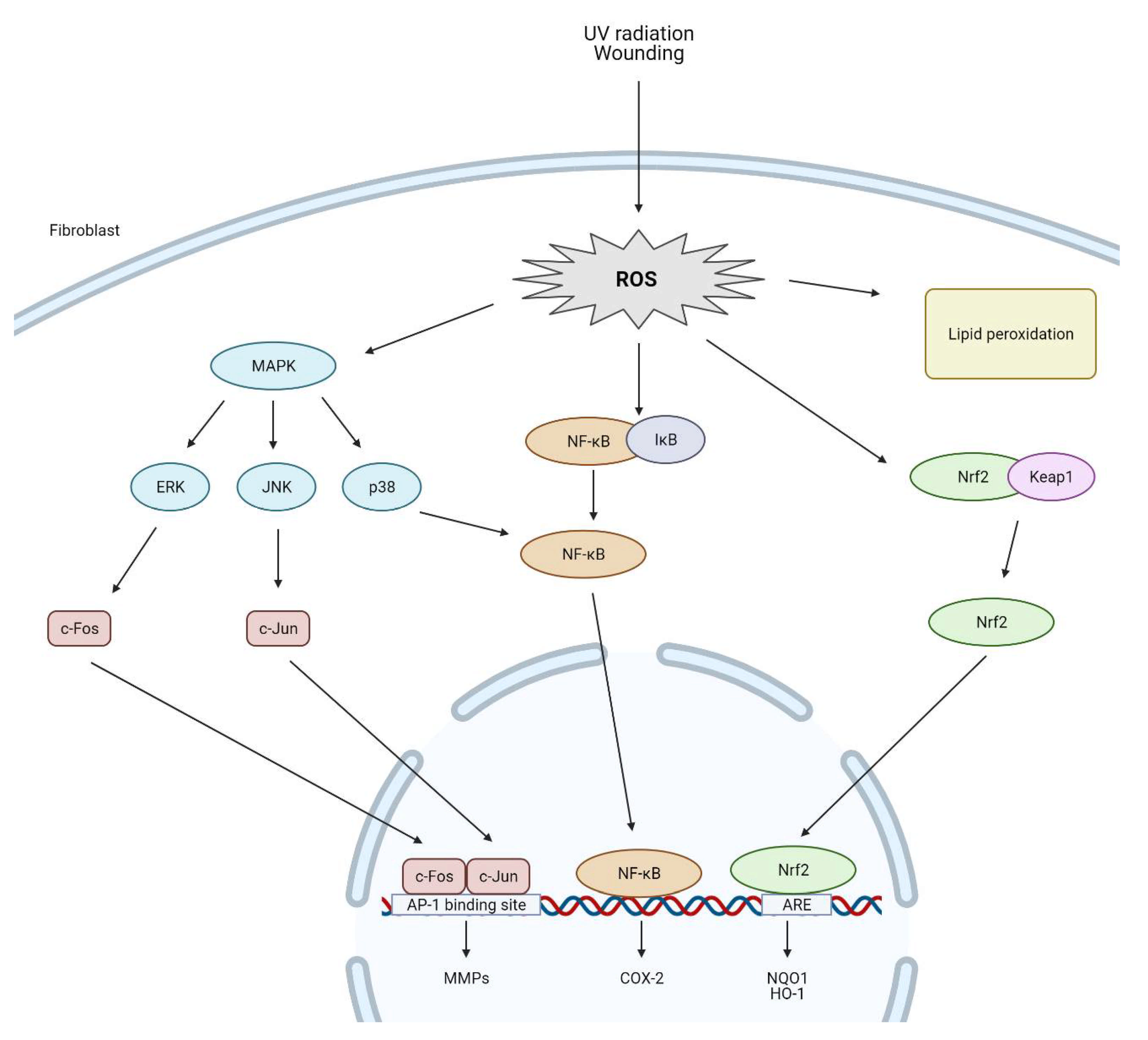
5. The Impact of Reactive Oxygen Species on Dermal Fibroblasts
6. Modulation of ROS Levels in Fibroblasts by Phenolic Compounds and Their Role in Regulation of the Wound Healing Process
7. Modulation of ROS Levels in Fibroblasts by Phenolic Compounds and Their Role in the Aging Process
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACTA2 | actin alpha 2 |
| AGEs | advanced glycation end products |
| Bfgf | basic fibroblast growth factor |
| CAT | catalase |
| CCL | C-C motif chemokine ligand |
| CX3CL | C-X3-C motif chemokine ligand |
| CXCL | C-X-C motif chemokine ligand |
| CXCR | C-X-C motif chemokine receptor |
| DLK1 | delta-like noncanonical Notch ligand 1 |
| DPP4 | dipeptidyl peptidase-4 |
| ECM | extracellular matrix |
| EMT | epithelial-mesenchymal transition |
| G-CSF | granulocyte colony-stimulating factor |
| GM-CSF | granulocyte/macrophage colony-stimulating factor |
| GPx | glutathione peroxidase |
| HDFs | human dermal fibroblasts |
| HO-1 | heme oxygenase-1 |
| IDH2 | isocitrate dehydrogenase (NADP(+)) |
| IGF1 | insulin like growth factor 1 |
| IKK | inhibitory kappa kinase |
| IL | interleukin |
| INF γ | interferon |
| ITGA1 | integrin subunit alpha 1 |
| Keap1 | Kelch-like ECH associated protein 1 |
| LPO | lipid peroxidation |
| MAF | musculoaponeurotic fibrosarcoma |
| MAPKs | mitogen-activated protein kinases |
| MET | mesenchymal-epithelial transition |
| MMPs | matrix metalloproteinases |
| NF-kB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NLR family pyrin domain containing 3 |
| NQO1 | NAD(P)H:quinone oxidoreductase 1 |
| Nrf2 | nuclear factors (erythroid-derived 2), factor 2 |
| PDGFR-α | platelet derived growth factor receptor alpha |
| PI3K | phosphatidylinositol-3-OH kinase |
| PTEN | reduced phosphatase and tensin homologs |
| SCA1 | spinocerebellar ataxia type 1 |
| SOD | superoxide dismutase |
| TGF-β | transforming growth factor |
| TIMPs | tissue inhibitors of metalloproteinase |
| TLR | toll-like receptors |
| TNF-α | tumor necrosis factor |
References
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabassum, N.; Hamdani, M. Plants used to treat skin diseases. Pharmacogn. Rev. 2014, 8, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sousa Araújo, T.A.; de Melo, J.G.; Ferreira Júnior, W.S.; Albuquerque, U.P. Medicinal plants. In Introduction to Ethnobiology; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar]
- Benzie, I.F.F.; Wachtel-Galor, S. Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Costa, A.; Bonner, M.Y.; Arbiser, J.L. Use of polyphenolic compounds in dermatologic oncology. Am. J. Clin. Dermatol. 2016, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Wang, J.H.C. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular mechanisms of dermal aging and antiaging approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- De Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet light induced generation of reactive oxygen species. In Advances in Experimental Medicine and Biology; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Okada, T.; Konishi, H.; Tsuji, T. The effect of reactive oxygen species on the biosynthesis of collagen and glycosaminoglycans in cultured human dermal fibroblasts. Arch. Dermatol. Res. 1993, 285, 352–355. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef] [Green Version]
- Limtrakul, P.; Yodkeeree, S.; Punfa, W.; Srisomboon, J. Inhibition of the MAPK signaling pathway by red rice extract in UVB-irradiated human skin fibroblasts. Nat. Prod. Commun. 2016, 11, 1877–1882. [Google Scholar] [CrossRef] [Green Version]
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y.K. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Gull, A.; Ahmad Lone, A.; Ul Islam Wani, N. Biotic and abiotic stresses in plants. In Abiotic and Biotic Stress in Plants; IntechOpen Limited: London, UK, 2019. [Google Scholar]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [Green Version]
- Merecz-Sadowska, A.; Sitarek, P.; Śliwiński, T.; Zajdel, R. Anti-inflammatory activity of extracts and pure compounds derived from plants via modulation of signaling pathways, especially PI3K/AKT in macrophages. Int. J. Mol. Sci. 2020, 21, 9605. [Google Scholar] [CrossRef]
- Sitarek, P.; Merecz-Sadowska, A.; Śliwiński, T.; Zajdel, R.; Kowalczyk, T. An in vitro evaluation of the molecular mechanisms of action of medical plants from the Lamiaceae family as effective sources of active compounds against human cancer cell lines. Cancers 2020, 12, 2957. [Google Scholar] [CrossRef]
- Sitarek, P.; Merecz-Sadowska, A.; Kowalczyk, T.; Wieczfinska, J.; Zajdel, R.; Śliwiński, T. Potential synergistic action of bioactive compounds from plant extracts against skin infecting microorganisms. Int. J. Mol. Sci. 2020, 21, 5105. [Google Scholar] [CrossRef]
- Sitarek, P.; Kowalczyk, T.; Wieczfinska, J.; Merecz-Sadowska, A.; Górski, K.; Śliwiński, T.; Skała, E. Plant extracts as a natural source of bioactive compounds and potential remedy for the treatment of certain skin diseases. Curr. Pharm. Des. 2020, 26, 2859–2875. [Google Scholar] [CrossRef]
- Zielinska-Blizniewska, H.; Sitarek, P.; Merecz-Sadowska, A.; Malinowska, K.; Zajdel, K.; Jablonska, M.; Sliwinski, T.; Zajdel, R. Plant extracts and reactive oxygen species as two counteracting agents with anti- and pro-obesity properties. Int. J. Mol. Sci. 2019, 20, 4556. [Google Scholar] [CrossRef] [Green Version]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, A.R.; El-Anssary, A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In Herbal Medicine; IntechOpen Limited: London, UK, 2019. [Google Scholar]
- Lattanzio, V. Phenolic compounds: Introduction. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer International Publishing: New York, NY, USA, 2013. [Google Scholar]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef] [Green Version]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Bian, Y.; Wei, J.; Zhao, C.; Li, G. Natural polyphenols targeting senescence: A novel prevention and therapy strategy for cancer. Int. J. Mol. Sci. 2020, 21, 684. [Google Scholar] [CrossRef] [Green Version]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosm. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Boo, Y.C. Can plant phenolic compounds protect the skin from airborne particulate matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef] [Green Version]
- Dick, M.K.; Miao, J.H.; Limaiem, F. Histology, Fibroblast; Stat Pearls: Treasure Island, FL, USA, 2020; Available online: https://www.ncbi.nlm.nih.gov/books/NBK541065 (accessed on 15 March 2021).
- Lynch, M.D.; Watt, F.M. Fibroblast heterogeneity: Implications for human disease. J. Clin. Investig. 2018, 128, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Ravikanth, M.; Soujanya, P.; Manjunath, K.; Saraswathi, T.R.; Ramachandran, C.R. Heterogenecity of fibroblasts. J. Oral Maxillofac. Pathol. 2011, 5, 247–250. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Li, H.; Guo, Z. Mesenchymal stem cell-like properties in fibroblasts. Cell. Physiol. Biochem. 2014, 34, 703–714. [Google Scholar] [CrossRef]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Korosec, A.; Frech, S.; Lichtenberger, B.M. Isolation of papillary and reticular fibroblasts from human skin by fluorescence-activated cell sorting. J. Vis. Exp. 2019, 147, e59372. [Google Scholar] [CrossRef] [Green Version]
- Thulabandu, V.; Chen, D.; Atit, R.P. Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Weinstein, G.D.; Boucek, R.J. Collagen and elastin of human dermis. J. Investig. Dermatol. 1960, 35, 227–229. [Google Scholar] [CrossRef] [Green Version]
- Bahar, M.A.; Bauer, B.; Tredget, E.E.; Ghahary, A. Dermal fibroblasts from different layers of human skin are heterogeneous in expression of collagenase and types I and III procollagen mRNA. Wound Repair Regen. 2004, 12, 175–182. [Google Scholar] [CrossRef]
- Stunova, A.; Vistejnova, L. Dermal fibroblasts—A heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor Rev. 2018, 39, 137–150. [Google Scholar] [CrossRef]
- Whitney, J.D. Overview: Acute and chronic wounds. Nurs. Clin. North Am. 2005, 40, 191–205. [Google Scholar] [CrossRef]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular matrix regulation of fibroblast function: Redefining our perspective on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birch, H.L. Extracellular matrix and ageing. Subcell. Biochem. 2018, 90, 169–190. [Google Scholar] [CrossRef]
- Bautista-Hernández, L.A.; Gómez-Olivares, J.L.; Buentello-Volante, B.; Bautista-de Lucio, V.M. Fibroblasts: The unknown sentinels eliciting immune responses against microorganisms. Eur. J. Microbiol. Immunol. 2017, 7, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Miteva, K.; Tschöpe, C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 2014, 102, 258–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avniel, S.; Arik, Z.; Maly, A.; Sagie, A.; Basst, H.B.; Yahana, M.D.; Weiss, I.D.; Pal, B.; Wald, O.; Ad-El, D.; et al. Involvement of the CXCL12/CXCR4 pathway in the recovery of skin following burns. J. Investig. Dermatol. 2006, 126, 468–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eklund, K.K.; Niemi, K.; Kovanen, P.T. Immune functions of serum amyloid A. Crit. Rev. Immunol. 2012, 32, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xue, W.; Wang, Y. Inhibition of miR-31a-5p decreases inflammation by down-regulating IL-25 expression in human dermal fibroblast cells (CC-2511 cells) under hyperthermic stress via Wnt/β-catenin pathway. Biomed. Pharmacother. 2018, 107, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zheng, Y.-W.; Liu, Q.; Liu, L.-P.; Luo, F.-L.; Zhou, H.-C.; Isoda, H.; Ohkohchi, N.; Li, Y.-M. Reactive Oxygen Species in Skin Repair, Regeneration, Aging, and Inflammation. In Reactive Oxygen Species (ROS) in Living Cells; IntechOpen Limited: London, UK, 2018. [Google Scholar]
- Ma, W.; Wlaschek, M.; Tantcheva-Poór, I.; Schneider, L.A.; Naderi, L.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Chronological ageing and photoageing of the fibroblasts and the dermal connective tissue. Clin. Exp. Dermatol. 2001, 26, 592–599. [Google Scholar] [CrossRef]
- Zaw, K.K.; Yokoyama, Y.; Abe, M.; Ishikawa, O. Catalase restores the altered mRNA expression of collagen and matrix metalloproteinases by dermal fibroblasts exposed to reactive oxygen species. Eur. J. Dermatol. 2006, 16, 375–379. [Google Scholar]
- Kawaguchi, Y.; Tanaka, H.; Okada, T.; Konishi, H.; Takashi, M.; Ito, M.; Asai, J. Effect of reactive oxygen species on the elastin mRNA expression in cultured human dermal fibroblasts. Free Radic. Biol. Med. 1997, 23, 162–165. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Tanaka, H.; Okada, T.; Konishi, H.; Takahashi, M.; Ito, M.; Asai, J. The effects of ultraviolet A and reactive oxygen species on the mRNA expression of 72-kDa type IV collagenase and its tissue inhibitor in cultured human dermal fibroblasts. Arch. Dermatol. Res. 1996, 288, 39–44. [Google Scholar] [CrossRef]
- Chen, Q.M.; Liu, J.; Merrett, J.B. Apoptosis or senescence-like growth arrest: Influence of cell-cycle position, p53, p21 and bax in H2O2 response of normal human fibroblasts. Biochem. J. 2000, 347, 543–551. [Google Scholar] [CrossRef]
- Lämmermann, I.; Terlecki-Zaniewicz, L.; Weinmüllner, R.; Schosserer, M.; Dellago, H.; de Matos Branco, A.D.; Autheried, D.; Sevcnikar, B.; Kleissl, L.; Berlin, I.; et al. Blocking negative effects of senescence in human skin fibroblasts with a plant extract. NPJ Aging Mech. Dis. 2018, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Harding, K.G.; Moore, K.; Phillips, T.J. Wound chronicity and fibroblast senescence—Implications for treatment. Int. Wound J. 2005, 2, 364–368. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Senescence in wound repair: Emerging strategies to target chronic healing wounds. Front. Cell Dev. Biol. 2020, 8, 773. [Google Scholar] [CrossRef]
- Gurjala, A.N.; Liu, W.R.; Mogford, J.E.; Procaccini, P.S.A.; Mustoe, T.A. Age-dependent response of primary human dermal fibroblasts to oxidative stress: Cell survival, pro-survival kinases, and entrance into cellular senescence. Wound Repair Regen. 2005, 13, 565–575. [Google Scholar] [CrossRef]
- Clark, R.A.F. Oxidative stress and “senescent” fibroblasts in non-healing wounds as potential therapeutic targets. J. Investig. Dermatol. 2008, 128, 2361–2364. [Google Scholar] [CrossRef] [Green Version]
- Hiebert, P.; Werner, S. Regulation of wound healing by the nrf2 transcription factor—More than cytoprotection. Int. J. Mol. Sci. 2019, 20, 3856. [Google Scholar] [CrossRef] [Green Version]
- Hiebert, P.; Wietecha, M.S.; Cangkrama, M.; Haertel, E.; Mavrogonatou, E.; Stumpe, M.; Steenbock, H.; Grossi, S.; Beer, H.D.; Angel, P.; et al. Nrf2-mediated fibroblast reprogramming drives cellular senescence by targeting the matrisome. Dev. Cell 2018, 46, 145–161. [Google Scholar] [CrossRef] [Green Version]
- McDonald, J.T.; Kim, K.; Norris, A.J.; Vlashi, E.; Phillips, T.M.; Lagadec, C.; Della Donna, L.; Ratikan, J.; Szelag, H.; Hlatky, L.; et al. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010, 70, 8886–8895. [Google Scholar] [CrossRef] [Green Version]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Whitmarsh, A.J.; Davis, R.J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 1996, 74, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Boyd, D.D. Regulation of matrix metalloproteinase expression. J. Cell. Physiol. 2007, 211, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Moon, K.C.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Cercato, L.M.; Araújo, J.M.D.; Oliveira, A.S.; Melo, A.J.O.; Lima, B.S.; dos Santos, E.W.P.; Agenor, A.G.; de Albuquerque-Júnior, R.L.C.; Duarte, M.C.; Araujo, A.A.S.; et al. Reduced cutaneous inflammation associated with antioxidant action after topical application of the aqueous extract of Annona muricata leaves. Inflammopharmacology 2021, 29, 307–315. [Google Scholar] [CrossRef]
- Csepregi, R.; Temesfői, V.; Das, S.; Alberti, Á.; Tóth, C.A.; Herczeg, R.; Papp, N.; Kőszegi, T. Cytotoxic, antimicrobial, antioxidant properties and effects on cell migration of phenolic compounds of selected Transylvanian medicinal plants. Antioxidants 2020, 9, 166. [Google Scholar] [CrossRef] [Green Version]
- Kanlayavattanakul, M.; Lourith, N.; Chaikul, P. Jasmine rice panicle: A safe and efficient natural ingredient for skin aging treatments. J. Ethnopharmacol. 2016, 193, 607–616. [Google Scholar] [CrossRef]
- Choi, S.-I.; Lee, J.S.; Lee, S.; Cho, B.Y.; Choi, S.H.; Han, X.; Sim, W.S.; Kim, Y.C.; Lee, B.Y.; Kang, I.J.; et al. Protective effects and mechanisms of Pourthiaea villosa (Thunb.) Decne. Extract on hydrogen peroxide-induced skin aging in human dermal fibroblasts. J. Med. Food 2019, 22, 841–850. [Google Scholar] [CrossRef]
- Dudonné, S.; Poupard, P.; Coutiére, P.; Woillez, M.; Richard, T.; Mérillon, J.M.; Vitrac, X. Phenolic composition and antioxidant properties of poplar bud (Populus nigra) extract: Individual antioxidant contribution of phenolics and transcriptional effect on skin aging. J. Agric. Food Chem. 2011, 59, 4527–4536. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin acute wound healing: A comprehensive review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Hinz, B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016, 64, 171–177. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in tissue repair. In Inflammation: From Molecular and Cellular Mechanisms to the Clinic; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2018. [Google Scholar]
- Gonzalez, A.C.D.O.; Andrade, Z.D.A.; Costa, T.F.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Janda, J.; Nfonsam, V.; Calienes, F.; Sligh, J.E.; Jandova, J. Modulation of ROS levels in fibroblasts by altering mitochondria regulates the process of wound healing. Arch. Dermatol. Res. 2016, 308, 239–248. [Google Scholar] [CrossRef]
- Park, G.; Oh, D.S.; Kim, Y.U.; Park, M.K. Acceleration of collagen breakdown by extracellular basic pH in human dermal fibroblasts. Skin Pharmacol. Physiol. 2016, 29, 204–209. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, J.W. IDH2 deficiency impairs cutaneous wound healing via ROS-dependent apoptosis. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165523. [Google Scholar] [CrossRef]
- Fujiwara, T.; Dohi, T.; Maan, Z.N.; Rustad, K.C.; Kwon, S.H.; Padmanabhan, J.; Whittam, A.J.; Suga, H.; Duscher, D.; Rodrigues, M.; et al. Age-associated intracellular superoxide dismutase deficiency potentiates dermal fibroblast dysfunction during wound healing. Exp. Dermatol. 2019, 28, 485–492. [Google Scholar] [CrossRef]
- Kunkemoeller, B.; Kyriakides, T.R. Redox signaling in diabetic wound healing regulates extracellular matrix deposition. Antioxid. Redox Signal. 2017, 27, 823–838. [Google Scholar] [CrossRef]
- Bitar, M. The GSK-3β/Fyn/Nrf2 pathway in fibroblasts and wounds of type 2 diabetes: On the road to an evidence-based therapy of non-healing wounds. Adipocyte 2012, 1, 161–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitar, M.S.; Al-Mulla, F. A defect in Nrf2 signaling constitutes a mechanism for cellular stress hypersensitivity in a genetic rat model of type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1119–E1129. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Xie, T.; Ge, K.; Lin, Y.; Lu, S. Effects of extracellular matrix glycosylation on proliferation and apoptosis of human dermal fibroblasts via the receptor for advanced glycosylated end products. Am. J. Dermatopathol. 2008, 30, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.S.; Al-Mulla, F. ROS constitute a convergence nexus in the development of IGF1 resistance and impaired wound healing in a rat model of type 2 diabetes. Dis. Model. Mech. 2012, 5, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Chen, H.; Chai, Y. Advanced Glycation End Products (AGEs) induce apoptosis of fibroblasts by activation of NLRP3 inflammasome via Reactive Oxygen Species (ROS) signaling pathway. Med. Sci. Monit. 2019, 25, 7499–7508. [Google Scholar] [CrossRef]
- Muniandy, K.; Gothai, S.; Tan, W.S.; Kumar, S.S.; Mohd Esa, N.; Chandramohan, G.; Al-Numair, K.S.; Arulselvan, P. In vitro wound healing potential of stem extract of Alternanthera sessilis. Evid. Based Complement. Altern. Med. 2018, 2018, 3142073. [Google Scholar] [CrossRef] [Green Version]
- Bueno, F.G.; Panizzon, G.P.; Mello, E.V.S.D.L.; Lechtenberg, M.; Petereit, F.; De Mello, J.C.P.; Hensel, A. Hydrolyzable tannins from hydroalcoholic extract from Poincianella pluviosa stem bark and its wound-healing properties: Phytochemical investigations and influence on in vitro cell physiology of human keratinocytes and dermal fibroblasts. Fitoterapia 2014, 99, 252–260. [Google Scholar] [CrossRef]
- Ustuner, O.; Anlas, C.; Bakirel, T.; Ustun-Alkan, F.; Sigirci, B.D.; Ak, S.; Akpulat, H.A.; Donmez, C.; Koca-Caliskan, U. In vitro evaluation of antioxidant, anti-inflammatory, antimicrobial and wound healing potential of thymus sipyleus boiss. subsp. rosulans (borbas) jalas. Molecules 2019, 24, 3353. [Google Scholar] [CrossRef] [Green Version]
- Tasić-Kostov, M.; Arsić, I.; Pavlović, D.; Stojanović, S.; Najman, S.; Naumović, S.; Tadić, V. Towards a modern approach to traditional use: In vitro and in vivo evaluation of Alchemilla vulgaris L. gel wound healing potential. J. Ethnopharmacol. 2019, 238, 111789. [Google Scholar] [CrossRef]
- Addis, R.; Cruciani, S.; Santaniello, S.; Bellu, E.; Sarais, G.; Ventura, C.; Maioli, M.; Pintore, G. Fibroblast proliferation and migration in wound healing by phytochemicals: Evidence for a novel synergic outcome. Int. J. Med. Sci. 2020, 17, 1030–1042. [Google Scholar] [CrossRef] [Green Version]
- Bayrami, Z.; Hajiaghaee, R.; Khalighi-Sigaroodi, F.; Rahimi, R.; Farzaei, M.H.; Hodjat, M.; Baeeri, M.; Rahimifard, M.; Navaei-Nigjeh, M.; Abdollahi, M. Bio-guided fractionation and isolation of active component from Tragopogon graminifolius based on its wound healing property. J. Ethnopharmacol. 2018, 226, 48–55. [Google Scholar] [CrossRef]
- Moghadam, S.E.; Ebrahimi, S.N.; Salehi, P.; Farimani, M.M.; Hamburger, M.; Jabbarzadeh, E. Wound healing potential of chlorogenic acid and myricetin-3-o-β-rhamnoside isolated from parrotia persica. Molecules 2017, 22, 1501. [Google Scholar] [CrossRef] [Green Version]
- Agyare, C.; Lechtenberg, M.; Deters, A.; Petereit, F.; Hensel, A. Ellagitannins from Phyllanthus muellerianus (Kuntze) Exell.: Geraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Phytomedicine 2011, 18, 617–624. [Google Scholar] [CrossRef]
- Ma, R.J.; Yang, L.; Bai, X.; Li, J.Y.; Yuan, M.Y.; Wang, Y.Q.; Xie, Y.; Hu, J.M.; Zhou, J. Phenolic constituents with antioxidative, tyrosinase inhibitory and anti-aging activities from Dendrobium loddigesii Rolfe. Nat. Prod. Bioprospect. 2019, 9, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Chiu, C.C.; Wu, C.P.; Chou, Y.T.; Wang, H.M. Enhancements of skin cell proliferations and migrations via 6-dehydrogingerdione. J. Agric. Food Chem. 2013, 61, 1349–1356. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transpl. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Quan, C.; Cho, M.K.; Perry, D.; Quan, T. Age-associated reduction of cell spreading induces mitochondrial DNA common deletion by oxidative stress in human skin dermal fibroblasts: Implication for human skin connective tissue aging. J. Biomed. Sci. 2015, 22, 62. [Google Scholar] [CrossRef] [Green Version]
- Stout, R.; Birch-Machin, M. Mitochondria’s role in skin ageing. Biology 2019, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- De Silva, S.A.M.; Leonardi, G.R.; Michniak-Kohn, B. An overview about oxidation in clinical practice of skin aging. An. Bras. Dermatol. 2017, 92, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Morliere, P.; Moysan, A.; Tirache, I. Action spectrum for UV-induced lipid peroxidation in cultured human skin fibroblasts. Free Radic. Biol. Med. 1995, 19, 365–371. [Google Scholar] [CrossRef]
- Schneider, L.A.; Dissemond, J.; Brenneisen, P.; Hainzl, A.; Briviba, K.; Wlaschek, M.; Scharffetter-Kochanek, K. Adaptive cellular protection against UVA-1-induced lipid peroxidation in human dermal fibroblasts shows donor-to-donor variability and is glutathione dependent. Arch. Dermatol. Res. 2006, 297, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective tissue and fibroblast senescence in skin aging. J. Invest. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Rodríguez, S.; Folgueras, A.R.; López-Otín, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef]
- Seo, S.W.; Park, S.K.; Oh, S.J.; Shin, O.S. TLR4-mediated activation of the ERK pathway following UVA irradiation contributes to increased cytokine and MMP expression in senescent human dermal fibroblasts. PLoS ONE 2019, 13, e0202323. [Google Scholar] [CrossRef] [Green Version]
- Surowiak, P.; Gansukh, T.; Donizy, P.; Halon, A.; Rybak, Z. Increase in cyclooxygenase-2 (COX-2) expression in keratinocytes and dermal fibroblasts in photoaged skin. J. Cosmet. Dermatol. 2014, 13, 195–201. [Google Scholar] [CrossRef]
- Mavrogonatou, E.; Konstantinou, A.; Kletsas, D. Long-term exposure to TNF-α leads human skin fibroblasts to a p38 MAPK- and ROS-mediated premature senescence. Biogerontology 2018, 19, 237–249. [Google Scholar] [CrossRef]
- Treiber, N.; Maity, P.; Singh, K.; Kohn, M.; Keist, A.F.; Ferchiu, F.; Sante, L.; Frese, S.; Bloch, W.; Kreppel, F.; et al. Accelerated aging phenotype in mice with conditional deficiency for mitochondrial superoxide dismutase in the connective tissue. Aging Cell 2011, 10, 239–254. [Google Scholar] [CrossRef]
- Noh, E.M.; Park, J.; Song, H.R.; Kim, J.M.; Lee, M.; Song, H.K.; Hong, O.Y.; Whang, P.H.; Han, M.K.; Kwon, K.B.; et al. Skin aging-dependent activation of the PI3K signaling pathway via downregulation of PTEN increases intracellular ROS in human dermal fibroblasts. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt signaling and redox metabolism in cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef]
- Shi, H.; Cheng, Y.; Ye, J.; Cai, P.; Zhang, J.; Li, R.; Yang, Y.; Wang, Z.; Zhang, H.; Lin, C.; et al. bFGF promotes the migration of human dermal fibroblasts under diabetic conditions through reactive oxygen species production via the PI3K/Akt-Rac1- JNK pathways. Int. J. Biol. Sci. 2015, 11, 845–859. [Google Scholar] [CrossRef]
- Liu, C.S.; Nam, T.G.; Han, M.W.; Ahn, S.M.; Choi, H.S.; Kim, T.Y.; Chun, O.K.; Koo, S.I.; Kim, D.O. Protective effect of detoxified Rhus verniciflua stokes on human keratinocytes and dermal fibroblasts against oxidative stress and identification of the bioactive phenolics. Biosci. Biotechnol. Biochem. 2013, 77, 1682–1688. [Google Scholar] [CrossRef]
- Cuelho, C.H.F.; de Alves, G.A.D.; Lovatto, M.O.; Bonilha, I.F.; Barbisan, F.; da Cruz, I.B.M.; Oliveira, S.M.; Fachinetto, R.; do Canto, G.S.; Manfron, M.P. Topical formulation containing Ilex Paraguariensis extract increases metalloproteinases and myeloperoxidase activities in mice exposed to UVB radiation. J. Photochem. Photobiol. B 2018, 189, 95–103. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.W.; Kim, E.K.; Lee, S.J.; Park, N.H.; Kim, H.S.; Kim, H.K.; Char, K.; Jang, Y.P.; Kim, J.W. Inhibition effect of Gynura procumbens extract on UV-B-induced matrix-metalloproteinase expression in human dermal fibroblasts. J. Ethnopharmacol. 2011, 137, 427–433. [Google Scholar] [CrossRef]
- Yoo, H.G.; Lee, B.H.; Kim, W.; Lee, J.S.; Kim, G.H.; Chun, O.K.; Koo, S.I.; Kim, D.O. Lithospermum erythrorhizon extract protects keratinocytes and fibroblasts against oxidative stress. J. Med. Food. 2014, 17, 1189–1196. [Google Scholar] [CrossRef]
- Gao, W.; Lin, P.; Hwang, E.; Wang, Y.; Yan, Z.; Ngo, H.T.T.; Yi, T.H. Pterocarpus santalinus L. regulated ultraviolet B irradiation-induced procollagen reduction and matrix metalloproteinases expression through activation of TGF-β/Smad and inhibition of the MAPK/AP-1 pathway in normal human dermal fibroblasts. Photochem. Photobiol. 2018, 94, 139–149. [Google Scholar] [CrossRef]
- Limtrakul, P.; Yodkeeree, S.; Thippraphan, P.; Punfa, W.; Srisomboon, J. Anti-aging and tyrosinase inhibition effects of Cassia fistula flower butanolic extract. BMC Complement. Altern. Med. 2016, 16, 497. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.Y.; Choi, D.I.; Lee, J.B.; Yun, S.J.; Lee, D.H.; Eun, J.B.; Lee, S.C. Ethanol extract of peanut sprout induces Nrf2 activation and expression of antioxidant and detoxifying enzymes in human dermal fibroblasts: Implication for its protection against UVB-irradiated oxidative stress. Photochem. Photobiol. 2013, 89, 453–460. [Google Scholar] [CrossRef]
- Chiang, H.M.; Chiu, H.H.; Liao, S.T.; Chen, Y.T.; Chang, H.C.; Wen, K.C. Isoflavonoid-rich Flemingia macrophylla extract attenuates UVB-induced skin damage by scavenging reactive oxygen species and inhibiting MAP kinase and MMP expression. Evid. Based Complement. Altern. Med. 2013, 2013, 696879. [Google Scholar] [CrossRef] [Green Version]
- Kurt-Celep, İ.; Celep, E.; Akyüz, S.; İnan, Y.; Barak, T.H.; Akaydın, G.; Telci, D.; Yesilada, E. Hypericum olympicum L. recovers DNA damage and prevents MMP–9 activation induced by UVB in human dermal fibroblasts. J. Ethnopharmacol. 2020, 246, 112202. [Google Scholar] [CrossRef]
- Ferreira, L.d.A.O.; de Melo, C.d.B.; Saito, P.; Iwanaga, C.C.; Nakamura, C.V.; Casagrande, R.; Truiti, M.d.T. Nectandra cuspidata fraction and the isolated polyphenols protect fibroblasts and hairless mice skin from UVB-induced inflammation and oxidative stress. J. Photochem. Photobiol. B Biol. 2020, 205, 111824. [Google Scholar] [CrossRef]
- De Oliveira, M.M.; Daré, R.G.; Barizão, É.O.; Visentainer, J.V.; Romagnolo, M.B.; Nakamura, C.V.; da Truiti, M.C.T. Photodamage attenuating potential of Nectandra hihua against UVB-induced oxidative stress in L929 fibroblasts. J. Photochem. Photobiol. B 2018, 181, 127–133. [Google Scholar] [CrossRef]
- De Souza, R.O.; Alves, G.d.A.D.; Aguillera, A.L.S.; Rogez, H.; Fonseca, M.J.V. Photochemoprotective effect of a fraction of a partially purified extract of Byrsonima crassifolia leaves against UVB-induced oxidative stress in fibroblasts and hairless mice. J. Photochem. Photobiol. B 2018, 178, 53–60. [Google Scholar] [CrossRef]
- Patwardhan, J.; Bhatt, P. Flavonoids derived from Abelmoschus esculentus attenuatesUV-B Induced cell damage in human dermal fibroblasts throughNrf2-ARE pathway. Pharmacogn. Mag. 2016, 12, S129–S138. [Google Scholar] [CrossRef] [Green Version]
- Patwardhan, J.; Bhatt, P. Ultraviolet-B protective effect of flavonoids from Eugenia Caryophylata on human dermal fibroblast cells. Pharmacogn. Mag. 2015, 11, S397–S406. [Google Scholar] [CrossRef]
- Ruszová, E.; Cheel, J.; Pávek, S.; Moravcová, M.; Hermannová, M.; Matějková, I.; Spilková, J.; Velebný, V.; Kubala, L. Epilobium angustifolium extract demonstrates multiple effects on dermal fibroblasts in vitro and skin photo-protection in vivo. Gen. Physiol. Biophys. 2013, 32, 347–359. [Google Scholar] [CrossRef] [Green Version]
- Bravo, K.; Duque, L.; Ferreres, F.; Moreno, D.A.; Osorio, E. Passiflora tarminiana fruits reduce UVB-induced photoaging in human skin fibroblasts. J. Photochem. Photobiol. B 2017, 168, 78–88. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Tulipani, S.; Gonzàles-Paramàs, A.M.; Santos-Buelga, C.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Photoprotective potential of strawberry (Fragaria × ananassa) extract against UV-A irradiation damage on human fibroblasts. J. Agric. Food Chem. 2012, 60, 2322–2327. [Google Scholar] [CrossRef]
- Kwak, C.S.; Yang, J.; Shin, C.Y.; Chung, J.H. Rosa multiflora Thunb flower extract attenuates ultraviolet-induced photoaging in skin cells and hairless mice. J. Med. Food 2020, 23, 988–997. [Google Scholar] [CrossRef]
- Liu, S.; You, L.; Zhao, Y.; Chang, X. Hawthorn polyphenol extract inhibits UVB-induced skin photoaging by regulating MMP expression and type I procollagen production in mice. J. Agric. Food Chem. 2018, 66, 8537–8546. [Google Scholar] [CrossRef]
- Wu, P.Y.; Huang, C.C.; Chu, Y.; Huang, Y.H.; Lin, P.; Liu, Y.H.; Wen, K.C.; Lin, C.Y.; Hsu, M.C.; Chiang, H.M. Alleviation of ultraviolet B-induced photodamage by Coffea arabica extract in human skin fibroblasts and hairless mouse skin. Int. J. Mol. Sci. 2017, 18, 782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, K.C.; Chiu, H.H.; Fan, P.C.; Chen, C.W.; Wu, S.M.; Chang, J.H.; Chiang, H.M. Antioxidant activity of Ixora parviflora in a cell/cell-free system and in UV-exposed human fibroblasts. Molecules 2011, 16, 5735–5752. [Google Scholar] [CrossRef] [PubMed]
- Brugè, F.; Tiano, L.; Astolfi, P.; Emanuelli, M.; Damiani, E. Prevention of UVA-induced oxidative damage in human dermal fibroblasts by new UV filters, assessed using a novel in vitro experimental system. PLoS ONE 2014, 9, e83401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poon, F.; Kang, S.; Chien, A.L. Mechanisms and treatments of photoaging. Photodermatol. Photoimmunol. Photomed. 2015, 31, 65–74. [Google Scholar] [CrossRef]
- Lopez Perez, R.; Brauer, J.; Rühle, A.; Rühle, A.; Trinh, T.; Sisombath, S.; Wuchter, P.; Grosu, A.-L.; Debus, J.; Saffrich, R.; et al. Human mesenchymal stem cells are resistant to UV-B irradiation. Sci. Rep. 2000, 9. [Google Scholar] [CrossRef]
- Pinnell, S.R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J. Am. Acad. Dermatol. 2003, 48, 1–19. [Google Scholar] [CrossRef]
- Lee, S.; Yu, J.S.; Phung, H.M.; Lee, J.G.; Kim, K.H.; Kang, K.S. Potential anti-skin aging effect of (-)-catechin isolated from the root bark of ulmus davidiana var. Japonica in tumor necrosis factor-α-stimulated normal human dermal fibroblasts. Antioxidants 2020, 9, 981. [Google Scholar] [CrossRef]
- Lyu, S.Y.; Park, W.B. Photoprotective potential of anthocyanins isolated from acanthopanax divaricatus var. albeofructus fruits against uv irradiation in human dermal fibroblast cells. Biomol. Ther. 2012, 20, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Parzonko, A.; Kiss, A.K. Caffeic acid derivatives isolated from Galinsoga parviflora herb protected human dermal fibroblasts from UVA-radiation. Phytomedicine 2019, 57, 215–222. [Google Scholar] [CrossRef]
| Name of the Families | Name of the Species (Common Names) | Part of the Plant | Type of Extract (Concentrations) | Identified Bioactive | Cell Lines | Mechanisms of Action | Ref. |
|---|---|---|---|---|---|---|---|
| Annonaceae | Annona muricata L. (soursop) | leaves | aqueous (12.5 to 200 µg/mL) | quercetin 3-glucoside, rutin, chlorogenic acid, catechin, and gallic acid | L929 fibroblasts exposed to 750 µmol/L H2O2 | Reduced ROS production | [76] |
| Onagraceae | Fuchsia magellanica Lam. (hardy fuchsia) | leaves | aqueous/ethanolic (1000 µg/mL) | various phenolic acid, flavonoid, and anthocyanin derivatives | 3T3 fibroblasts exposed to 1 mM 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) | Reduced ROS production | [77] |
| Poaceae | Oryza sativa L. (rice) | panicles | ethyl acetate (0.1 to 100 µg/mL) | gallic, protocatechuic, chlorogenic, caffeic, syringic, p-coumaric, ferulic, sinapic and rosmarinic acids, vanillin, and quercetin | HDFs exposed to 150 µmol/L H2O2 | Reduced oxidative stress | [78] |
| Rosaceae | Pourthiaea villosa (Thunb.) Decne. (oriental photinia) | leaves | ethanolic (25 to 100 µg/mL) | p-coumaric acid, caffeic acid, chlorogenic acid, patuletin, catechin, epicatechin, eriodictyol, naringenin, quercetin, and quercetin derivatives | HDFs exposed to 1 mmol/L H2O2 | Reduced ROS production | [79] |
| Salicaceae | Populus nigra L. (Lombardy poplar) | whole plant | aqueous (25 to 200 μg/mL) | caffeic and p-coumaric acids | HDFs exposed to 100 µmol/L AAPH | Reduced ROS production | [80] |
| Name of the Families | Name of the Species (Common Names) | Part of the Plant | Type of Extract (Concentrations) | Identified Bioactive | Cell Lines | Mechanisms of Action | Ref. |
|---|---|---|---|---|---|---|---|
| Amaranthaceae | Alternanthera sessilis (L.) R.Br. ex DC. (sessile joyweed) | stems | Ethanolic (12.5 to 50 μg/mL) | 2,4-dihydroxy-2,5-dimethyl-3(2H)-furan-3-one, hexadecanoic acid, 2-1,2,4-trioxolane,3-phenyl, palmitate ethyl, and L-glutamic acid | HDFs and diabetic HDFs | Increased migratory rate | [95] |
| Fabaceae | Cenostigma pluviosum (DC.) Gagnon & G.P.Lewis | stem bark | ethanolic | pyrogallol, gallic acid, gallic acid methyl ester, ellagic acid, corilagin, 1,4,6-tri-O-galloyl-glucose, tellimagrandin I, 1,2,3,6-tetra-O-galloyl-glucose, mallotinic acid, tellimagrandin II, 1,2,3,4,6-penta-O-galloyl-glucose, geraniin, and mallotusinic acid | HDFs | Increased cell proliferation rate | [96] |
| Lamiaceae | Thymus sipyleus Boiss. | aerial parts | ethanolic (50 to 200 μg/mL) | luteolin-7-O-glucoside | 3T3 fibroblasts | Increased migratory rate | [97] |
| Primulaceae | Lysimachia nummularia L. (creeping jenny) | leaves | ethanolic (10 to 50 μg/mL) | various phenolic acid, flavonoid, and anthocya-nin derivatives | 3T3 fibroblasts | Increased migratory rate | [77] |
| Rosaceae | Alchemilla vulgaris L. | whole plant | ethanolic | kaempferol, luteolin, apigenin-7-Oglucoside, luteolin-7-O-glucoside, isoquercetin, and ellagic acid | L929 fibroblasts | Increased migratory rate | [98] |
| Name of the Families | Name of the Species (Common Names) | Part of the Plant | Type of Extract (Concentration) | Identified Bioactive | Cell Lines | Mechanisms of Action | Ref. |
|---|---|---|---|---|---|---|---|
| Anacardiaceae | Toxicodendron vernicifluum (Stokes) F.A.Barkley (Chinese lacquer) | rhus | methanolic (1 to 50 μg/mL) | gallic acid, 2-(ethoxymethoxy)-3-hydroxyphenol, fustin, a fustin isomer, tetragalloyl glucose, pentagalloyl glucose, fisetin, sulfuretin, a sulfuretin isomer, and butein | HDFs exposed to UVA radiation | Reduced MMP-1 expression | [121] |
| Aquifoliaceae | Ilex paraguariensis A.St.-Hil. (mate) | leaves | ethanolic (40 to 400 μg/mL) | chlorogenic acid and caffeic acid | HFF-1 fibroblasts exposed to UVB radiation | Not cytotoxic for non-irradiated cells; photostable and non-phototoxic for radiated cells | [122] |
| Asteraceae | Gynura procumbens Merr. | leaves | ethanolic (1 to 20 μg/mL) | quercetin 3-O-rutinoside and isobioquercetin, kaempferol 3-O-rutinoside | HDFs exposed to UVB radiation | Reduced MMP-1 and MMP-9 production | [123] |
| Boraginaceae | Lithospermum erythrorhizon Siebold & Zucc. (Lithospermum) | whole plant | methanolic (0.1 to 10 mg/mL) | rabdosiin, rosmarinic acid, lithospermic acid, lithospermic acid B, salvianolic acid A, and acetylshikonin, isomers of lithospermic acid, shikonofuran E, b-hydroxyisovalerylshikonin, isobutylshikonin, b,b-dimethylacrylshikonin, and isovalerylshikonin | HDFs exposed to UVA radiation | Reduced MMP-1 expression | [124] |
| Fabaceae | Pterocarpus santalinus L.f. (red sandalwood) | heartwoods | ethanolic (10 μg/mL) | taxifolin, quercetin, and naringenin | HDFs exposed to UVB radiation | Reduced MMP-1, MMP-3, IL-6, AP-1 and MAPKs expression Increased Nrf2 activity | [125] |
| Fabaceae | Cassia fistula L. (golden shower) | flowers | butanolic (25 to 200 μg/mL) | vanillic acid and protocatechuic acid, gallic acid, coumaric acid, ferulic acid, and chlorogenic acid, catechin | HDFs | Increased collagen and hyaluronic acid synthesis Reduced ollagenase and MMP-2 activity | [126] |
| Fabaceae | Arachis hypogaea L. (peanut) | sprout | ethanolic (0.005% to 2.5% from the stock) | trans-resveratrol | HDFs exposed to UVB radiation | Reduced ROS production Increased Nrf2 activity | [127] |
| Fabaceae | Flemingia macrophylla (Willd.) Kuntze ex Merr. | stems | aqueous (10 to 500 μg/mL) | daidzin, genistin, | Hs68 fibroblasts exposed to UVB radiation | Reduced elastase and collagenase activity, MAPKs, MMP-1, MMP-3, MMP-9 expression Increased type I procollagen expression | [128] |
| Hypericaceae | Hypericum olympicum L. | flowering aerial parts | methanolic (0.5 to 1.5 mg/mL) | chlorogenic acid and quercetin glycosides (rutin, hyperoside, isoquercitrin) | HDFs exposed to UVB radiation | Reduced MMP-9 concentrations | [129] |
| Lauraceae | Nectandra cuspidata Nees & Mart. | leaves | ethyl acetate fraction (6.54 μg/mL) | epicatechin, isovitexin, and vitexin | L-929 fibroblasts exposed to UVB radiation | Reduced ROS production, LPO inhibition | [130] |
| Lauraceae | Nectandra hihua (Ruiz & Pav.) Rohwer (shinglewood) | leaves | ethyl acetate fraction (10 μg/mL) | quercitrin, avicularin, juglalin, afzelin, and astragalin | L929 fibroblasts exposed to UVB radiation | Reduced ROS production, LPO inhibition | [131] |
| Malpighiaceae | Byrsonima crassifolia (L.) Kunth (maricao cimun) | leaves | ethanolic—partially purified (0.6 to 5 µg/mL) | catechin, epigallocatechin gallate, quercetin 3-O-β-D-glucopyranoside | L929 fibroblasts exposed to UVB radiation | Prevented the decrease in reduced GSH levels | [132] |
| Malvaceae | Abelmoschus esculentus (L.) Moench (okra) | fruits | ethyl acetate fraction (5 to 30 µg/mL) | rutin | HDFs exposed to UVB radiation | Prevented: UV-induced depletion of endogenous enzymatic antioxidants Reduced oxidative DNA damage, ROS production, apoptotic changes Increased Nrf2 activity | [133] |
| Myrtaceae | Syzygium aromaticum (L.) Merr. & L.M.Perry (clove) | clove buds (5 to 40 µg/mL) | methanolic | flavonoid-enriched fraction: quercetin, kaempferol, gallic acid | HDFs exposed to UVB radiation | Prevented: UV-induced depletion of endogenous enzymatic antioxidants Reduced xidative DNA damage, ROS production, apoptotic changes Increased Nrf2 activity | [134] |
| Onagraceae | Epilobium angustifolium L. | aerial parts | Isopropylalcohol (10 μg/mL) | gallic acid, oenothein B, chlorogenic acid, myricetin-3-O-hexoside, myricetin-3-O-pentoside, myricetin-3-O-rhamnoside, quercetin-7-O-glucuronide, quercetin-3-Opentoside, kaempferol-3-O-hexoside, kaempferol-7-O-glucuronide; and kaempferol-3-O-rhamnoside | UV-irradiated HDFs | Reduced MMP-1, hyaluronidase 2 gene expression Increased TIMP-1, TIMP-2 gene expression | [135] |
| Passifloraceae | Passiflora tarminiana Coppens & V.E. Barney (banana passionflower) | fruits | aqueous (2.5 to 10 μg/mL) | dimeric proanthocyanidins of flavan-3-ols, flavone derivatives | HDFs exposed to UVB radiation | Reduced ROS production, MMP-1 expression Increased procollagen production | [136] |
| Rosaceae | Fragaria × ananassa (Duchesne ex Weston) Duchesne ex Rozier (strawberry) | fruits | Methanolic (0.05 to 0.5 mg/mL) | anthocyanins | HDFs exposed to UVA radiation | Reduced DNA damages | [137] |
| Rosaceae | Rosa multiflora Thunb. (multiflora rose) | flowers | ethanolic (1 to 10 μg/mL) | quercitrin and hyperin | HDFs exposed to UVB radiation | Reduced MMP-1 expression Increased type I procollagen expression | [138] |
| Rosaceae | Crataegus pinnatifida Bunge (Chinese haw) | fruits | Ethanolic (5 to 10 μg/mL) | chlorogenic acid, procyanidin B2, and epicatechin | HDFs exposed to UVB radiation | Reduced MMP-1 expression, ROS production | [139] |
| Rubiaceae | Coffea arabica L. (Arabian coffee) | leaves | Methanolic (1 to 50 μg/mL) | chlorogenic acid | Hs68 cells exposed to UVB radiation | Reduced ROS production, COX-2 level, translocation of NF-κB to the nucleus | [140] |
| Rubiaceae | Ixora parviflora Lam. | leaves | methanolic (1 to 50 μg/mL) | chlorogenic acid | Hs68 cells exposed to UVB | Reduced ROS production | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Cegliński, T.; Zajdel, R. Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells. Antioxidants 2021, 10, 726. https://doi.org/10.3390/antiox10050726
Merecz-Sadowska A, Sitarek P, Kucharska E, Kowalczyk T, Zajdel K, Cegliński T, Zajdel R. Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells. Antioxidants. 2021; 10(5):726. https://doi.org/10.3390/antiox10050726
Chicago/Turabian StyleMerecz-Sadowska, Anna, Przemysław Sitarek, Ewa Kucharska, Tomasz Kowalczyk, Karolina Zajdel, Tomasz Cegliński, and Radosław Zajdel. 2021. "Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells" Antioxidants 10, no. 5: 726. https://doi.org/10.3390/antiox10050726
APA StyleMerecz-Sadowska, A., Sitarek, P., Kucharska, E., Kowalczyk, T., Zajdel, K., Cegliński, T., & Zajdel, R. (2021). Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells. Antioxidants, 10(5), 726. https://doi.org/10.3390/antiox10050726








