A Two-Step Microwave Annealing Process for PAN Pre-Oxidation through a TM-Mode Cavity
Abstract
:1. Introduction
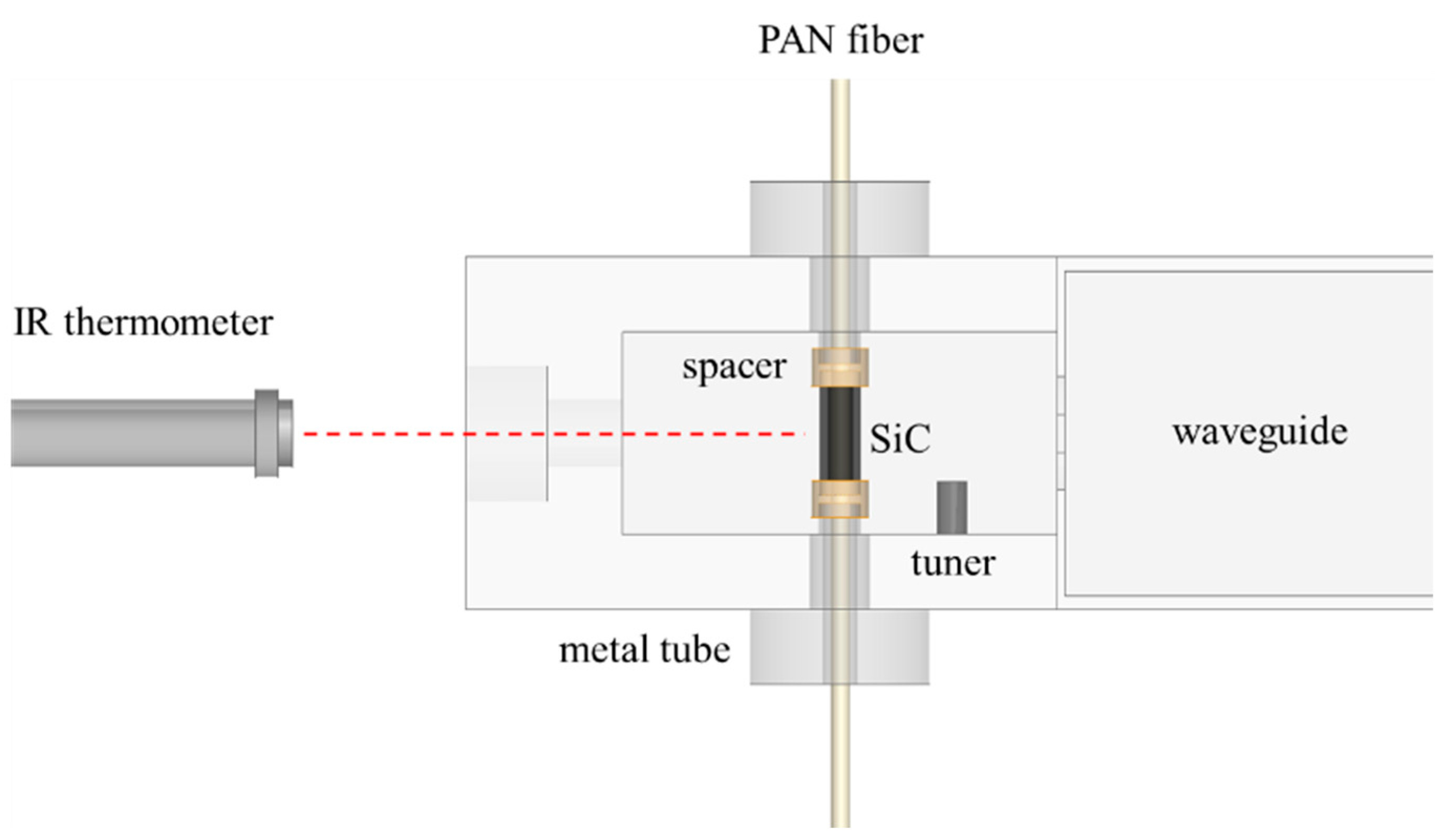
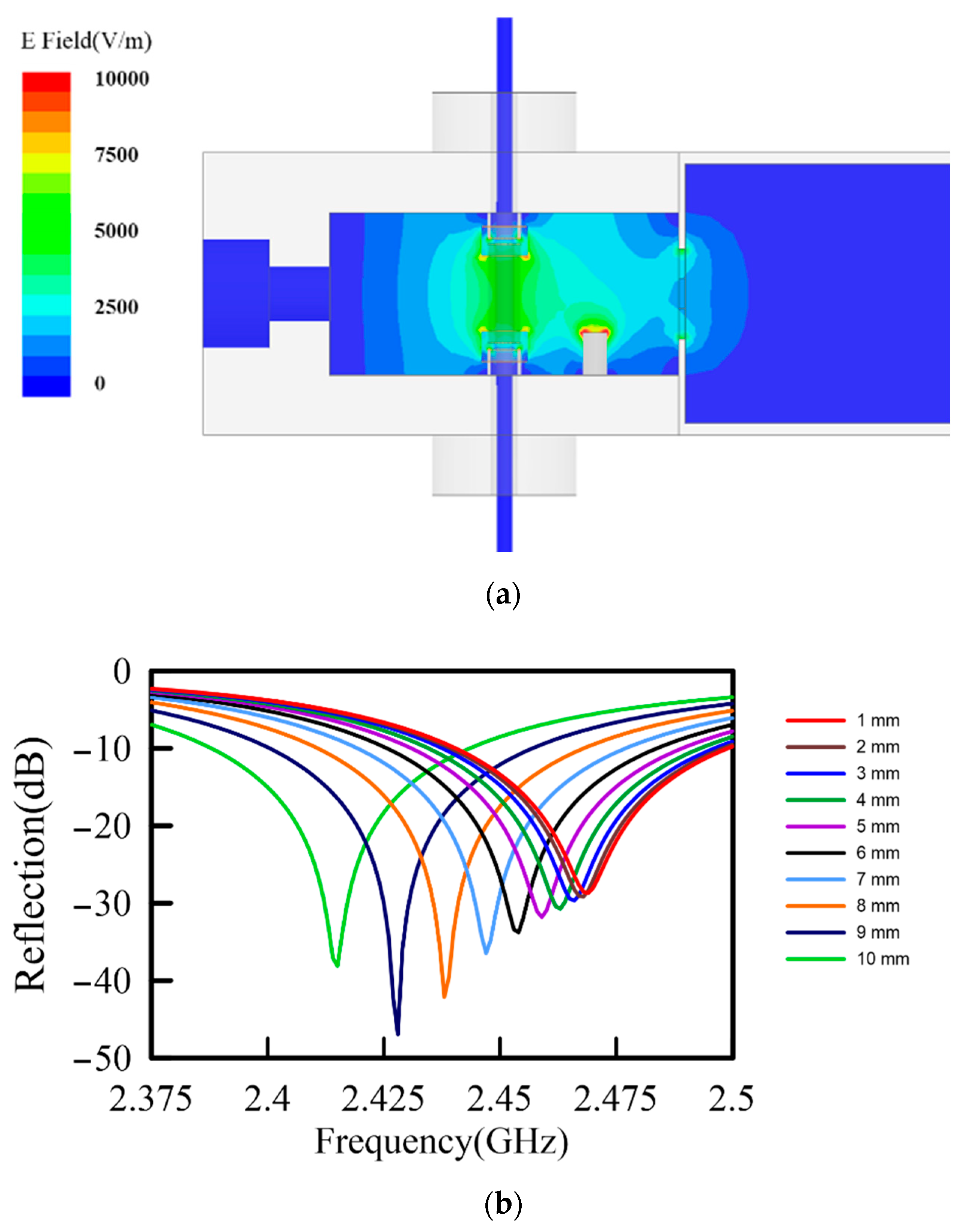
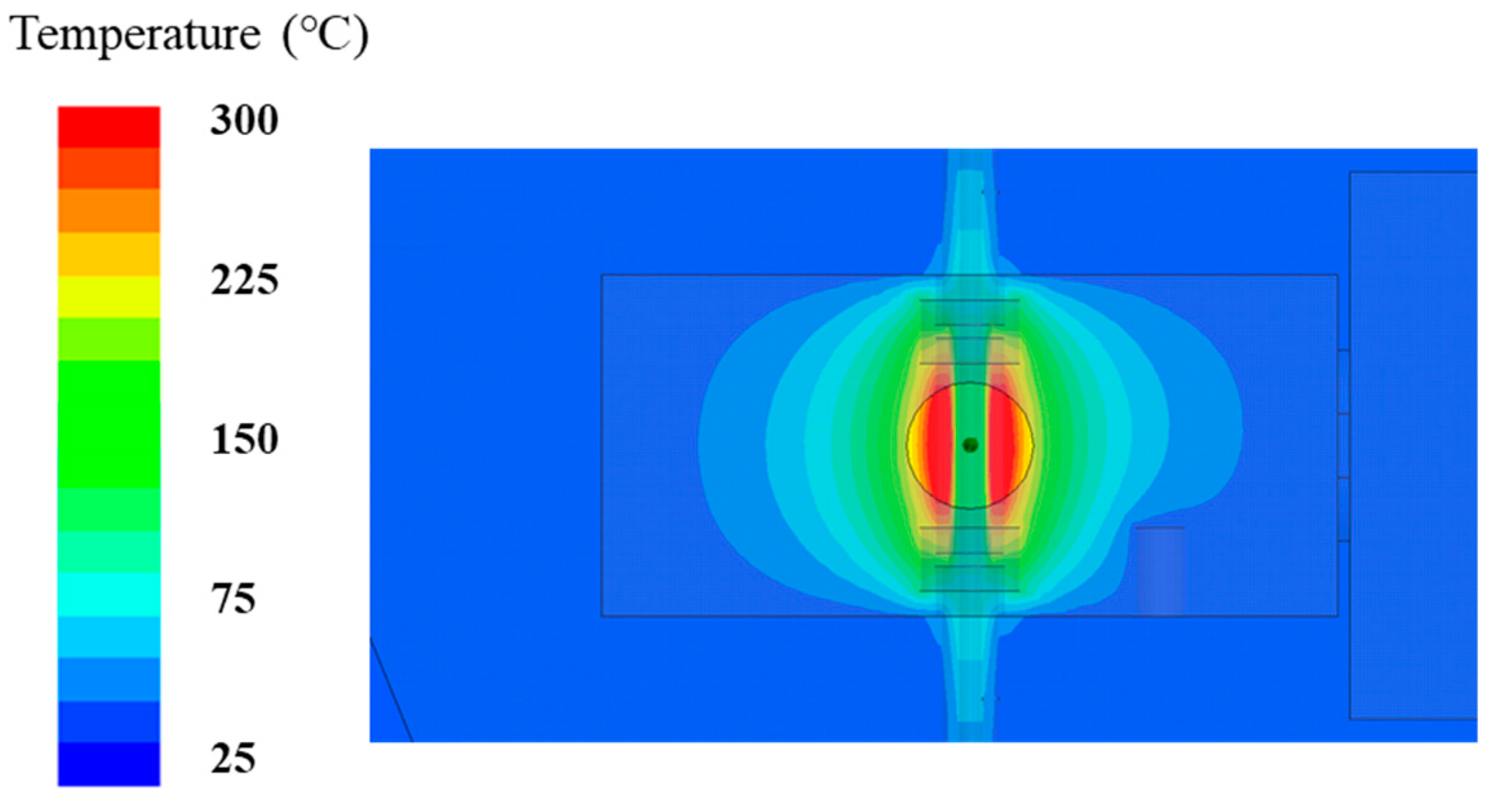
2. Characteristics of a Well-Defined TM-Mode Resonant Cavity
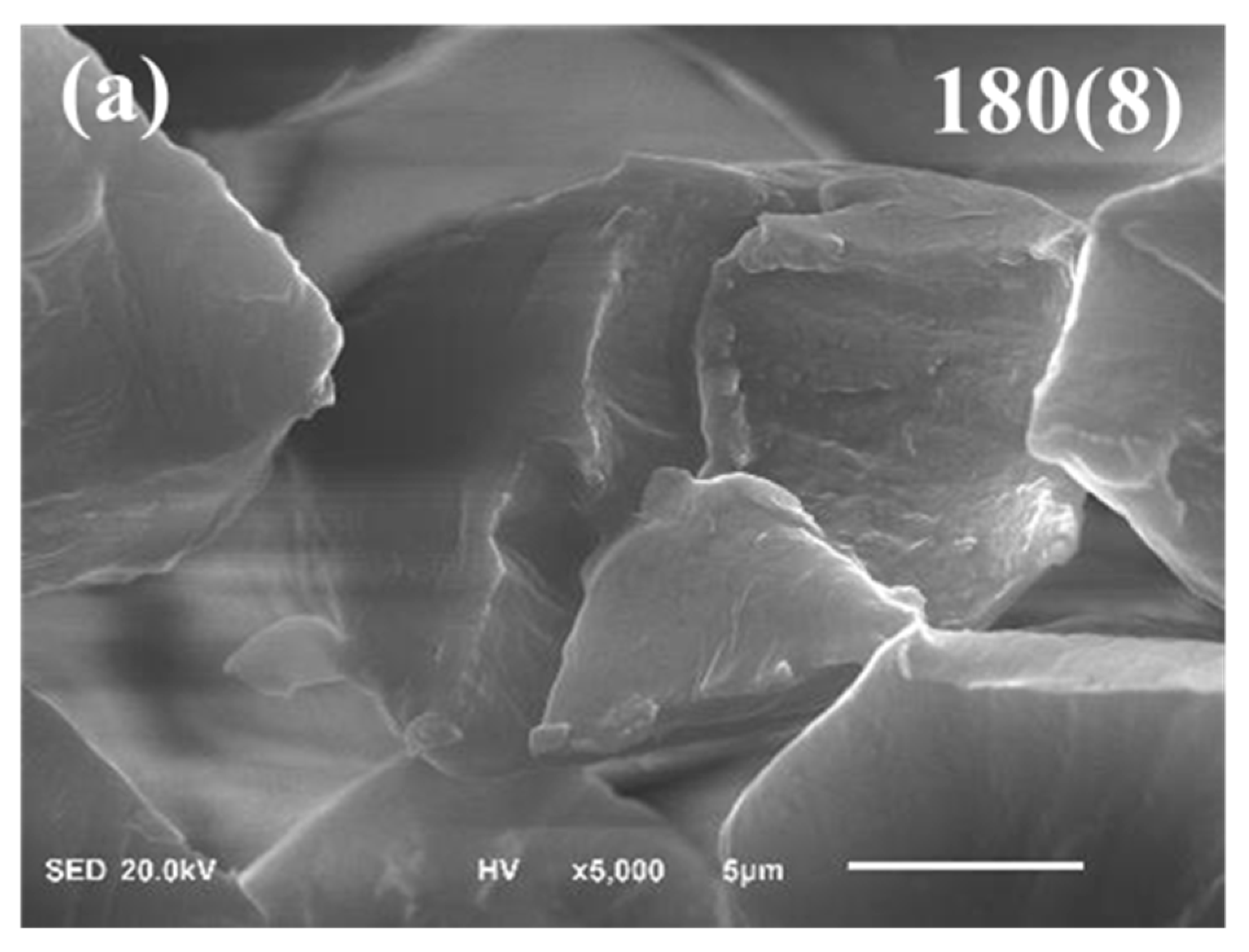
3. Specimen Preparation and Analysis
4. Two-Step Microwave Pre-Oxidation Process of PAN Fiber
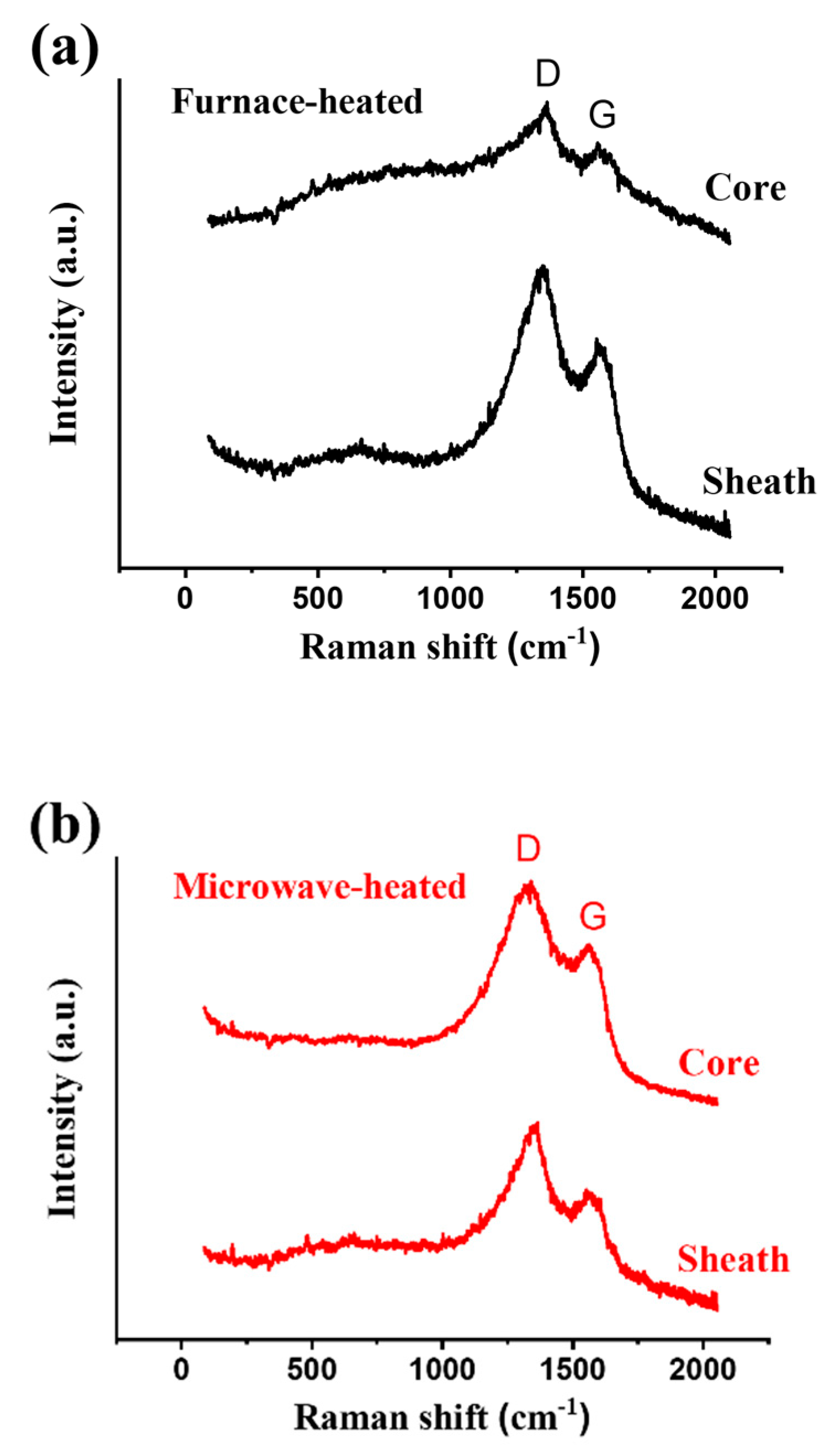
5. Comparison with Conventional Heating Process
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donnet, J.B.; Bansal, R.C. Carbon Fibers; Marcel Dekker Inc.: New York, NY, USA, 1990. [Google Scholar]
- Kinoshita, K. Carbon: Electrochemical and Physicochemical Properties; Wiley-Interscience: Hoboken, NJ, USA, 1998. [Google Scholar]
- Morgan, P. Carbon Fibers and Their Composites; Taylor & Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Ouyang, Q.; Cheng, L.; Wang, H.; Li, K. Mechanism and kinetics of the stabilization reactions of itaconic acid-modified polyacrylonitrile. Polym. Degrad. Stab. 2008, 93, 1415–1421. [Google Scholar] [CrossRef]
- Frank, E.; Hermanutz, F.; Buchmeiser, M.R. Carbon fibers: Precursors, manufacturing, and properties. Macromol. Mater. Eng. 2012, 297, 493–501. [Google Scholar] [CrossRef]
- Qiao, M.; Kong, H.; Ding, X.; Hu, Z.; Zhang, L.; Yu, M. Effect of graphene oxide coatings on the structure of polyacrylonitrile fibers during pre-oxidation process. RSC Adv. 2019, 9, 28146–28152. [Google Scholar] [CrossRef] [Green Version]
- Elagib, T.; Hassan, E.; Fan, C.; Han, K.; Yu, M. Microwave pre-oxidation for polyacrylonitrile precursor coated with nano-carbon black. Polym. Eng. Sci. 2019, 59, 457–464. [Google Scholar] [CrossRef]
- Bykov, Y.V.; Rybakov, K.I.; Semenov, V.E. High-temperature microwave processing of materials. J. Phys. D 2001, 34, 55. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Semenov, V.E.; Link, G.; Thumm, M. Preferred orientation of pores in ceramics under heating by a linearly polarized microwave field. J. Appl. Phys. 2007, 101, 084915. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Eremeev, A.G.; Egorov, S.V.; Bykov, Y.V.; Pajkic, Z.; Willert-Porada, M. Effect of microwave heating on phase transformations in nanostructured alumina. J. Phys. D Appl. Phys. 2008, 41, 1. [Google Scholar] [CrossRef]
- Fong, S.C.; Wang, C.Y.; Chang, T.H.; Chin, T.S. Crystallization of amorphous Si film with SiC susceptor by microwave annealing. Appl. Phys. Lett. 2009, 94, 102104. [Google Scholar] [CrossRef] [Green Version]
- Bhaskar, A.; Chang, T.H.; Chang, H.Y.; Cheng, S.Y. Pb(Zr0.53Ti0.47)O3 thin films with different thickness obtained at low-temperature by microwave irradiation. Appl. Surf. Sci. 2009, 255, 3795. [Google Scholar] [CrossRef]
- Bhaskar, A.; Chang, H.Y.; Chang, T.H.; Cheng, S.Y. Microwave annealing of YAG: Ce nanophosphors. Mater. Lett. 2012, 78, 124–126. [Google Scholar] [CrossRef]
- Liu, J.H.; Xiao, S.J.; Shen, Z.G.; Xu, L.; Zhang, L.B.; Peng, J.H. Study on the oxidative stabilization of polyacrylonitrile fibers by microwave heating. Polym. Degrad. Stab. 2018, 150, 86–91. [Google Scholar] [CrossRef]
- Chang, T.H.; Li, C.H.; Wu, C.N.; Yu, C.F. Generating pure circular TEmn modes using Y-type power dividers. IEEE Trans. Microw. Theory Tech. 2010, 58, 1543. [Google Scholar] [CrossRef]
- Chang, T.H.; Chao, H.W.; Syu, F.H.; Chiang, W.Y.; Fong, S.C.; Chin, T.S. Efficient heating with a controlled microwave field. Rev. Sci. Instrum. 2011, 82, 124703. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.M.; Hong, T.; Liao, Y.H.; Chen, F.Y.; Ye, J.H.; Zhu, H.C.; Huang, K.M. Frequency-selected method to improve microwave heating performance. Appl. Therm. Eng. 2018, 131, 642–648. [Google Scholar] [CrossRef]
- Barham, J.P.; Koyama, E.; Norikane, Y.; Oneda, N.; Yoshimura, T. Microwave Flow: A perspective on reactor and microwave configurations and the emergence of tunable single-mode heating toward large-scale applications. Chem. Rec. 2019, 19, 188–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, C.; Lu, S.; Appleyard, S.P.; Rand, B. The stabilisation of carbon fibres studied by micro-thermal analysis. Carbon 2003, 41, 165–171. [Google Scholar] [CrossRef]
- Lv, M.Y.; Ge, H.Y.; Chen, J. Study on the chemical structure and skin-core structure of polyacrylonitrile-based fibers during stabilization. J. Polym. Res. 2009, 16, 513–517. [Google Scholar] [CrossRef]
- Qiao, M.; Kong, H.; Ding, X.; Hu, Z.; Zhang, L.; Cao, Y.; Yu, M. Study on the changes of structures and properties of PAN fibers during the cyclic reaction in supercritical carbon dioxide. Polymers 2019, 11, 402. [Google Scholar] [CrossRef] [Green Version]
- Ko, T.H.; Day, T.C.; Perng, J.A.; Lin, M.F. The characterization of PAN-based carbon fibers developed by two-stage continuous carbonization. Carbon 1993, 31, 765–771. [Google Scholar]
- Yu, M.J.; Bai, Y.J.; Wang, C.G.; Xu, Y.; Guo, P.Z. A new method for the evaluation of stabilization index of polyacrylonitrile fibers. Mater. Lett. 2007, 61, 2292–2294. [Google Scholar] [CrossRef]
- Panapoy, M.; Dankeaw, A.; Ksapabutr, B. Electrical conductivity of PAN-based carbon nanofibers prepared by electrospinning method. Thammasat. Int. J. Sc. Tech. 2008, 13, 11–17. [Google Scholar]
- Lee, S.; Kim, J.H.; Ku, B.C.; Kim, J.K.; Joh, H.I. Structural evolution of polyacrylonitrile fibers in stabilization and carbonization. Chem. Eng. J. 2012, 2, 18911. [Google Scholar] [CrossRef] [Green Version]
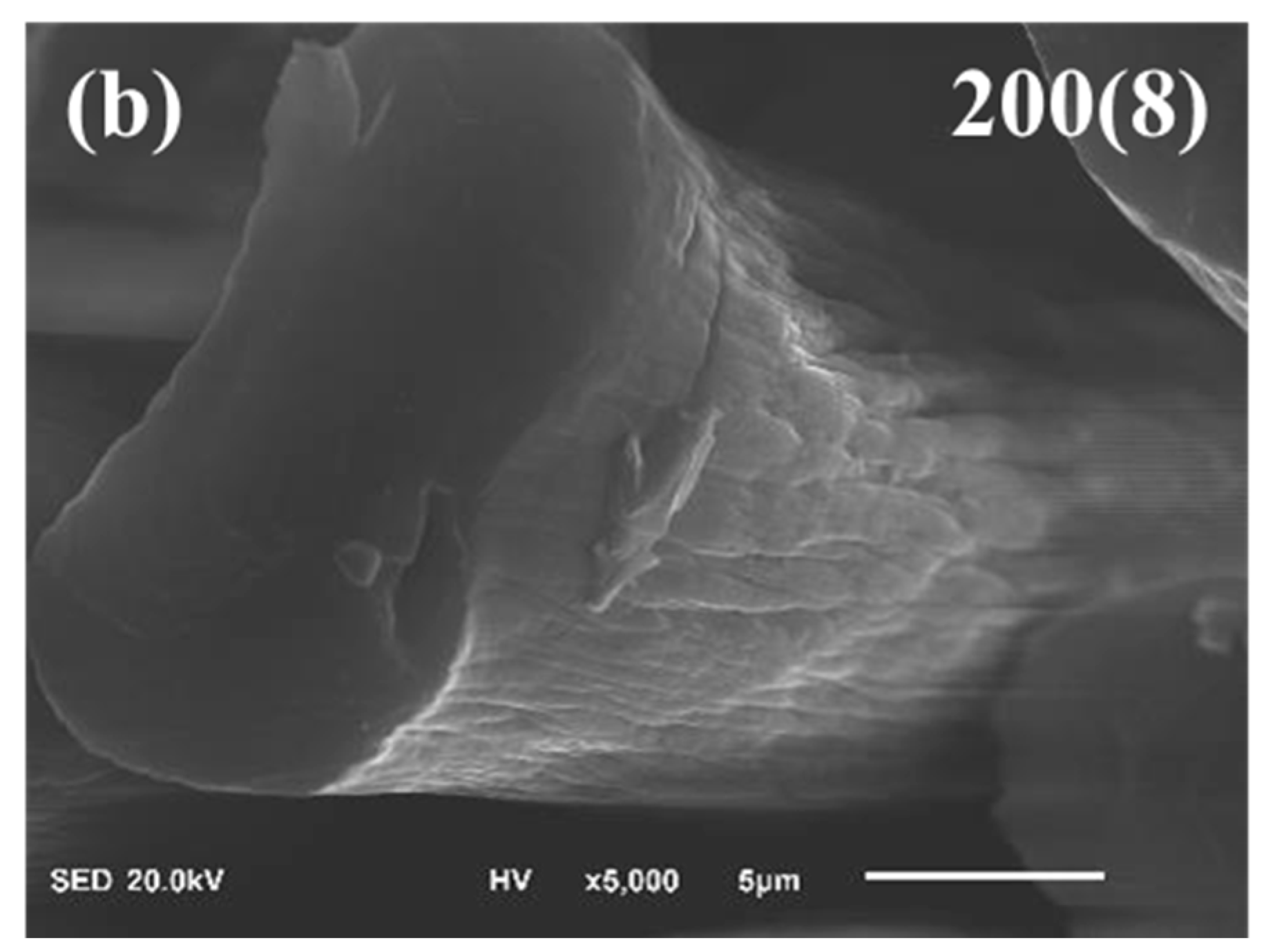
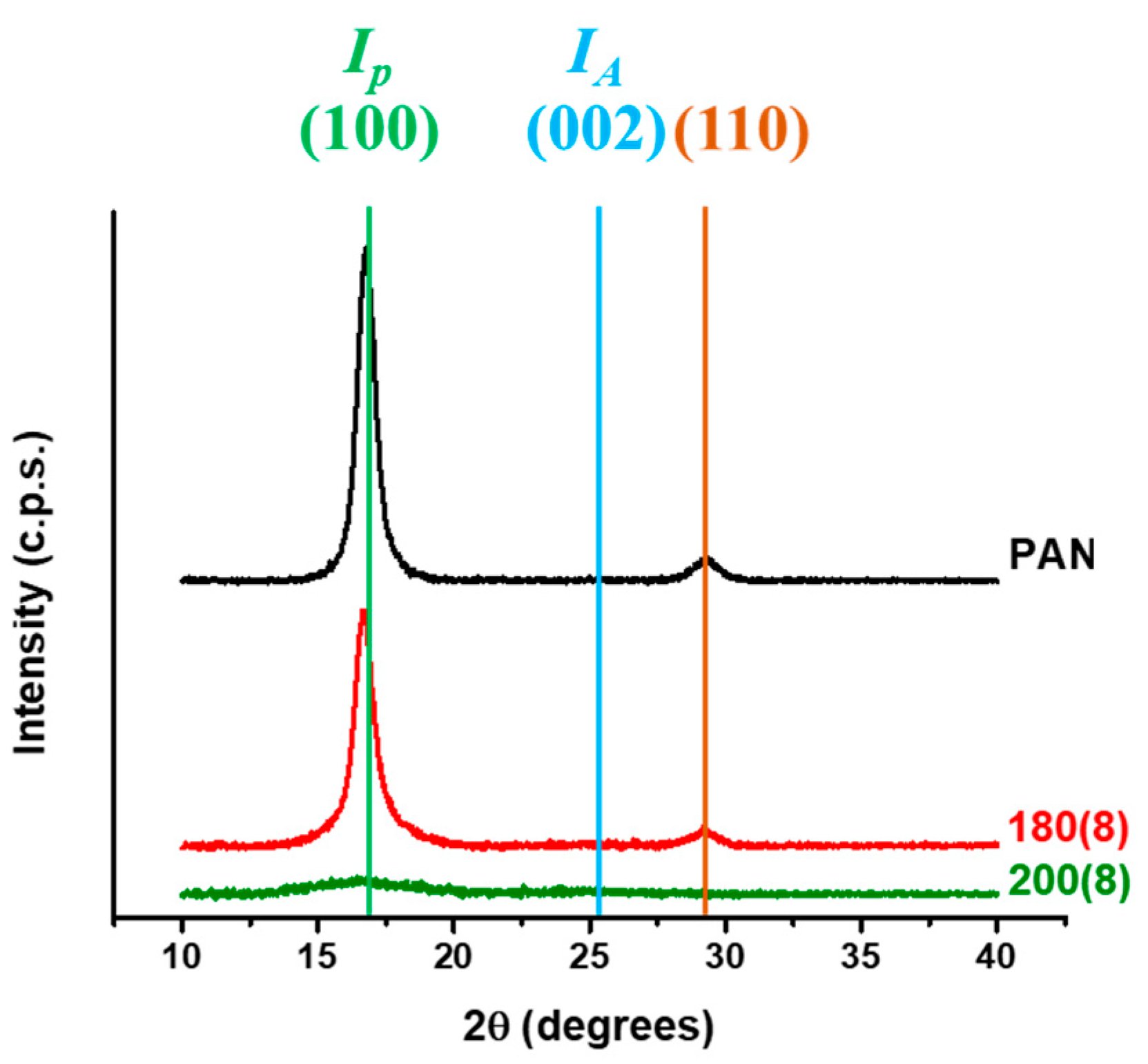


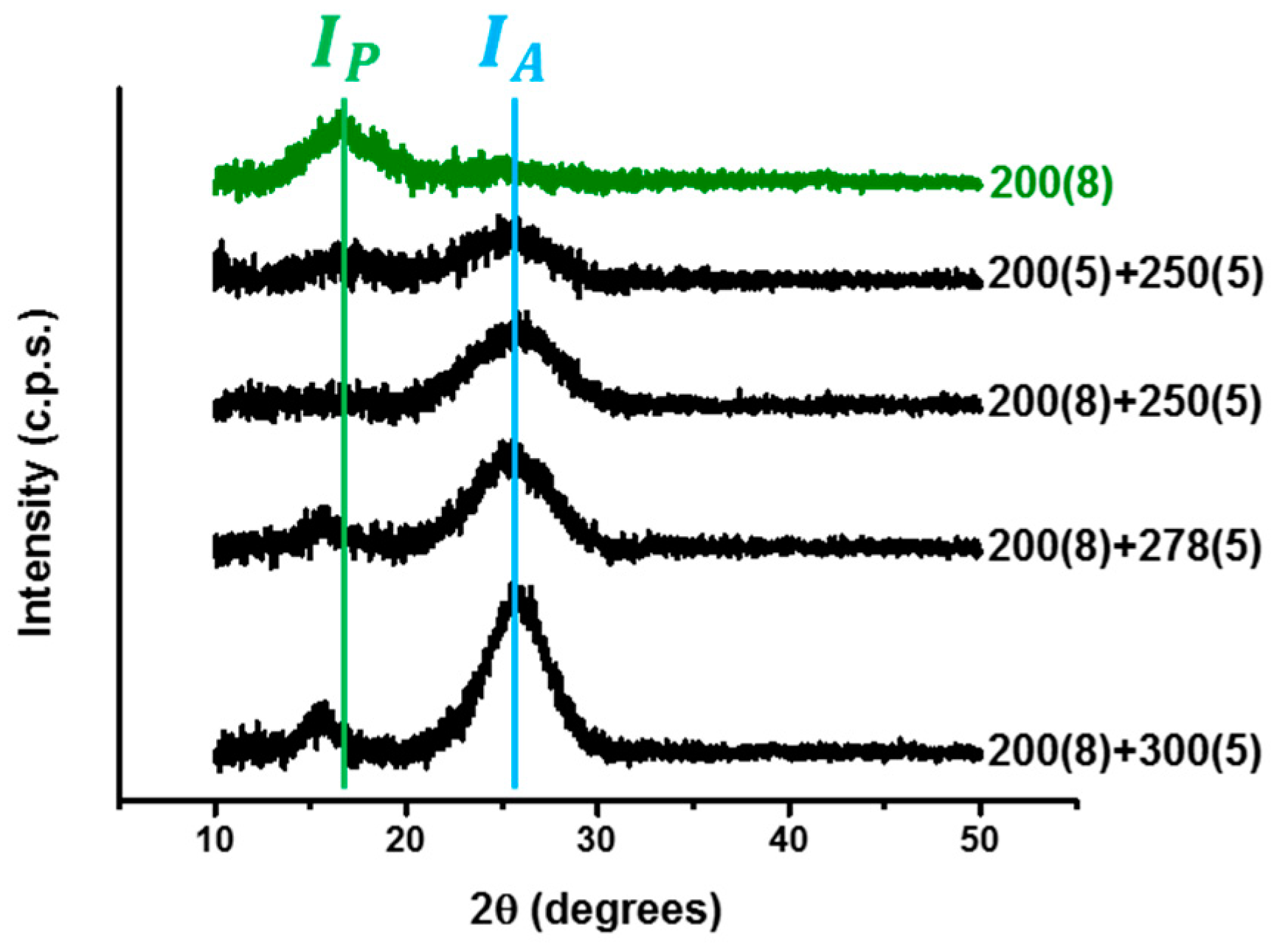

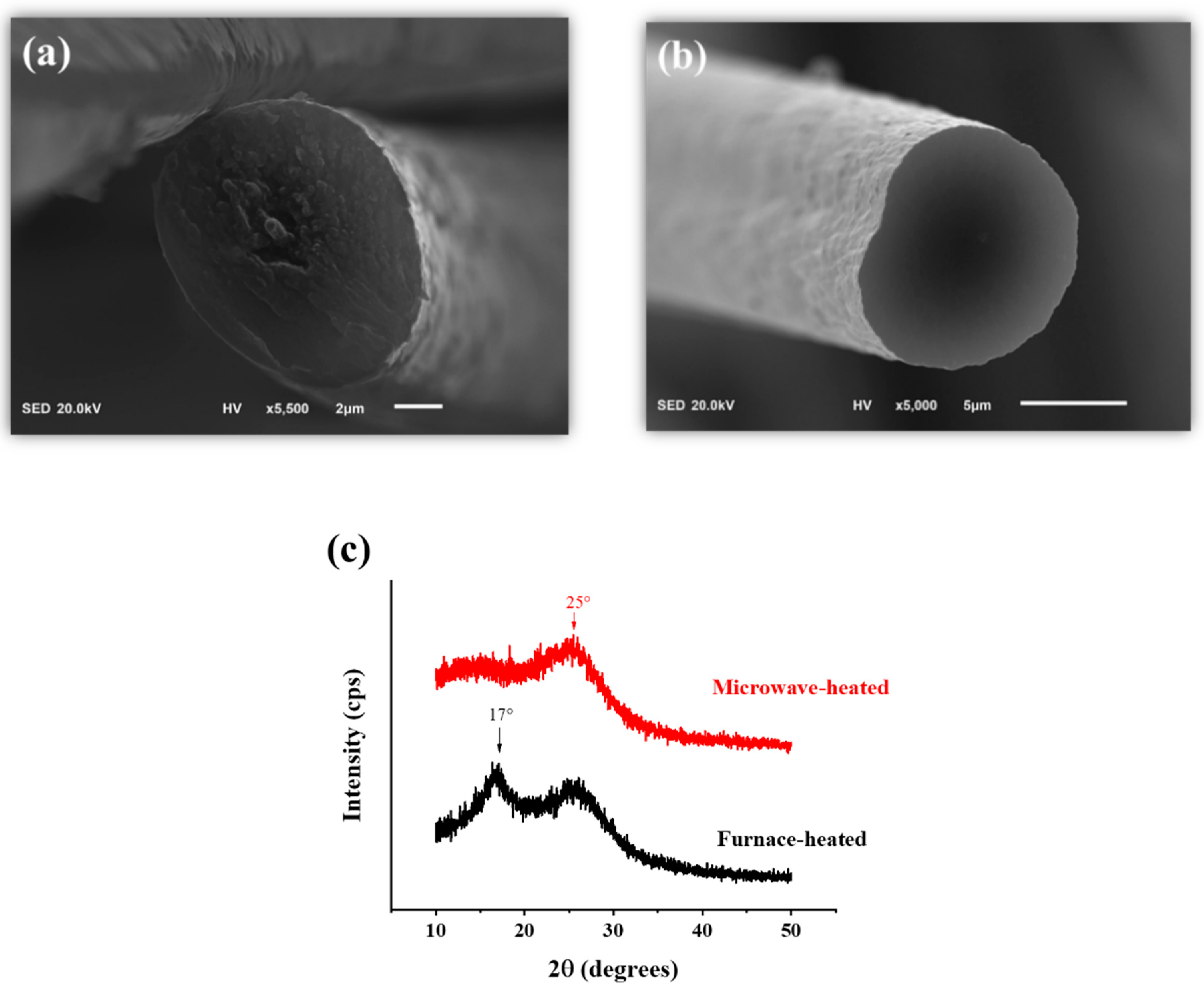
| Specimens | Aromatic Index (%) |
|---|---|
| PAN | - |
| 180(8) | N/A |
| 180(8) + 250(5) | 51.07 |
| 180(8) + 300(5) | 65.45 |
| 200(8) | 25.31 |
| 200(5) + 250(5) | 57.48 |
| 200(8) + 250(5) | 66.39 |
| 200(8) + 278(5) | 70.90 |
| 200(8) + 300(5) | 71.36 |
| Specimens | ΔH(J) | AI(%) |
|---|---|---|
| PAN | 803 | - |
| 200(8) + 230(5) | 440 | 45.3 |
| 200(8) + 240(5) | 319 | 60.3 |
| 200(8) + 250(5) | 136 | 83.1 |
| Specimens | R of Sheath | R of Core | S-C Factor |
|---|---|---|---|
| furnace-heated | 1.3 | 1.15 | 1.13 |
| microwave-heated | 1.24 | 1.33 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-R.; Chao, H.-W.; Hsu, H.-C.; Chan, C.-H.; Lin, W.-H.; Tsai, C.-W.; Chang, T.-H. A Two-Step Microwave Annealing Process for PAN Pre-Oxidation through a TM-Mode Cavity. Polymers 2021, 13, 1476. https://doi.org/10.3390/polym13091476
Chen Y-R, Chao H-W, Hsu H-C, Chan C-H, Lin W-H, Tsai C-W, Chang T-H. A Two-Step Microwave Annealing Process for PAN Pre-Oxidation through a TM-Mode Cavity. Polymers. 2021; 13(9):1476. https://doi.org/10.3390/polym13091476
Chicago/Turabian StyleChen, Yan-Ren, Hsien-Wen Chao, Hung-Chun Hsu, Cheng-Hsuan Chan, Wei-Hsiang Lin, Che-Wei Tsai, and Tsun-Hsu Chang. 2021. "A Two-Step Microwave Annealing Process for PAN Pre-Oxidation through a TM-Mode Cavity" Polymers 13, no. 9: 1476. https://doi.org/10.3390/polym13091476
APA StyleChen, Y.-R., Chao, H.-W., Hsu, H.-C., Chan, C.-H., Lin, W.-H., Tsai, C.-W., & Chang, T.-H. (2021). A Two-Step Microwave Annealing Process for PAN Pre-Oxidation through a TM-Mode Cavity. Polymers, 13(9), 1476. https://doi.org/10.3390/polym13091476







