Effects of Circuit Weight-Interval Training on Physical Fitness, Cardiac Autonomic Control, and Quality of Life in Sedentary Workers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Outcome Measures
2.3. Physical Fitness Evaluations
2.4. Heart Rate Variability Analyses
2.5. Statistical Analysis
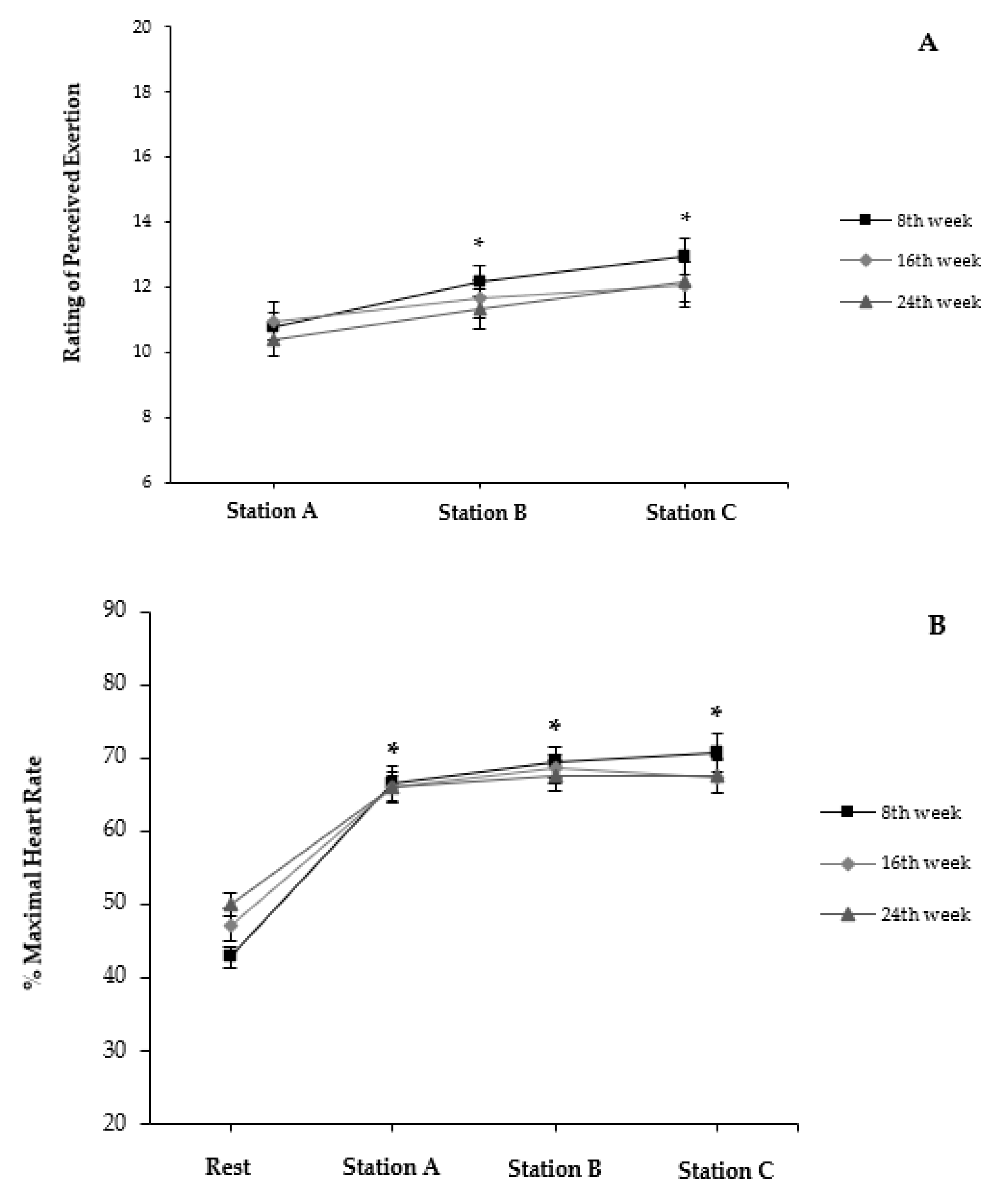
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Owen, N.; Healy, G.N.; Matthews, C.E.; Dunstan, D.W. Too much sitting. Exerc. Sport Sci. Rev. 2010, 38, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.J.; Bauman, A.E.; Owen, N. Stand up, Sit down, keep moving: Turning circles in physical activity research? Br. J. Sports Med. 2008, 43, 86–88. [Google Scholar] [CrossRef]
- Cheema, B.S.; Marshall, P.W.; Chang, D.; Colagiuri, B.; Machliss, B. Effect of an office worksite-based yoga program on heart rate variability: A randomized controlled trial. BMC Public Health 2011, 11, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Després, J.-P. Physical activity, sedentary behaviours, and cardiovascular health: When Will cardiorespiratory fitness become a vital sign? Can. J. Cardiol. 2016, 32, 505–513. [Google Scholar] [CrossRef]
- Blangsted, A.K.; Søgaard, K.; Hansen, E.A.; Hannerz, H.; Sjøgaard, G. One-year randomized controlled trial with different physical-activity programs to reduce musculoskeletal symptoms in the neck and shoulders among office workers. Scand. J. Work. Environ. Health 2008, 34, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Coury, H.J.C.G.; Moreira, R.F.C.; Dias, N.B. Efetividade do exercício físico em ambiente ocupacional para controle da dor cervical, lombar e do ombro: Uma revisão sistemática. Rev. Bras. Fisioter. 2009, 13, 461–479. [Google Scholar] [CrossRef] [Green Version]
- Andersen, L.L.; Saervoll, C.A.; Mortensen, O.S.; Poulsen, O.M.; Hannerz, H.; Zebis, M.K. Effectiveness of small daily amounts of progressive resistance training for frequent neck/shoulder pain: Randomised controlled trial. Pain 2011, 152, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Sihawong, R.; Janwantanakul, P.; Sitthipornvorakul, E.; Pensri, P. Exercise therapy for office workers with nonspecific neck pain: A systematic review. J. Manip. Physiol. 2011, 34, 62–71. [Google Scholar] [CrossRef]
- Heart Rate Variability: Standards of Measurement, Physiological Interpretation and Clinical Use. Task Force of the European society of cardiology and the North American Society of pacing and electrophysiology. Circulation 1996, 93, 1043–1065. [CrossRef] [Green Version]
- Xhyheri, B.; Manfrini, O.; Mazzolini, M.; Pizzi, C.; Bugiardini, R. Heart Rate Variability Today. Prog. Cardiovasc. Dis. 2012, 55, 321–331. [Google Scholar] [CrossRef]
- Tonello, L.; Rodrigues, F.B.; Souza, J.W.S.; Campbell, C.S.G.; Leicht, A.S.; Boullosa, D.A. The role of physical activity and heart rate variability for the control of work related stress. Front. Physiol. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaffalon Júnior, J.R.; Viana, A.O.; de Melo, G.E.L.; De Angelis, K. The impact of sedentarism on Heart Rate Variability (HRV) at rest and in response to mental stress in young women. Physiol. Rep. 2018, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.M.; Vanderlei, F.M.; Valenti, V.E.; Vanderlei, L.C.M. Non-linear regulation of cardiac autonomic modulation in obese youths: Interpolation of ultra-short time series. Cardiol. Young 2019, 29, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Hjortskov, N.; Rissen, D.; Blangsted, A.K.; Fallentin, N.; Lundberg, U.; Søgaard, K. The effect of mental stress on heart rate variability and blood pressure during computer work. Eur. J. Appl. Physiol. 2004, 92, 84–89. [Google Scholar] [CrossRef]
- Järvelin-Pasanen, S.; Sinikallio, S.; Tarvainen, M.P. Heart rate variability and occupational stress—Systematic Review. Ind. Health 2018, 56, 500–511. [Google Scholar] [CrossRef] [Green Version]
- Uusitalo, A.; Mets, T.; Martinmäki, K.; Mauno, S.; Kinnunen, U.; Rusko, H. Heart rate variability related to effort at work. Appl. Erg. 2011, 42, 830–838. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Verheyden, B.; Aubert, A.E.; Fagard, R.H. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J. Hum. Hypertens. 2010, 24, 175–182. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Goetschalckx, K.; Verheyden, B.; Aubert, A.E.; Arnout, J.; Persu, A.; Rademakers, F.; Fagard, R.H. Effect of endurance training on blood pressure regulation, biomarkers and the heart in subjects at a higher age. Scand. J. Med. Sci. Sports 2011, 21, 526–534. [Google Scholar] [CrossRef]
- Alansare, A.; Alford, K.; Lee, S.; Church, T.; Jung, H. The effects of high-intensity interval training vs. moderate-intensity continuous training on heart rate variability in physically inactive adults. Int. J. Environ. Res. Public Health 2018, 15, 1508. [Google Scholar] [CrossRef] [Green Version]
- Besnier, F.; Labrunée, M.; Richard, L.; Faggianelli, F.; Kerros, H.; Soukarié, L.; Bousquet, M.; Garcia, J.-L.; Pathak, A.; Gales, C.; et al. Short-term effects of a 3-week interval training program on heart rate variability in chronic heart failure. A randomised controlled trial. Ann. Phys. Rehabil. Med. 2019, 62, 321–328. [Google Scholar] [CrossRef]
- Kingsley, J.D.; Figueroa, A. Acute and training effects of resistance exercise on heart rate variability. Clin. Physiol. Funct. Imaging 2016, 36, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Flatt, A.A.; Globensky, L.; Bass, E.; Sapp, B.L.; Riemann, B.L. Heart rate variability, neuromuscular and perceptual recovery following resistance training. Sports 2019, 7, 225. [Google Scholar] [CrossRef] [Green Version]
- Proper, K.I.; Staal, B.J.; Hildebrandt, V.H.; van der Beek, A.J.; van Mechelen, W. Effectiveness of physical activity programs at worksites with respect to work-related outcomes. Scand. J. Work. Environ. Health 2002, 28, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Hautala, A.J.; Karjalainen, J.; Kiviniemi, A.M.; Kinnunen, H.; Mäkikallio, T.H.; Huikuri, H.V.; Tulppo, M.P. Physical activity and heart rate variability measured simultaneously during waking hours. Am. J. Physiol. Circ. Physiol. 2010, 298, H874–H880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcão, J.; Sinzato, C.; Massuda, K.; Masunaga, D.; Oliveira Júnior, S.; Christofoletti, G.; Carregaro, R. Impactos físicos e mentais de um programa de exercícios terapêuticos direcionado aos servidores de uma instituição pública de mato grosso do sul. Rev. Bras. Ativ. Física Saúde 2013, 18, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Boullosa, D.; Barros, E.; del Rosso, S.; Nakamura, F.; Leicht, A. Reliability of heart rate measures during walking before and after running maximal efforts. Int. J. Sports Med. 2014, 35, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.; Bertollo, M.; Reichert, F.F.; Boullosa, D.A.; Nakamura, F.Y. Reliability of heart rate variability in children: Influence of Sex and Body Position During Data Collection. Pediatr. Exerc. Sci. 2017, 29, 228–236. [Google Scholar] [CrossRef]
- Rave, G.; Boullosa, D.A.; Saeidi, A. Heart rate variability is correlated with perceived physical fitness in elite soccer players heart rate variability is correlated with perceived physical fitness in elite soccer players. J. Hum. Kinet. 2019, 72, 141–150. [Google Scholar] [CrossRef] [Green Version]
- De Sousa, A.F.M.; Medeiros, A.R.; Benitez-Flores, S.; Del Rosso, S.; Stults-Kolehmainen, M.; Boullosa, D.A. Improvements in attention and cardiac autonomic modulation after a 2-weeks sprint interval training program: A fidelity approach. Front. Physiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Barak, O.F.; Jakowvljevic, D.G.; Gacesa, J.Z.P.; Ovcin, Z.B.; Brodie, D.A.; Grujic, N.G. Heart Rate Variability Before and After Cycle Exercise in relation to Different Body Positions. J. Sports. Sci. Med. 2010, 9, 176–182. [Google Scholar]
- Medeiros, A.R.; Leicht, A.S.; Michael, S.; Boullosa, D. Weekly vagal modulations and their associations with physical fitness and physical activity. Eur. J. Sport Sci. 2020, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, B.L.; Jones, M.T.; Blegen, M.; Matthews, T.D. Acute effects of three different circuit weight training protocols on blood lactate, heart rate, and rating of perceived exertion in recreationally active women. J. Sport. Sci. Med. 2012, 11, 660–668. [Google Scholar]
- Paoli, A.; Paccelli, F.; Bargossi, A.M.; Marcolin, G.; Guzzinati, S.; Neri, M.; Bianco, A.; Palma, A. Effects of three distinct protocols of fitness training on body composition, strength and blood lactate. J. Sports Med. Phys Fit. 2010, 50, 43–51. [Google Scholar]
- Gist, N.H.; Fedewa, M.V.; Dishman, R.K.; Cureton, K.J. Sprint interval training effects on aerobic capacity: A systematic review and meta-analysis. Sports Med. 2014, 44, 269–279. [Google Scholar] [CrossRef]
- Abel, M.G.; Mortara, A.J.; Pettitt, R.W. Evaluation of circuit-training intensity for firefighters. J. Strength Cond. Res. 2011, 25, 2895–2901. [Google Scholar] [CrossRef]
- Masroor, S.; Bhati, P.; Verma, S.; Khan, M.; Hussain, M.E. Heart rate variability following combined aerobic and resistance training in sedentary hypertensive women: A randomised control trial. Indian Heart J. 2018, 70, S28–S35. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S.S.; Alemdaroglu, I.; Karaduman, A.A.; Yilmaz, Ö.T. The effects of physical activity on sleep quality, job satisfaction, and quality of life in office workers. Work 2019, 63, 3–7. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020; p. 103. [Google Scholar]
- Shephard, R.J. PAR-Q, Canadian home fitness test and exercise screening alternatives. Sport. Med. 1988, 5, 185–195. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J. Clin. Epidemiol. 2010, 63, 834–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherkas, A.; Abrahamovych, O.; Golota, S.; Nersesyan, A.; Pichler, C.; Serhiyenko, V.; Knasmüller, S.; Zarkovic, N.; Eckl, P. The correlations of glycated hemoglobin and carbohydrate metabolism parameters with heart rate variability in apparently healthy sedentary young male subjects. Redox Biol. 2015, 5, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Scherr, J.; Wolfarth, B.; Christle, J.W.; Pressler, A.; Wagenpfeil, S.; Halle, M. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur. J. Appl. Physiol. 2013, 113, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Tingelstad, H.C.; Theoret, D.; Spicovck, M.; Haman, F. Explaining performance on military tasks in the Canadian armed forces: The importance of morphological and physical fitness characteristics. Mil. Med. 2016, 181, e1623–e1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, C.L.K.; Gandek, B.; Ren, X.S.; Chan, M.S. Tests of scaling assumptions and construct validity of the Chinese (HK) version of the sf-36 health survey. J. Clin. Epidemiol. 1998, 51, 1139–1147. [Google Scholar] [CrossRef] [Green Version]
- Kline, G.M.; Porcari, J.P.; Hintermeister, R.; Freedson, P.S.; Ward, A.; McCarron, R.F.; Ross, J.; Rippe, J.M. Estimation of VO2max from a one-mile track walk, gender, age, and body weight. Med. Sci. Sports Exerc. 1987, 19, 253–259. [Google Scholar] [CrossRef]
- Faulkner, R.A.; Sprigings, E.J.; McQuarrie, A.; Bell, R.D. A partial curl-up protocol for adults based on an analysis of two procedures. Can. J. Sport Sci. 1989, 14, 135–141. [Google Scholar]
- Vanderburgh, P.M.; Mahar, M.T.; Chou, C.H. Allometric scaling of grip strength by body mass in college-age men and women. Res. Q. Exerc. Sport 1995, 66, 80–84. [Google Scholar] [CrossRef]
- Wells, K.F.; Dillon, E.K. The sit and reach—A test of back and leg flexibility. Res. Q. Am. Assoc. Heal. Phys. Educ. Recreat. 1952, 23, 115–118. [Google Scholar] [CrossRef]
- Giles, D.; Draper, N.; Neil, W. Validity of the polar V800 heart rate monitor to measure RR intervals at rest. Eur. J. Appl. Physiol. 2016, 116, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Quintana, D.S.; Heathers, J.A.J. Considerations in the assessment of heart rate variability in biobehavioral research. Front. Psychol. 2014, 5, 805. [Google Scholar] [CrossRef]
- Lima-Borges, D.S.; Martinez, P.F.; Vanderlei, L.C.M.; Barbosa, F.S.S.; Oliveira-Junior, S.A. Autonomic modulations of heart rate variability are associated with sports injury incidence in sprint swimmers. Phys. Sportsmed. 2018, 46, 374–384. [Google Scholar] [CrossRef]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Edu. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Pollock, M.L.; Gaesser, G.A.; Butcher, J.D.; Després, J.-P.; Dishman, R.K.; Franklin, B.A.; Garber, C.E. ACSM position stand: The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med. Sci. Sport. Exerc. 1998, 30, 975–991. [Google Scholar] [CrossRef]
- Gotshalk, L.A.; Berger, R.A.; Kraemer, W.J. Cardiovascular responses to a high-volume continuous circuit resistance training protocol. J. Strength Cond. Res. 2004, 18, 760. [Google Scholar] [CrossRef] [PubMed]
- Kay, G.N.; Ashar, M.S.; Bubien, R.S.; Dailey, S.M. Relationship between heart rate and oxygen kinetics during constant workload exercise. Pacing Clin. Electrophysiol. 1995, 18, 1853–1860. [Google Scholar] [CrossRef]
- Gearhart, R.E.; Goss, F.L.; Lagally, K.M.; Jakicic, J.M.; Gallagher, J.; Robertson, R.J. Standardized scaling procedures for rating perceived exertion during resistance exercise. J. Strength Cond. Res. 2001, 15, 320–325. [Google Scholar]
- Focht, B.C. Perceived exertion and training load during self-selected and imposed-intensity resistance exercise in untrained women. J. Strength Cond. Res. 2007, 21, 183–187. [Google Scholar] [CrossRef]
- Giannaki, C.D.; Aphamis, G.; Sakkis, P.; Hadjicharalambous, M. Eight weeks of a combination of high intensity interval training and conventional training reduce visceral adiposity and improve physical fitness: A group-based intervention. J. Sports Med. Phys. Fit. 2016, 56, 483–490. [Google Scholar]
- Monks, L.; Seo, M.-W.; Kim, H.-B.; Jung, H.C.; Song, J.K. High-intensity interval training and athletic performance in taekwondo athletes. J. Sports Med. Phys. Fit. 2017, 57, 1252–1260. [Google Scholar] [CrossRef]
- Kozey Keadle, S.; Lyden, K.; Staudenmayer, J.; Hickey, A.; Viskochil, R.; Braun, B.; Freedson, P.S. The independent and combined effects of exercise training and reducing sedentary behavior on cardiometabolic risk factors. Appl. Physiol. Nutr. Metab. 2014, 39, 770–780. [Google Scholar] [CrossRef] [Green Version]
- Burrup, R.; Tucker, L.A.; Cheminant, J.D.L.E.; Bailey, B.W. Strength training and body composition in middle-age women. J. Sports Med. Phys. Fit. 2018, 58, 82–91. [Google Scholar] [CrossRef]
- Saanijoki, T.; Nummenmaa, L.; Eskelinen, J.-J.; Savolainen, A.M.; Vahlberg, T.; Kalliokoski, K.K.; Hannukainen, J.C. Affective responses to repeated sessions of high-intensity interval training. Med. Sci. Sport. Exerc. 2015, 47, 2604–2611. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, K.; McAuliffe, S.; DeBurca, N. The effects of eccentric training on lower limb flexibility: A systematic review. Br. J. Sports Med. 2012, 46, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Herdy, A.H.; Caixeta, A. Brazilian cardiorespiratory fitness classification based on maximum oxygen consumption. Arq. Bras. Cardiol. 2016. [Google Scholar] [CrossRef]
- Shepherd, S.O.; Wilson, O.J.; Taylor, A.S.; Thøgersen-Ntoumani, C.; Adlan, A.M.; Wagenmakers, A.J.M.; Shaw, C.S. Low-volume high-intensity interval training in a gym setting improves cardio-metabolic and psychological health. PLoS ONE 2015, 10, e0139056. [Google Scholar] [CrossRef] [Green Version]
- Vrachimis, A.; Hadjicharalambous, M.; Tyler, C. The effect of circuit training on resting heart rate variability, cardiovascular disease risk factors and physical fitness in healthy untrained adults. Health 2016, 8, 144–155. [Google Scholar] [CrossRef] [Green Version]
- Goldberger, J.J.; Challapalli, S.; Tung, R.; Parker, M.A.; Kadish, A.H. Relationship of Heart rate variability to parasympathetic effect. Circulation 2001, 103, 1977–1983. [Google Scholar] [CrossRef] [Green Version]
- Sandercock, G.R.H.; Bromley, P.D.; Brodie, D.A. The reliability of short-term measurements of heart rate variability. Int. J. Cardiol. 2005, 103, 238–247. [Google Scholar] [CrossRef]
- Tonello, L.; Reichert, F.F.; Oliveira-Silva, I.; Del Rosso, S.; Leicht, A.S.; Boullosa, D.A. Correlates of heart rate measures with incidental physical activity and cardiorespiratory fitness in overweight female workers. Front. Physiol. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Plews, D.J.; Laursen, P.B.; Le Meur, Y.; Hausswirth, C.; Kilding, A.E.; Buchheit, M. Monitoring training with heart-rate variability: How much compliance is needed for valid assessment? Int. J. Sports Physiol. Perform. 2014, 9, 783–790. [Google Scholar] [CrossRef]
- Flatt, A.A.; Esco, M.R. Smartphone-derived heart-rate variability and training load in a women’s soccer team. Int. J. Sports Physiol. Perform. 2015, 10, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Abad, C.; Kobal, R.; Kitamura, K.; Gil, S.; Pereira, L.; Loturco, I.; Nakamura, F. Heart rate variability in elite sprinters: Effects of gender and body position. Clin. Physiol. Funct. Imaging 2017, 37, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Vanderlei, L.C.M.; Pastre, C.M.; Hoshi, R.A.; de Carvalho, T.D.; de Godoy, M.F. Noções básicas de variabilidade da frequência cardíaca e sua aplicabilidade clínica. Rev. Bras. Cir. Cardiovasc. 2009, 24, 205–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sequence | Activity | |||
|---|---|---|---|---|
| 1. | Data collection (rest) (HR, blood pressure and Borg’s 6–20 RPE scale) | |||
| 2. | Warm-up (5 min at 60–70% HRmax in the cycle ergometer) | |||
| 3. Station A | Triceps bench dips * | Sessions 1–8 (1st month) | Sessions 9–16 (2nd month) | Sessions 17–24 (3rd month) |
| Knees flexed (90°) (3 × 8 reps.; relief intervals: 15 s) | Knees slightly flexed (3 × 10 reps.; relief intervals: 15 s) | Knees completely extended (3 × 13 reps.; relief intervals: 15 s) | ||
| Hip lifts * | Feet on the floor (3 × 8 reps.; relief intervals: 15 s) | Feet on the swiss ball (3 × 10 reps.; relief intervals: 15 s) | Feet on the swiss ball (3 × 13 reps.; relief intervals: 15 s) | |
| Prone planks * | 5 × 10–15 s hold (relief intervals: 10 s) | 3 × 20 s hold (relief intervals: 15 s) | 3 × 30 s hold (relief intervals: 15 s) | |
| Cycle ergometer | 55 s (60–70% HRmax); 5 s maximal sprint (no load); 30 s maximal sprint (2.5% body mass) | 55 s (60–70% HRmax); 5 s maximal sprint (no load); 30 s maximal sprint (4% body mass) | 55 s (60–70% HRmax); 5 s maximal sprint (no load); 30 s maximal sprint (5% body mass) | |
| Data collection (HRmax) | ||||
| Cycle ergometer | 3 min easy (no load) intensity cycling (50–70 rpm) | |||
| Data collection (HR and Borg’s 6–20 RPE scale) | ||||
| 4. Station B | Standing biceps curl | Load: 1 kg (3 × 8–10 reps.; intervals: 15 s) | Load: 1 kg (3 × 10–13 reps.; relief intervals: 15 s) | Load: 2 kg (3 × 13 reps.; relief intervals: 15 s) |
| Dumbbell squats | Load: 0–1 kg (3 × 8–10 reps.; relief intervals: 15 s) | Load: 0–1 kg (3 × 10–13 reps.; intervals: 15 s) | Load: 1–2 kg (3 × 13 reps.; relief intervals: 15 s) | |
| Pushups * | Knees on the floor (3 × 8–10 reps.; relief intervals: 15 s) | Knees on the floor (3 × 10–13 reps.; relief intervals: 15 s) | Feet on the floor (3 × 8–10 reps.; relief intervals: 15 s) | |
| Cycle ergometer | 55 s (60–70% HRmax); 5 s maximal sprint (no load); 30 s maximal sprint (2.5% body mass); 3 min easy intensity cycling (50–70 rpm) | 55 s (60–70% HRmax); 5 s maximal sprint (no load); 30 s maximal sprint (4.0% body mass); 3 min easy intensity cycling (50–70 rpm) | 55 s (60–70% HRmax); 5 s maximal sprint (no load); 30 s maximal sprint (5.0% body mass); 3 min easy intensity cycling (50–70 rpm) | |
| Data collection (HRmax) | ||||
| Cycle ergometer | 3 min easy (no load) intensity cycling (50–70 rpm) | |||
| Data collection (HR and Borg’s 6–20 RPE scale) | ||||
| 5. Station C | Standing dumbbell lateral raise | Load: 1 kg (3 × 8–10 reps.; relief intervals: 15 s) | Load: 1 kg (3 × 10–13 reps.; relief intervals: 15 s) | Load: 1 kg (3 × 13 reps.; relief intervals: 15 s) |
| Dumbbell split squat R leg | Load: 1–2 kg (3 × 13 reps.; relief intervals: 15 s) | |||
| Dumbbell split squat L leg | Load: 1–2 kg (3 × 13 reps.; relief intervals: 15 s) | |||
| Standing dumbbell bent-over row | Load: 2 kg (3 × 13 reps.; relief intervals: 15 s) | |||
| Cycle ergometer | 55 s (60–70% HRmax); 5 s maximal sprint (no load); 30 s maximal sprint (2.5% body mass); 3 min easy intensity cycling (50–70 rpm) | 55 s (60–70% HRmax); 5 s maximal sprint (no load); 30 s maximal sprint (4.0% body mass); 3 min easy intensity cycling (50–70 rpm) | 55 s (60–70% HRmax); 5 s maximal sprint (no load); 30 s maximal sprint (5.0% body mass); 3 min easy intensity cycling (50–70 rpm) | |
| Data collection (HRmax) | ||||
| Cycle ergometer | 3 min easy (no load) intensity cycling (50–70 rpm) | |||
| Data collection (HR and Borg’s 6–20 RPE scale) | ||||
| 6. | Cooling down (5 min at an “easy”—no load—intensity in the cycle ergometer) | |||
| 7. | Data collection (rest) (blood pressure, HRmax and mean HR) | |||
| 8. | Active stretching exercises (9 min; 1 rep × 45 s per position), including muscle in sites of trunk, upper limbs, and lower limbs following this sequence. | |||
| Variables | Moment | Group | Effect Size (ηp2) | |
|---|---|---|---|---|
| Control | Experimental | |||
| Body mass (kg) | Initial | 78.9 ± 14.7 | 76.5 ± 15.8 | 0.005 |
| Final | 78.8 ± 15.0 | 76.6 ± 15.8 | 0.005 | |
| ηp2 | 0.001 | 0.001 | ||
| BMI (kg/m²) | Initial | 27.7 ± 3.9 | 27.2 ± 4.4 | 0.003 |
| Final | 27.7 ± 4.1 | 27.2 ± 4.4 | 0.003 | |
| ηp2 | 0.000 | 0.000 | ||
| Arm circumference (cm) | Initial | 33.6 ± 3.9 | 32.8 ± 5.5 | 0.006 |
| Final | 34.2 ± 3.9 | 33.4 ± 4.3 | 0.007 | |
| ηp2 | 0.020 | 0.056 | ||
| Leg circumference (cm) | Initial | 39.4 ± 2.0 | 39.8 ± 3.6 | 0.003 |
| Final | 39.3 ± 1.9 | 39.6 ± 3.5 | 0.002 | |
| ηp2 | 0.005 | 0.021 | ||
| Waist circumference (cm) | Initial | 94.5 ± 11.9 | 88.3 ± 14.3 | 0.047 |
| Final | 93.4 ± 12.7 | 87.6 ± 13.5 | 0.043 | |
| ηp2 | 0.049 | 0.045 | ||
| Hip circumference (cm) | Initial | 104.9 ± 6.4 | 105.0 ± 7.1 | 0.000 |
| Final | 105.4 ± 6.6 | 105.5 ± 7.2 | 0.000 | |
| ηp2 | 0.011 | 0.019 | ||
| Waist-to-hip ratio | Initial | 0.90 ± 0.07 | 0.84 ± 0.10 | 0.087 |
| Final | 0.88 ± 0.07 | 0.83 ± 0.09 | 0.071 | |
| ηp2 | 0.068 | 0.062 | ||
| Variables | Moment | Group | Effect Size (ηp2) | |
|---|---|---|---|---|
| Control | Experimental | |||
| Handgrip strength (kgf) | Initial | 35.7 ± 14.8 | 30.9 ± 11.4 | 0.033 |
| Final | 37.9 ± 14.8 | 32.6 ± 11.6 # | 0.039 | |
| ηp2 | 0.116 | 0.163 | ||
| Sit and reach test (cm) | Initial | 22.9 ± 12.5 | 22.6 ± 11.4 | 0.000 |
| Final | 23.2 ± 11.6 | 25.3 ± 10.1 # | 0.009 | |
| ηp2 | 0.004 | 0.538 | ||
| 1-min curl-ups test (repetitions) | Initial | 15.4 ± 7.9 | 21.5 ± 8.6 | 0.107 |
| Final | 15.5 ± 11.1 | 24.7 ± 8.0 *,# | 0.191 | |
| ηp2 | 0.000 | 0.242 | ||
| VO2max (mL/kg/min) | Initial | 33.92 ± 11.47 | 34.03 ± 5.36 | 0.000 |
| Final | 33.10 ± 11.93 | 36.45 ± 6.05 # | 0.037 | |
| ηp2 | 0.018 | 0.275 | ||
| Time for completion Rockport Fitness Test (min) | Initial | 16.60 ± 1.05 | 15.90 ± 1.34 | 0.064 |
| Final | 16.80 ± 1.30 | 15.37 ± 1.59 * | 0.166 | |
| ηp2 | 0.009 | 0.139 | ||
| Scores | Moment | Groups | Effect Size (ηp2) | |
|---|---|---|---|---|
| Control | Experimental | |||
| Physical functioning | Initial | 69.4 ± 10.1 | 71.6 ± 11.4 | 0.009 |
| Final | 66.9 ± 15.8 | 76.6 ± 8.8 # | 0.144 | |
| ηp2 | 0.025 | 0.196 | ||
| Physical role limitations | Initial | 87.5 ± 35.3 | 76.3 ± 32.8 | 0.024 |
| Final | 84.3 ± 26.5 | 93.4 ± 14.0 # | 0.052 | |
| ηp2 | 0.003 | 0.194 | ||
| Body pain | Initial | 77.9 ± 22.2 | 70.2 ± 18.6 | 0.033 |
| Final | 68.3 ± 28.4 | 77.4 ± 18.2 | 0.039 | |
| ηp2 | 0.053 | 0.068 | ||
| General health perceptions | Initial | 71.1 ± 14.2 | 75.6 ± 14.9 | 0.020 |
| Final | 71.5 ± 21.4 | 81.7 ± 13.3 # | 0.084 | |
| ηp2 | 0.000 | 0.220 | ||
| Vitality | Initial | 64.4 ± 21.4 | 59.7 ± 17.4 | 0.014 |
| Final | 59.4 ± 28.6 | 72.4 ± 13.4 # | 0.096 | |
| ηp2 | 0.024 | 0.276 | ||
| Social functioning | Initial | 78.1 ± 30.4 | 87.5 ± 19.9 | 0.035 |
| Final | 76.5 ± 29.4 | 90.1 ± 11.5 | 0.109 | |
| ηp2 | 0.003 | 0.020 | ||
| Emotional role functioning | Initial | 87.5 ± 35.3 | 82.4 ± 30.1 | 0.006 |
| Final | 75.0 ± 38.8 | 89.5 ± 22.3 | 0.057 | |
| ηp2 | 0.047 | 0.035 | ||
| Mental health | Initial | 73.5 ± 27.9 | 73.9 ± 14.7 | 0.000 |
| Final | 80.5 ± 19.7 | 83.1 ± 10.6 # | 0.008 | |
| ηp2 | 0.054 | 0.193 | ||
| Variables | Moment | Groups | Effect Size (ηp2) | ||
|---|---|---|---|---|---|
| Control | Experimental | ||||
| Time domain | SDNN (ms) | Initial | 35.70 ± 13.07 | 31.74 ± 17.50 | 0.034 |
| Final | 37.04 ± 12.71 | 41.42 ± 22.85 # | 0.000 | ||
| ηp2 | 0.006 | 0.328 | |||
| RMSSD (ms) | Initial | 37.46 ± 19.40 | 36.51 ± 25.64 | 0.010 | |
| Final | 37.45 ± 14.38 | 42.31 ± 23.57 | 0.003 | ||
| ηp2 | 0.002 | 0.199 | |||
| Frequency domain | LF (ms2) | Initial | 602 ± 334 | 358 ± 423 | 0.149 |
| Final | 645 ± 457 | 841 ± 1044 # | 0.004 | ||
| ηp2 | 0.000 | 0.369 | |||
| HF (ms2) | Initial | 655 ± 660 | 629 ± 722 | 0.015 | |
| Final | 585 ± 522 | 945 ± 1090 | 0.018 | ||
| ηp2 | 0.018 | 0.202 | |||
| Total (ms2) | Initial | 1306 ± 798 | 1024 ± 1065 | 0.064 | |
| Final | 1294 ± 939 | 1916 ± 2316 # | 0.003 | ||
| ηp2 | 0.006 | 0.293 | |||
| LF/HF | Initial | 1.38 ± 0.74 | 0.90 ± 0.75 | 0.086 | |
| Final | 1.51 ± 0.83 | 1.09 ± 0.82 | 0.057 | ||
| ηp2 | 0.012 | 0.059 | |||
| Non-Linear | SampEn | Initial | 1.792 ± 0.137 | 1.698 ± 0.277 | 0.032 |
| Final | 1.673 ± 0.212 | 1.629 ± 0.295 | 0.006 | ||
| ηp2 | 0.044 | 0.036 | |||
| DFA Alfa 1 | Initial | 0.988 ± 0.218 | 0.836 ± 0.275 | 0.071 | |
| Final | 0.991 ± 0.100 | 0.948 ± 0.280 | 0.007 | ||
| ηp2 | 0.000 | 0.194 | |||
| Variables | Moment | Groups | Effect Size (ηp2) | ||
|---|---|---|---|---|---|
| Control | Experimental | ||||
| Time domain | SDNN (ms) | Initial | 38.45 ± 12.70 | 31.04 ± 12.12 | 0.086 |
| Final | 36.90 ± 15.53 | 39.43 ± 17.88 # | 0.003 | ||
| ηp2 | 0.018 | 0.348 | |||
| RMSSD (ms) | Initial | 34.56 ± 11.11 | 29.05 ± 12.75 | 0.058 | |
| Final | 29.04 ± 12.37 | 34.30 ± 13.73 # | 0.032 | ||
| ηp2 | 0.088 | 0.147 | |||
| Frequency domain | LF (ms2) | Initial | 907 ± 872 | 486 ± 569 | 0.136 |
| Final | 875 ± 756 | 795 ± 789 # | 0.012 | ||
| ηp2 | 0.001 | 0.403 | |||
| HF (ms2) | Initial | 562 ± 398 | 434 ± 497 | 0.064 | |
| Final | 460 ± 389 | 541 ± 397 | 0.005 | ||
| ηp2 | 0.047 | 0.120 | |||
| Total (ms2) | Initial | 1555 ± 1069 | 995 ± 1066 | 0.117 | |
| Final | 1407 ± 1101 | 1426 ± 1088 # | 0.000 | ||
| ηp2 | 0.016 | 0.343 | |||
| LF/HF | Initial | 1.86 ± 1.36 | 1.52 ± 1.08 | 0.018 | |
| Final | 2.55 ± 2.22 | 1.60 ± 1.10 | 0.082 | ||
| ηp2 | 0.078 | 0.003 | |||
| Non-Linear | SampEn | Initial | 1.628 ± 0.175 | 1.596 ± 0.200 | 0.092 |
| Final | 1.490 ± 0.225 | 1.556 ± 0.266 | 0.020 | ||
| ηp2 | 0.006 | 0.015 | |||
| DFA Alfa 1 | Initial | 1.059 ± 0.186 | 1.007 ± 0.300 | 0.008 | |
| Final | 1.214 ± 0.232 | 1.083 ± 0.296 | 0.047 | ||
| ηp2 | 0.126 | 0.075 | |||
| Variables | Moment | Groups | Effect Size (EF) | ||
|---|---|---|---|---|---|
| Control | Experimental | ||||
| Time domain | SDNN 1 | Initial | 11.6 (−27.0–50.0) | 11.2 (−45.7–100.0) | 0.097 |
| Final | −2.2 (−24.2–24.9) | −1.1 (−16.3–5.9) | 0.056 | ||
| EF | 0.035 | 0.013 | |||
| RMSSD 2 | Initial | −0.23 ± 25.39 | −5.65 ± 31.02 | 0.008 | |
| Final | −22.64 ± 10.65 # | −10.34 ± 29.39 | 0.050 | ||
| EF | 0.162 | 0.020 | |||
| Frequency domain | LF 1 | Initial | 46.3 (−48.5–189.2) | 29.3 (−62.9–423.5) | 0.046 |
| Final | 55.8 (−46.1–207.4) | 29.4 (−54.4–166.4) | 0.066 | ||
| EF | 0.035 | 0.966 | |||
| HF 2 | Initial | 19.2 ± 85.7 | 4.7 ± 66.4 | 0.009 | |
| Final | 25.8 ± 144.4 | 5.2 ± 74.8 | 0.010 | ||
| EF | 0.003 | 0.000 | |||
| Total 2 | Initial | 31.9 ± 64.0 | 34.5 ± 82.7 | 0.000 | |
| Final | 30.7 ± 67.4 | 21.6 ± 67.1 | 0.004 | ||
| EF | 0.000 | 0.021 | |||
| LF/HF 1 | Initial | 59 (−44–196) | 50 (−77–1445) | 0.148 | |
| Final | 56 (−63–548) | 49 (−20–1758) | 0.005 | ||
| EF | 0.035 | 0.013 | |||
| Non-Linear | SampEn 1 | Initial | −9.2 (−20.8–−2.5) | −8.3 (−23.4–72.6) | 0.105 |
| Final | −13.4 (−29.1–35.5) | −2.4 (−22.3–17.2) | 0.065 | ||
| EF | 0.128 | 0.250 | |||
| DFA Alfa 1 1 | Initial | 4.3 (−24.4–49.6) | 13.0 (−17.1–245.2) | 0.169 | |
| Final | 15.8 (−1.9–55.1) | 10.8 (−4.0–44.2) | 0.179 | ||
| EF | 0.385 | 0.104 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira-Junior, S.A.; Boullosa, D.; Mendonça, M.L.M.; Vieira, L.F.C.; Mattos, W.W.; Amaral, B.O.C.; Lima-Borges, D.S.; Reis, F.A.; Cezar, M.D.M.; Vanderlei, L.C.M.; et al. Effects of Circuit Weight-Interval Training on Physical Fitness, Cardiac Autonomic Control, and Quality of Life in Sedentary Workers. Int. J. Environ. Res. Public Health 2021, 18, 4606. https://doi.org/10.3390/ijerph18094606
Oliveira-Junior SA, Boullosa D, Mendonça MLM, Vieira LFC, Mattos WW, Amaral BOC, Lima-Borges DS, Reis FA, Cezar MDM, Vanderlei LCM, et al. Effects of Circuit Weight-Interval Training on Physical Fitness, Cardiac Autonomic Control, and Quality of Life in Sedentary Workers. International Journal of Environmental Research and Public Health. 2021; 18(9):4606. https://doi.org/10.3390/ijerph18094606
Chicago/Turabian StyleOliveira-Junior, Silvio A., Daniel Boullosa, Maria L. M. Mendonça, Larissa F. C. Vieira, Wania W. Mattos, Bruna O. C. Amaral, Dayanne S. Lima-Borges, Filipe A. Reis, Marcelo D. M. Cezar, Luiz C. M. Vanderlei, and et al. 2021. "Effects of Circuit Weight-Interval Training on Physical Fitness, Cardiac Autonomic Control, and Quality of Life in Sedentary Workers" International Journal of Environmental Research and Public Health 18, no. 9: 4606. https://doi.org/10.3390/ijerph18094606







