Expiration Date of Ready-to-Eat Salads: Effects on Microbial Load and Biochemical Attributes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Microbiological Analyses
2.2.1. Isolation and Identification of Salmonella spp. and Listeria spp.
2.2.2. Molecular Confirmation of Salmonella enterica and Listeria monocytogenes
2.3. Carbon Dioxide Production
2.4. Polyphenol Content and Antioxidant Activity of Ready-to-Eat Salads
2.5. Damage Index
2.6. Statistical Analysis
3. Results
3.1. Effects of Season
3.1.1. Microbiological Analysis
Salad Producer
Type of Salad
3.1.2. Total Phenols Content, Antioxidants, CO2, H2O2 and Lipid Peroxidation
Salad Producer
Type of Salad
3.2. Effects of Shelf Life
3.2.1. Microbiological Analysis
Salad Producer
Type of Salad
3.2.2. Total Phenolic Content, Antioxidants, CO2, H2O2 and Lipid Peroxidation
Salad Producer
Type of Salad
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cömert, E.D.; Mogol, B.A.; Gökmen, V. Relationship between color and antioxidant capacity of fruits and vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). A Brief History of USDA Food Guides. Available online: https://www.choosemyplate.gov/eathealthy/brief-history-usda-food-guides (accessed on 28 June 2020).
- EFSA. Scientific Opinion on establishing Food-Based Dietary Guidelines. EFSA J. 2010, 8, 1–42. [Google Scholar]
- Balali, G.I.; Yar, D.; Dela, V.G.A.; Adjei-Kusi, P. Microbial Contamination, an Increasing Threat to the Consumption of Fresh Fruits and Vegetables in Today’s World. Int. J. Microbiol. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate Outbreaks of Foodborne Illness in the United States Associated with Fresh Produce From 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriquez, T.; Lenzi, A.; Baldi, A.; Marvasi, M. Frontiers in Plant Breeding: Perspectives for the Selection of Vegetables Less Susceptible to Enteric Pathogens. Front. Microbiol. 2020, 11, 1087. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Iwu, C.D.; Okoh, A.I. Preharvest Transmission Routes of Fresh Produce Associated Bacterial Pathogens with Outbreak Potentials: A Review. Int. J. Environ. Res. Public Health 2019, 16, 4407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xylia, P.; Clark, A.; Chrysargyris, A.; Romanazzi, G.; Tzortzakis, N. Quality and safety attributes on shredded carrots by using Origanum majorana and ascorbic acid. Postharvest Biol. Technol. 2019, 155, 120–129. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the risk posed by pathogens in food of non-animal origin. Part 1 (outbreak data analysis and risk ranking of food/pathogen combinations). EFSA J. 2013, 11, 1–138. [Google Scholar]
- Soon, J.M.; Manning, L.; Davies, W.P.; Baines, R. Fresh produce-associated outbreaks: A call for HACCP on farms? Br. Food J. 2012, 114, 553–597. [Google Scholar] [CrossRef]
- Uyttendaele, M.; Jaykus, L.-A.; Amoah, P.; Chiodini, A.; Cunliffe, D.; Jacxsens, L.; Holvoet, K.; Korsten, L.; Lau, M.; McClure, P.; et al. Microbial Hazards in Irrigation Water: Standards, Norms, and Testing to Manage Use of Water in Fresh Produce Primary Production. Compr. Rev. Food Sci. Food Saf. 2015, 14, 336–356. [Google Scholar] [CrossRef]
- Gorny, J.R.; Giclas, H.; Gombas, D.; Means, K. Commodity Specific Food Safety Guidelines for the Lettuce and Leafy Greens Supply Chain; US Food and Drug Administration: Rockwell, MD, USA, 2006; p. 45.
- Martínez-Vaz, B.M.; Fink, R.C.; Diez-Gonzalez, F.; Sadowsky, M.J. Enteric Pathogen-Plant Interactions: Molecular Connections Leading to Colonization and Growth and Implications for Food Safety. Microbes Environ. 2014, 29, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Kharousi, Z.S.; Guizani, N.; Al-Sadi, A.M.; Al-Bulushi, I.M.; Shaharoona, B. Hiding in Fresh Fruits and Vegetables: Opportunistic Pathogens May Cross Geographical Barriers. Int. J. Microbiol. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO/WHO. Microbiological Hazards in Fresh Leafy Vegetables and Herbs; Meeting report; Microbiological Risk Assessment Series No. 14; Nutrition and Consumer Protection Division Food and Agriculture Organization of the United Nations: Rome, Italy, 2008. [Google Scholar]
- Dankwa, A.; Machado, R.; Perry, J. Sources of food contamination in a closed hydroponic system. Lett. Appl. Microbiol. 2020, 70, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Yeni, F.; Yavaş, S.; Alpas, H.; Soyer, Y. Most Common Foodborne Pathogens and Mycotoxins on Fresh Produce: A Review of Recent Outbreaks. Crit. Rev. Food Sci. Nutr. 2016, 56, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Brandl, M.T.; Cox, C.E.; Teplitski, M. Salmonella Interactions with Plants and Their Associated Microbiota. Phytopathology 2013, 103, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Cox, C.E.; Brandl, M.T.; de Moraes, M.H.; Gunasekera, S.; Teplitski, M. Production of the plant hormone auxin by Salmonella and its role in the interactions with plants and animals. Front. Microbiol. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- CDC Foodborne Outbreaks. Available online: https://www.cdc.gov/foodsafety/outbreaks/index.html (accessed on 11 November 2020).
- Hernández-Reyes, C.; Schikora, A. Salmonella, a cross-kingdom pathogen infecting humans and plants. FEMS Microbiol. Lett. 2013, 343, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bell, L.; Yahya, H.N.; Oloyede, O.O.; Methven, L.; Wagstaff, C. Changes in rocket salad phytochemicals within the commercial supply chain: Glucosinolates, isothiocyanates, amino acids and bacterial load increase significantly after processing. Food Chem. 2017, 221, 521–534. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.; García-Viguera, C. Health-Promoting Compounds in Broccoli as Influenced by Refrigerated Transport and Retail Sale Period. J. Agric. Food Chem. 2003, 51, 3029–3034. [Google Scholar] [CrossRef]
- McDowell, D.; Maloney, M.; Swan, L.; Erwin, P. A Review of the Fruit and Vegetable Food Chain; Safefood: Cork, Ireland, 2007; pp. 1–102. [Google Scholar]
- Caponigro, V.; Ventura, M.; Chiancone, I.; Amato, L.; Parente, E.; Piro, F. Variation of microbial load and visual quality of ready-to-eat salads by vegetable type, season, processor and retailer. Food Microbiol. 2010, 27, 1071–1077. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Cocetta, G.; Bulgari, R.; Spinardi, A.; Ferrante, A. Identification of innovative potential quality markers in rocket and melon fresh-cut produce. Food Chem. 2015, 188, 225–233. [Google Scholar] [CrossRef]
- Nousiainen, L.-L.; Joutsen, S.; Lunden, J.; Hänninen, M.-L.; Fredriksson-Ahomaa, M. Bacterial quality and safety of packaged fresh leafy vegetables at the retail level in Finland. Int. J. Food Microbiol. 2016, 232, 73–79. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Botsaris, G.; Tzortzakis, N.; Tzortzakis, N. Potential application of spearmint and lavender essential oils for assuring endive quality and safety. Crop. Prot. 2017, 102, 94–103. [Google Scholar] [CrossRef]
- Xylia, P.; Botsaris, G.; Chrysargyris, A.; Skandamis, P.; Tzortzakis, N. Variation of microbial load and biochemical activity of ready-to-eat salads in Cyprus as affected by vegetable type, season, and producer. Food Microbiol. 2019, 83, 200–210. [Google Scholar] [CrossRef]
- ISO 6579. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection of Salmonella spp; International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- ISO 11290-1. Modification of the Isolation Media and the Haemolysis Test, and Inclusion of Precision Data ISO 11290-1: 1996/Amd 1: 2004; International Organization for Standardization: Geneva, Switzerland, 2004. [Google Scholar]
- Rossmanith, P.; Krassnig, M.; Wagner, M.; Hein, I. Detection of Listeria monocytogenes in food using a combined enrichment/real-time PCR method targeting the prfA gene. Res. Microbiol. 2006, 157, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Tzortzakis, N.G.; Tzanakaki, K.; Economakis, C.D. Effect of Origanum Oil and Vinegar on the Maintenance of Postharvest Quality of Tomato. Food Nutr. Sci. 2011, 2, 974–982. [Google Scholar] [CrossRef] [Green Version]
- Chrysargyris, A.; Panayiotou, C.; Tzortzakis, N. Nitrogen and phosphorus levels affected plant growth, essential oil composition and antioxidant status of lavender plant (Lavandula angustifolia Mill.). Ind. Crop. Prod. 2016, 83, 577–586. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene Produced by Leaves Protects the Photosynthetic Apparatus against Ozone Damage, Quenches Ozone Products, and Reduces Lipid Peroxidation of Cellular Membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Neto, A.D.D.A.; Prisco, J.T.; Enéas-Filho, J.; De Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Santos, M.; Cavaco, A.; Gouveia, J.; Novais, M.; Nogueira, P.; Pedroso, L.; Ferreira, M. Evaluation of minimally processed salads commercialized in Portugal. Food Control. 2012, 23, 275–281. [Google Scholar] [CrossRef]
- Pothakos, V.; Snauwaert, C.; De Vos, P.; Huys, G.; Devlieghere, F. Monitoring psychrotrophic lactic acid bacteria contamination in a ready-to-eat vegetable salad production environment. Int. J. Food Microbiol. 2014, 185, 7–16. [Google Scholar] [CrossRef] [PubMed]
- De Corato, U. Fungal Population Dynamics in Ready-to-eat Salads during a Shelf-life in Italy. J. Agric. Sci. Technol. 2012, 2, 569–576. [Google Scholar]
- Kang, H.-M.; Saltveit, M.E. Antioxidant Capacity of Lettuce Leaf Tissue Increases after Wounding. J. Agric. Food Chem. 2002, 50, 7536–7541. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.; Martinetti, L.; Maggiore, T. Biochemical changes in cut vs. intact lamb’s lettuce (Valerianella olitoria) leaves during storage. Int. J. Food Sci. Technol. 2009, 44, 1050–1056. [Google Scholar] [CrossRef]
- Amodio, M.; DeRossi, A.; Mastrandrea, L.; Colelli, G. A study of the estimated shelf life of fresh rocket using a non-linear model. J. Food Eng. 2015, 150, 19–28. [Google Scholar] [CrossRef]
- Fröder, H.; Martins, C.G.; De Souza, K.L.O.; Landgraf, M.; Franco, B.D.G.M.; Destro, M.T. Minimally Processed Vegetable Salads: Microbial Quality Evaluation. J. Food Prot. 2007, 70, 1277–1280. [Google Scholar] [CrossRef]
- De Giusti, M.; Solimini, A.G.; Cottarelli, A.; De Vito, C.; Aurigemma, C.; Tufi, D.; Piccinato, L.; Boccia, A.; Marinelli, L. Temporal pattern of microbial indicators of ready-to-eat rocket salads during shelf life. Ann. dell’Istituto Super. Sanità 2014, 50, 90–95. [Google Scholar]
- Deza-Durand, K.M.; Petersen, M.A. The effect of cutting direction on aroma compounds and respiration rate of fresh-cut iceberg lettuce (Lactuca sativa L.). Postharvest Biol. Technol. 2011, 61, 83–90. [Google Scholar] [CrossRef]
- Edelenbos, M.; Løkke, M.M.; Seefeldt, H.F. Seasonal variation in color and texture of packaged wild rocket (Diplotaxis tenuifolia L.). Food Packag. Shelf Life 2017, 14, 46–51. [Google Scholar] [CrossRef]
- Hunter, P.J.; Atkinson, L.D.; Vickers, L.; Lignou, S.; Oruna-Concha, M.J.; Pink, D.; Hand, P.; Barker, G.; Wagstaff, C.; Monaghan, J.M. Oxidative discolouration in whole-head and cut lettuce: Biochemical and environmental influences on a complex phenotype and potential breeding strategies to improve shelf-life. Euphytica 2017, 213, 180. [Google Scholar] [CrossRef] [Green Version]
- Sant’Ana, A.S.; Barbosa, M.S.; Destro, M.T.; Landgraf, M.; Franco, B.D.G.M. Growth potential of Salmonella spp. and Listeria monocytogenes in nine types of ready-to-eat vegetables stored at variable temperature conditions during shelf-life. Int. J. Food Microbiol. 2012, 157, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.S.; Bouletis, A.D.; Papa, E.A.; Gkagtzis, D.C.; Hadjichristodoulou, C.; Papaloucas, C. Microbial and sensory quality of “Lollo verde” lettuce and rocket salad stored under active atmosphere packaging. Anaerobe 2011, 17, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Viñas, I. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int. J. Food Microbiol. 2008, 123, 121–129. [Google Scholar] [CrossRef]
- Preti, R.; Vinci, G. Nutritional and sensory evaluation of ready-to-eat salads during shelf life. Agro Food Ind. Hi. Tech. 2016, 27, 26–31. [Google Scholar]
 ) and summer (
) and summer (  ) among salad producers/packagers (A–E). Results include all samples for each microorganism tested and are the mean value ± standard deviation. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. *, ** and *** indicate significant differences at p ≤ 5%, 1% and 0.1%.
) among salad producers/packagers (A–E). Results include all samples for each microorganism tested and are the mean value ± standard deviation. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. *, ** and *** indicate significant differences at p ≤ 5%, 1% and 0.1%.
 ) and summer (
) and summer (  ) among salad producers/packagers (A–E). Results include all samples for each microorganism tested and are the mean value ± standard deviation. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. *, ** and *** indicate significant differences at p ≤ 5%, 1% and 0.1%.
) among salad producers/packagers (A–E). Results include all samples for each microorganism tested and are the mean value ± standard deviation. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. *, ** and *** indicate significant differences at p ≤ 5%, 1% and 0.1%.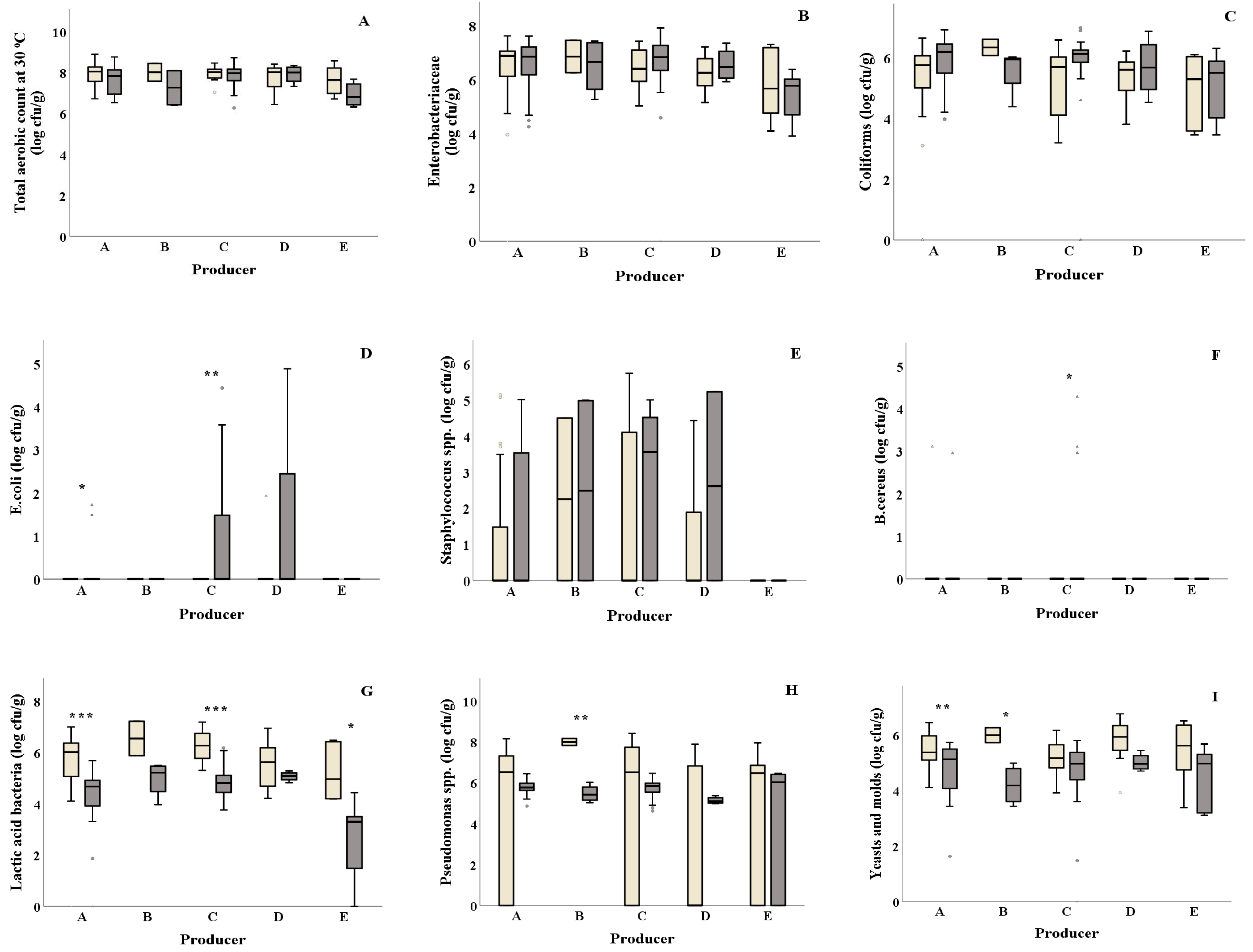
 ) and summer (
) and summer (  ). Results include only positive samples for each microorganism tested and are the mean value ± standard deviation. Other = lettuce + 2 or more ingredients. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. *, ** and *** indicate significant differences at p ≤ 5%, 1% and 0.1%.
). Results include only positive samples for each microorganism tested and are the mean value ± standard deviation. Other = lettuce + 2 or more ingredients. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. *, ** and *** indicate significant differences at p ≤ 5%, 1% and 0.1%.
 ) and summer (
) and summer (  ). Results include only positive samples for each microorganism tested and are the mean value ± standard deviation. Other = lettuce + 2 or more ingredients. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. *, ** and *** indicate significant differences at p ≤ 5%, 1% and 0.1%.
). Results include only positive samples for each microorganism tested and are the mean value ± standard deviation. Other = lettuce + 2 or more ingredients. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. *, ** and *** indicate significant differences at p ≤ 5%, 1% and 0.1%.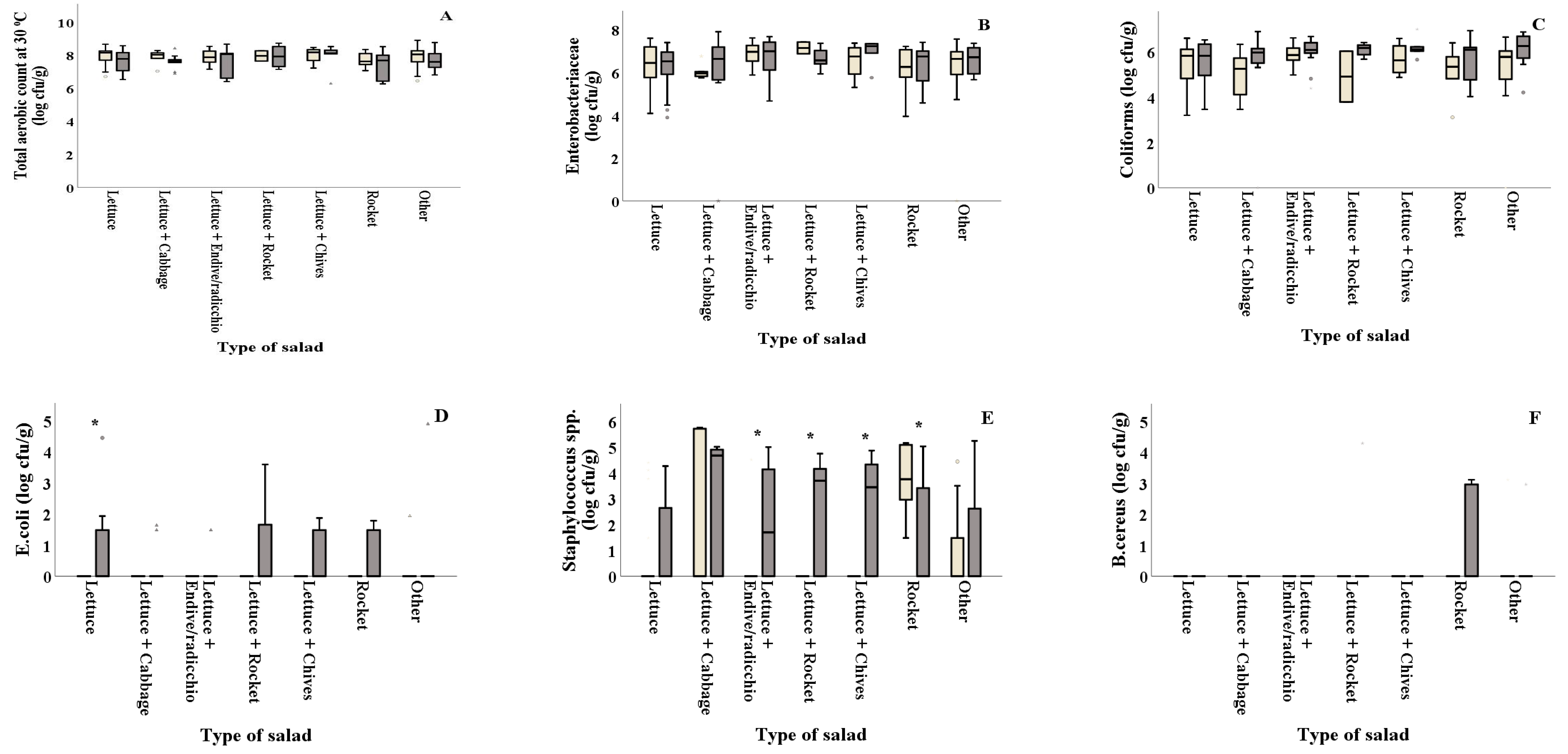
 ) and summer (
) and summer (  ) among salad producers/packagers (A, B, C, D, and E). Results include all samples for each microorganism tested and are the mean value ± standard deviation. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. * and *** indicate significant differences at p ≤ 5% and 0.1%.
) among salad producers/packagers (A, B, C, D, and E). Results include all samples for each microorganism tested and are the mean value ± standard deviation. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. * and *** indicate significant differences at p ≤ 5% and 0.1%.
 ) and summer (
) and summer (  ) among salad producers/packagers (A, B, C, D, and E). Results include all samples for each microorganism tested and are the mean value ± standard deviation. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. * and *** indicate significant differences at p ≤ 5% and 0.1%.
) among salad producers/packagers (A, B, C, D, and E). Results include all samples for each microorganism tested and are the mean value ± standard deviation. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. * and *** indicate significant differences at p ≤ 5% and 0.1%.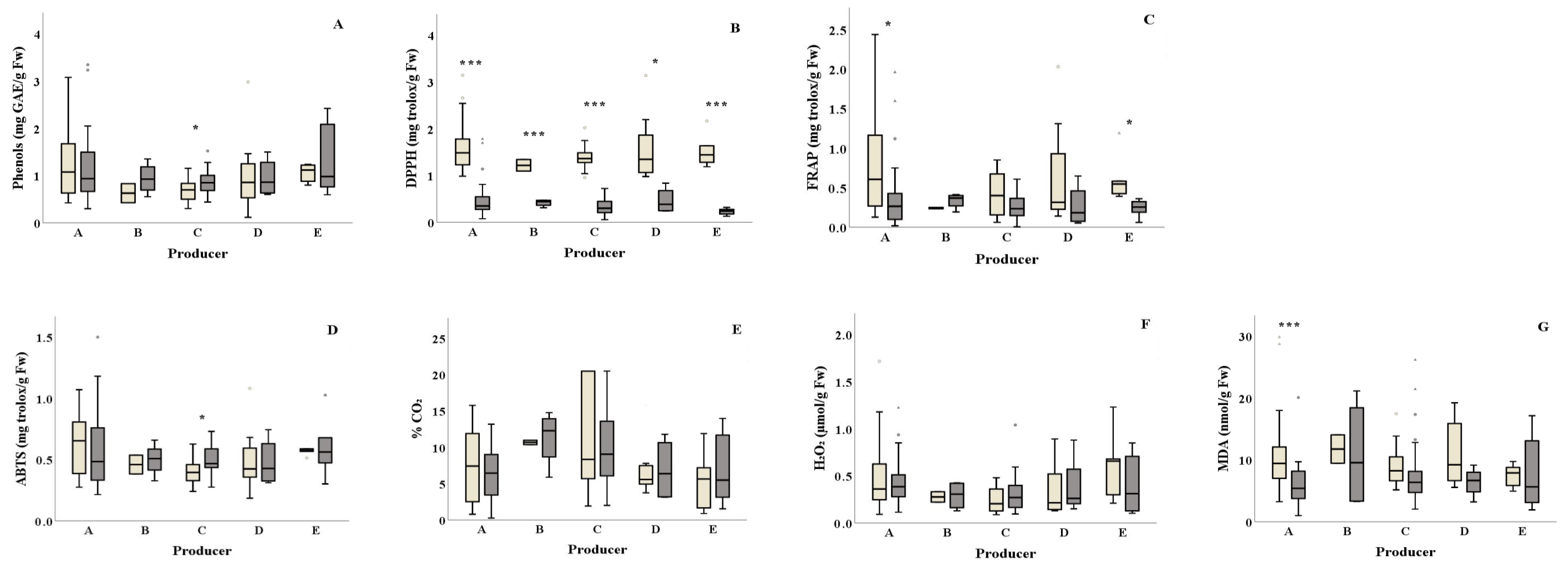
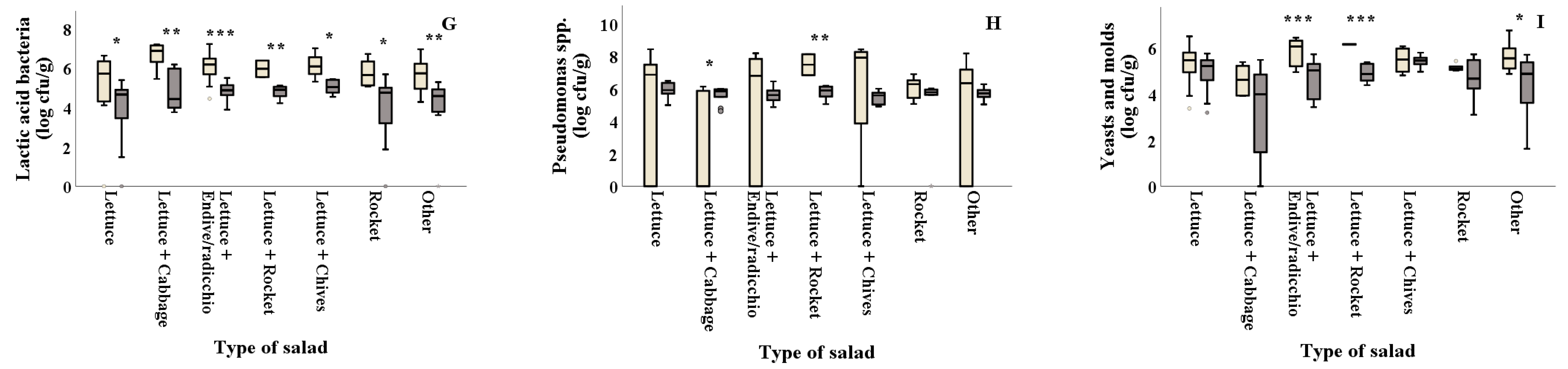
 ) and summer (
) and summer (  ) among types of salads. Results include all samples for each microorganism tested and are the mean value ± standard deviation. Other = lettuce + 2 or more ingredients. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. *, ** and *** indicate significant differences at p ≤ 5%, 1% and 0.1%.
) among types of salads. Results include all samples for each microorganism tested and are the mean value ± standard deviation. Other = lettuce + 2 or more ingredients. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. *, ** and *** indicate significant differences at p ≤ 5%, 1% and 0.1%.
 ) and summer (
) and summer (  ) among types of salads. Results include all samples for each microorganism tested and are the mean value ± standard deviation. Other = lettuce + 2 or more ingredients. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. *, ** and *** indicate significant differences at p ≤ 5%, 1% and 0.1%.
) among types of salads. Results include all samples for each microorganism tested and are the mean value ± standard deviation. Other = lettuce + 2 or more ingredients. Each box contains 50 percent of cases, and whiskers represent the rest. The line across the inside of the box represents the median value. *, ** and *** indicate significant differences at p ≤ 5%, 1% and 0.1%.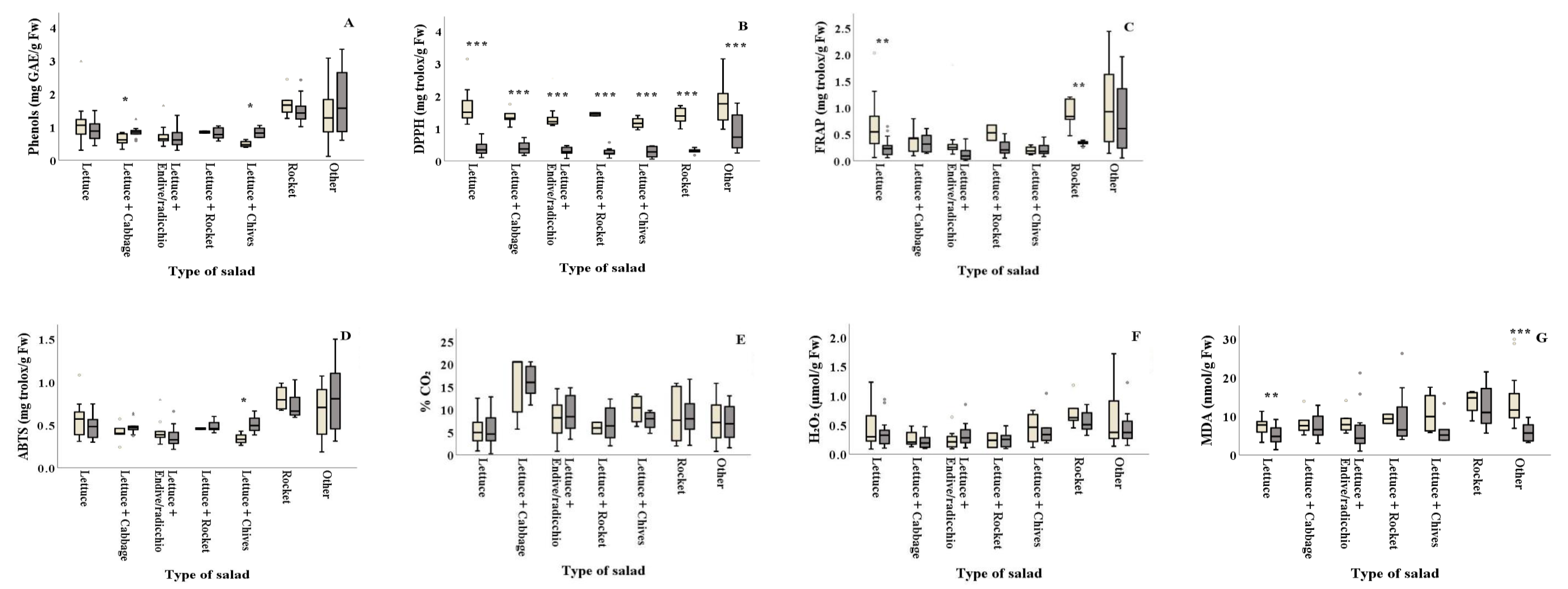
| Producer/Packager | |||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| TVC | 0.081 | 0.322 | 0.470 | 0.662 | 0.080 |
| Enterobacteriaceae | 0.750 | 0.706 | 0.858 | 0.492 | 0.608 |
| Coliforms | 0.105 | 0.280 | 0.080 | 0.527 | 0.829 |
| E. coli | 0.022 | ni | 0.001 | 0.485 | ni |
| Staphylococcus spp. | 0.399 | 0.937 | 0.114 | 0.285 | ni |
| B. cereus | 0.717 | ni | 0.047 | ni | ni |
| Lactic acid bacteria | 0.000 | 0.079 | 0.000 | 0.225 | 0.010 |
| Pseudomonas spp. | 0.100 | 0.002 | 0.568 | 0.107 | 0.817 |
| Yeasts and molds | 0.002 | 0.033 | 0.171 | 0.147 | 0.236 |
| Phenols | 0.786 | 0.327 | 0.040 | 0.853 | 0.479 |
| DPPH | 0.000 | 0.001 | 0.000 | 0.017 | 0.000 |
| FRAP | 0.010 | 0.277 | 0.093 | 0.324 | 0.015 |
| ABTS | 0.662 | 0.734 | 0.020 | 0.880 | 0.837 |
| CO2 | 0.365 | 0.837 | 0.690 | 0.992 | 0.605 |
| H2O2 | 0.708 | 0.877 | 0.297 | 0.838 | 0.284 |
| MDA | 0.001 | 0.871 | 0.436 | 0.139 | 0.930 |
| Type of Salad | |||||||
|---|---|---|---|---|---|---|---|
| Lettuce | Lettuce + Cabbage | Lettuce + Endive/Radicchio | Lettuce + Rocket | Lettuce + Chives | Rocket | Other | |
| TVC | 0.131 | 0.244 | 0.373 | 0.949 | 0.827 | 0.343 | 0.511 |
| Enterobacteriaceae | 0.605 | 0.941 | 0.599 | 0.221 | 0.391 | 0.692 | 0.480 |
| Coliforms | 0.705 | 0.681 | 0.430 | 0.480 | 0.210 | 0.344 | 0.147 |
| E. coli | 0.044 | 0.168 | 0.336 | 0.408 | 0.178 | 0.082 | 0.432 |
| Staphylococcus spp. | 0.465 | 0.483 | 0.016 | 0.012 | 0.028 | 0.032 | 0.589 |
| B. cereus | ni | ni | ni | 0.645 | ni | 0.081 | 0.530 |
| Lactic acid bacteria | 0.029 | 0.001 | 0.000 | 0.004 | 0.011 | 0.037 | 0.001 |
| Pseudomonas spp. | 0.291 | 0.035 | 0.656 | 0.003 | 0.793 | 0.201 | 0.078 |
| Yeasts and molds | 0.254 | 0.092 | 0.000 | 0.000 | 0.887 | 0.089 | 0.046 |
| Phenols | 0.279 | 0.026 | 0.662 | 0.760 | 0.005 | 0.389 | 0.376 |
| DPPH | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
| FRAP | 0.002 | 0.510 | 0.085 | 0.060 | 0.777 | 0.004 | 0.396 |
| ABTS | 0.230 | 0.177 | 0.353 | 0.589 | 0.021 | 0.184 | 0.320 |
| CO2 | 0.771 | 0.989 | 0.464 | 0.745 | 0.179 | 0.955 | 0.897 |
| H2O2 | 0.531 | 0.470 | 0.228 | 0.933 | 0.939 | 0.171 | 0.647 |
| MDA | 0.002 | 0.531 | 0.315 | 0.948 | 0.155 | 0.607 | 0.003 |
| Producer/Packager | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | ||||||
| Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | |
| TVC | 0.003 | 0.036 | ni | 0.119 | 0.691 | 0.303 | 0.058 | 0.837 | 0.953 | 0.777 |
| Enterobacteriaceae | 0.025 | 0.194 | ni | 0.391 | 0.418 | 0.702 | 0.397 | 0.892 | 0.522 | 0.153 |
| Coliforms | 0.061 | 0.432 | ni | 0.464 | 0.203 | 0.519 | 0.182 | 0.984 | 0.219 | 0.127 |
| E. coli | ni | 0.667 | ni | ni | ni | 0.506 | 0.391 | 0.500 | ni | ni |
| Staphylococcus spp. | 0.167 | 0.014 | ni | 0.500 | 0.443 | 0.732 | 0.927 | 0.500 | ni | ni |
| B. cereus | 0.329 | 0.339 | ni | ni | ni | 0.162 | ni | ni | ni | ni |
| Lactic acid bacteria | 0.007 | 0.574 | ni | 0.313 | 0.999 | 0.359 | 0.394 | 0.086 | 0.813 | 0.956 |
| Pseudomonas spp. | 0.692 | 0.237 | ni | 0.833 | 0.077 | 0.204 | 0.576 | 0.181 | 0.121 | 0.632 |
| Yeasts and molds | 0.682 | 0.093 | ni | 0.045 | 0.451 | 0.490 | 0.068 | 0.864 | 0.496 | 0.300 |
| Phenols | 0.062 | 0.665 | ni | 0.055 | 0.868 | 0.752 | 0.687 | 0.585 | 0.123 | 0.759 |
| DPPH | 0.446 | 0.444 | ni | 0.310 | 0.459 | 0.619 | 0.462 | 0.486 | 0.105 | 0.798 |
| FRAP | 0.203 | 0.312 | ni | 0.607 | 0.654 | 0.750 | 0.283 | 0.571 | 0.358 | 0.516 |
| ABTS | 0.091 | 0.952 | ni | 0.059 | 0.904 | 0.975 | 0.726 | 0.420 | 0.328 | 0.691 |
| CO2 | 0.000 | 0.000 | ni | 0.226 | 0.016 | 0.000 | 0.117 | 0.525 | 0.018 | 0.048 |
| H2O2 | 0.000 | 0.000 | ni | 0.407 | 0.000 | 0.000 | 0.011 | 0.09 | 0.044 | 0.155 |
| MDA | 0.000 | 0.001 | ni | 0.366 | 0.000 | 0.000 | 0.047 | 0.224 | 0.009 | 0.204 |
| Type of Salad | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lettuce | Lettuce + Cabbage | Lettuce + Endive/Radicchio | Lettuce + Rocket | Lettuce + Chives | Rocket | Other | ||||||||
| Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | |
| TVC | 0.386 | 0.898 | 0.361 | 0.263 | 0.029 | 0.027 | ni | 0.012 | 0.294 | 0.384 | 0.036 | 0.810 | 0.019 | 0.943 |
| Enterobacteriaceae | 0.244 | 0.313 | 0.516 | 0.366 | 0.011 | 0.024 | ni | 0.206 | 0.286 | 0.389 | 0.482 | 0.999 | 0.121 | 0.871 |
| Coliforms | 0.039 | 0.145 | 0.686 | 0.364 | 0.001 | 0.276 | ni | 0.261 | 0.130 | 0.453 | 0.500 | 0.602 | 0.146 | 0.189 |
| E. coli | ni | 0.181 | ni | 0.178 | ni | 0.356 | ni | 0.437 | ni | 0.423 | ni | 0.648 | 0.343 | 0.391 |
| Staphylococcus spp. | 0.647 | 0.213 | 0.423 | 0.698 | 0.356 | 0.065 | ni | 0.667 | ni | 0.186 | 0.363 | 0.632 | 0.686 | 0.391 |
| B. cereus | ni | ni | ni | ni | ni | ni | ni | 0.391 | ni | ni | ni | 0.348 | 0.343 | 0.391 |
| Lactic acid bacteria | 0.197 | 0.784 | 0.307 | 0.551 | 0.105 | 0.206 | ni | 0.025 | 0.051 | 0.469 | 0.223 | 0.985 | 0.060 | 0.391 |
| Pseudomonas spp. | 0.880 | 0.397 | 0.423 | 0.778 | 0.298 | 0.135 | ni | 0.040 | 0.551 | 0.089 | 0.856 | 0.220 | 0.633 | 0.547 |
| Yeasts and molds | 0.188 | 0.159 | 0.406 | 0.180 | 0.329 | 0.309 | ni | 0.001 | 0.855 | 0.705 | 0.306 | 0.167 | 0.685 | 0.952 |
| Phenols | 0.982 | 0.496 | 0.487 | 0.884 | 0.168 | 0.988 | ni | 0.796 | 0.164 | 0.010 | 0.768 | 0.342 | 0.174 | 0.207 |
| DPPH | 0.797 | 0.419 | 0.161 | 0.512 | 0.234 | 0.583 | ni | 0.466 | 0.071 | 0.241 | 0.978 | 0.925 | 0.759 | 0.243 |
| FRAP | 0.821 | 0.355 | 0.013 | 0.843 | 0.384 | 0.696 | ni | 0.256 | 0.227 | 0.149 | 0.497 | 0.044 | 0.271 | 0.223 |
| ABTS | 0.883 | 0.725 | 0.059 | 0.563 | 0.293 | 0.660 | ni | 0.387 | 0.092 | 0.101 | 0.085 | 0.534 | 0.059 | 0.371 |
| CO2 | 0.000 | 0.014 | 0.192 | 0.098 | 0.017 | 0.016 | ni | 0.016 | 0.173 | 0.035 | 0.004 | 0.085 | 0.001 | 0.050 |
| H2O2 | 0.000 | 0.002 | 0.005 | 0.016 | 0.000 | 0.040 | ni | 0.128 | 0.193 | 0.014 | 0.035 | 0.003 | 0.000 | 0.020 |
| MDA | 0.000 | 0.000 | 0.029 | 0.001 | 0.000 | 0.023 | ni | 0.069 | 0.314 | 0.105 | 0.004 | 0.006 | 0.000 | 0.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xylia, P.; Botsaris, G.; Skandamis, P.; Tzortzakis, N. Expiration Date of Ready-to-Eat Salads: Effects on Microbial Load and Biochemical Attributes. Foods 2021, 10, 941. https://doi.org/10.3390/foods10050941
Xylia P, Botsaris G, Skandamis P, Tzortzakis N. Expiration Date of Ready-to-Eat Salads: Effects on Microbial Load and Biochemical Attributes. Foods. 2021; 10(5):941. https://doi.org/10.3390/foods10050941
Chicago/Turabian StyleXylia, Panayiota, George Botsaris, Panagiotis Skandamis, and Nikolaos Tzortzakis. 2021. "Expiration Date of Ready-to-Eat Salads: Effects on Microbial Load and Biochemical Attributes" Foods 10, no. 5: 941. https://doi.org/10.3390/foods10050941






